文章信息
- 王建, 闫静, 张庆玲, 郑爱琴, 强胜, 刘琳莉, 伏建国, 宋小玲
- WANG Jian, YAN Jing, ZHANG Qinling, ZHENG Aiqin, QIANG Sheng, LIU Linli, FU Jianguo, SONG Xiaoling
- 从亲和性及F1的适合度评价抗除草剂转基因油菜向不同种群野芥菜的基因漂移风险
- Risk assessment on gene flow from transgenic oilseed rape to different wild Brassica juncea based on the compatibility and fitness of F1
- 南京农业大学学报, 2016, 39(4): 563-572
- Journal of Nanjing Agricultural University, 2016, 39(4): 563-572.
- http://dx.doi.org/10.7685/jnau.201601050
-
文章历史
- 收稿日期:2016-01-26
油菜属于常异花授粉作物,且能产生大量的花粉,同时在自然环境中存在许多野生近缘种,因此抗除草剂转基因油菜(Brassica napus,2n=38,AACC)的抗性基因漂移到野生近缘种,特别是近缘杂草中倍受关注[1]。抗性基因能否成功漂移到野生近缘种取决于两个方面,一是抗性作物和野生近缘种的亲和性,二是杂交和回交后代的适合度。抗性作物的抗性基因通过花粉传播到野生近缘种或近缘杂草中,产生携带抗性基因的杂交种是发生基因漂移的第一步,因此亲和性是抗性基因漂移的首要条件[2-4]。适合度是个体在特定环境条件下的生存和繁殖能力,决定着杂交或回交代能否在自然界中生存定居并建立种群[5],携带转基因杂种的适合度是决定抗性基因能否成功漂移到野生近缘种的重要因素。
转基因油菜和不同基因组野生近缘种的杂交亲和性受到基因组同源程度的显著影响[4, 6-7]。除基因组外,亲本的基因型对亲和性也有明显影响[8-9]。此外,杂交方向对亲和性也有一定的影响[10-11]。携带抗性基因的杂交及回交代的适合度依赖亲本基因组[12-14]、基因型[15-16]和测试环境条件[17-18]等。
在我国与甘蓝型油菜亲缘关系较近的杂草中最重要的是野芥菜(B.juncea var.gracilis)。野芥菜是芥菜型栽培油菜自逸的产物,20世纪80年代至今在西北地区严重危害小麦、青稞、油菜等作物[19]。近20年来,野芥菜已扩散到长江流域,成为广泛分布于农田和荒地的重要杂草,并呈现形态特点和遗传的多样性[20-21],且部分种群已对草甘膦产生了抗性[22]。如果抗除草剂转基因油菜特别是抗草甘膦转基因油菜的抗性基因能渗入到野芥菜中,将会对我国农田生态环境产生很大的负面影响;因此,不能忽视抗除草剂油菜的抗性基因向野芥菜的漂移。
甘蓝型油菜和芥菜(B.juncea,AABB,2n=36)有一个共同的基因组,增加了种间杂交和基因漂移的可能性。在田间自然条件下抗性油菜和芥菜能自发杂交[23-24]。在我国转基因油菜的抗性基因向野芥菜的漂移已有报道[25]。宋小玲等[7, 26]研究发现以江浦野芥菜为母本,甘蓝型油菜为父本很容易获得F1,但F1的结实率很低。如果油菜为转基因品种,F1全部表现了对除草剂的抗性[26]。F1和野芥菜回交[27-28],随着回交次数的增加,后代的结实率不断增加,回交3代生长健壮且结实率与野芥菜没有明显差异[28]。
以上研究的野芥菜种群数量有限,且杂交后代的生长都是在温室套袋情况下获得的结果,尚不清楚不同种群野芥菜和转基因油菜的杂交亲和性是否存在差异,以及F1在田间情况下是否能产生后代及后代的适合度。本文以我国不同省份采集的17个种群野芥菜为母本,以抗草甘膦和草丁膦转基因油菜为父本,研究不同种群野芥菜与2种转基因油菜的亲和性,F1在温室套袋自交和田间开放授粉情况下的生长和结实能力,以及F1后代的出苗率及抗性筛选后的存活植株率,明确不同种群野芥菜与转基因油菜的亲和性差异及F1在温室和田间的适合度等,为深入开展转基因油菜抗性基因漂移的研究提供资料。
1 材料与方法 1.1 材料转基因油菜:2种抗除草剂转基因油菜(Brassica napus L.;genome,AACC)都来自加拿大,抗草甘膦转基因油菜(DS-Roughr ider,Roundup Ready,event RT73)和抗草丁膦转基因油菜(Swallow,Liberty Link,event HCN92)都是纯合的。前者含有1个完整的cp4 epsps和gox基因以及它们各自的调控基因;后者含有2个拷贝的(连锁)的pat基因(http://www.agbios.com/dbase.php)。
各种群野芥菜信息见表 1。17个野芥菜种群分别采集于江苏、四川和陕西等省份。
| 序号No. | 采集地Location | 代号Abbreviation | 经纬度Latitude(°N)/Longitude(°E) |
| 1 | 江苏句容茅山Maoshan,Jurong,Jiangsu | JSMS | 31°57′/119°10′ |
| 2 | 江苏南通Nantong,Jiangsu | JSNT | 31°01′/120°51′ |
| 3 | 四川眉山Meishan,Sichuan | SCMS | 30°04′/103°50′ |
| 4 | 陕西汉中Hanzhong,Shaanxi | SXHZ | 34°14′/109°03′ |
| 5 | 陕西西安Xi′an,Shaanxi | SXXA | 33°05′/107°01′ |
| 6 | 青海西宁Xining,Qinghai | QHXN | 36°34′/101°49′ |
| 7 | 青海化隆Hualong,Qinghai | QHHL | 36°06′/102°16′ |
| 8 | 湖北十堰Shiyan,Hubei | HBSY | 30°21′/114°51′ |
| 9 | 湖北荆州Jingzhou,Hubei | HBJZ | 31°02′/112°12′ |
| 10 | 湖北鄂州Ezhou,Hubei | HBEZ | 31°40′/117°40′ |
| 11 | 安徽巢湖Chaohu,Anhui | HBCH | 31°40′/117°44′ |
| 12 | 安徽滁州Chuzhou,Anhui | AHCZ | 32°18′/118°18′ |
| 13 | 河南洛阳Luoyang,Henan | AHLY | 34°39′/112°24′ |
| 14 | 河南周口Zhoukou,Henan | HNZK | 33°22′/114°22′ |
| 15 | 浙江金华Jinhua,Zhejiang | ZJJH | 29°04′/119°39′ |
| 16 | 湖南岳阳Yueyang,Hunan | HNYY | 29°37′/113°01′ |
| 17 | 贵州贵阳Guiyang,Guizhou | GZGY | 26°28′/106°30′ |
2012 年3 月底于亲本第一朵花开后1 周开始进行自交和杂交试验。选取野芥菜成熟的未开花蕾,小心去掉雄蕊后立即授野芥菜自生或转基因油菜花粉,授粉后套袋挂牌。每种组合中父本分别和10株野芥菜进行杂交,每株至少杂交30 个花蕾,总计不少于300 个花蕾,野芥菜自交不少于200 个花蕾。同时对各野芥菜种群花蕾去雄50 个,不授粉检查去雄率。授粉的花蕾结实成熟后仔细收集每个角果,统计有效角果数、每角果饱满种子粒数及角果长度。比较各种群野芥菜授转基因油菜花粉与自花授粉的每角饱粒数及角果长的差异。
1.2.2 F1的出苗率及携带抗性基因的植株比例于2013年9月中下旬种植F1种子,用底部打孔的一次性塑料小杯(口径6.5 cm,深度9 cm)装满没有野芥菜发生的菜园土,浇足水后,每杯播种1粒,盖浅土。之后放置在温室中,进行正常的水分管理,15 d后统计出苗率。待植株长至4叶期时分别用草甘膦和草丁膦筛选2次,参照Song等[28]的方法进行。每次施用选择各自的母本野芥菜和相应的转基因油菜至少各10株作为阴阳对照。在药害症状明显时统计死亡和存活植株数量,对存活植株抽样进行分子检测,确认其是否携带抗性基因。分子检测的方法:对存活下来的后代植株随机抽样,每种后代选取5株以上,以野芥菜为阴性对照,相应的抗性油菜为阳性对照,采用PCR方法,确认抗性基因epsps和pat的存在。其中确认抗性基因epsps的方法同郑爱琴等[16]的方法。pat的扩增引物P1:5′AGGACAGAGCCACAAACAC-CAC3′,P2:5′ACCAACATCATGCCATCCACCA3′。反应体系与检测EPSPS的体系相同。反应条件:94 ℃ 5 min;94 ℃ 30 s,58 ℃ 30 s,72 ℃ 1 min,35个循环;72 ℃ 10 min。扩增获得的390 bp产物在10 g·L-1琼脂糖凝胶和100 V电压条件下电泳30 min,EB染色后在紫外灯下观察并拍照。
1.2.3 F1的温室和田间种植从经过抗性筛选和抗性检测后存活下来的植株中挑选生长健壮、大小适中的植株进行盆钵移栽。盆钵中所用土壤来源一致,均为菜园土,与腐殖质按质量比为1∶1混合均匀。所用盆钵大小一致(口径23 cm,深24 cm)。每盆移栽1株。每种群野芥菜及各自的F1分别移栽10株,随机排列。开花期时对各植株进行套袋自交防止窜粉,成熟后整株收获考种并收取种子。
田间试验在南京农业大学牌楼试验基地进行。从经过抗性筛选和抗性检测后存活下来的植株中挑选生长健壮、大小适中的植株按照试验设计进行田间移栽。每小区面积为1.2 m×2.5 m,种植5行、3列(每种群野芥菜及各自的F1各移栽5株,野芥菜种植在小区中间,各自的2种F1种植在母本两侧),每个处理重复3次,随机排列。试验地设专人看管,并设置围栏防止人为或动物破坏,试验地四周1 000 m内无十字花科植物生长。需要收获的材料单独收获和保藏,并由专业技术人员运输和保管。检测试验完毕后,除需要保留的材料外,其余材料一律烧毁。试验地保留边界标记,收获后及时翻耕。
1.2.4 生长指标的测量所测指标包括营养生长期指标(株高、茎粗、一次分枝数、地上部干生物量)和生殖生长期指标(单株有效角果数、角果长、每角果饱粒数)。具体方法同郑爱琴等[16]的方法。
1.2.5 F1后代的出苗率及携带抗性基因的植株比例获得的F1后代种植后统计出苗数,计算出苗率,并和各自的亲本野芥菜的出苗率进行比较。对后代饱满种子数量大于100粒的,每种F1种植25粒为1次重复,共重复4次;对后代饱满种子数量小于100粒的,每种F1种植50粒,10粒为1次重复,共重复5次。对出苗的幼苗进行管理,待长至3叶期喷施除草剂进行抗性筛选,统计存活率,并对存活植株进行抗性分子检测,方法同1.2.2节。
1.3 数据统计与分析数据的统计分析采用SPSS 17.0统计软件进行,多重比较采用Duncan′s新复极差测验。总适合度的计算方法同郑爱琴等[16]的方法。
2 结果与分析 2.1 杂交结实情况各种群野芥菜人工授粉的自交亲和性都很好,能结出12.11~19.14粒饱满种子,说明各种群野芥菜具有较高的自交亲和性。另外,去雄不授粉的花蕾子房不能正常发育,也没有结实,说明去雄彻底。各种群野芥菜自交及杂交结实情况见表 2。从表 2可以看出:17个野芥菜种群中有5个种群(分别为SCMS、HBEZ、HNLY、HNZK和HNYY)授2种转基因油菜花粉后及HBJZ授抗草丁膦转基因油菜的花粉所结角果的长度显著短于各自的野芥菜,其余各杂交组合所结角果长度和各自的野芥菜无显著差异。12个种群野芥菜授2种转基因油菜花粉后每角饱粒数和各自的野芥菜无显著差异,都能产生10.16~15.90粒饱满种子;只有2个种群(HNZK和GZGY)授两种转基因油菜的花粉及3个种群(SXHZ、HBJZ和HBEZ)授抗草丁膦转基因油菜的花粉每角饱粒数显著小于各自的野芥菜,但这些杂交组合的每角饱粒数至少也在8粒以上。这说明供试各种群野芥菜接受2种转基因油菜的花粉后都能产生大量后代。
| 自交或杂交Self-pollination orcrossing | 角果长/cmSiliquelength | 每角饱粒数Full seed numberper silique | 自交或杂交Self-pollination orcrossing | 角果长/cmSiliquelength | 每角饱粒数Full seed numberper silique | |
| JSMS | 3.83a | 13.44a | HBEZ | 4.25a | 13.94ab | |
| JSMS×L | 3.66a | 12.43a | HBEZ×L | 3.47c | 12.27b | |
| JSMS×R | 3.85a | 13.03a | HBEZ×R | 3.90b | 14.14a | |
| JSNT | 3.81a | 14.16a | AHCH | 4.21a | 13.35a | |
| JSNT×L | 3.53a | 13.53a | AHCH×L | 3.98a | 12.88a | |
| JSNT×R | 3.39a | 13.39a | AHCH×R | 4.01a | 13.34a | |
| SCMS | 4.44a | 12.67a | AHCZ | 3.72a | 13.99a | |
| SCMS×L | 3.75b | 10.16a | AHCZ×L | 3.26a | 12.04a | |
| SCMS×R | 3.87b | 10.57a | AHCZ×R | 3.27a | 11.51a | |
| SXHZ | 4.10a | 15.61a | HNLY | 4.24a | 19.14a | |
| SXHZ×L | 3.70a | 13.21b | HNLY×L | 3.62b | 15.52a | |
| SXHZ×R | 3.64a | 13.8ab | HNLY×R | 3.60b | 15.90a | |
| SXXA | 3.45a | 12.44a | HNZK | 4.24a | 13.23a | |
| SXXA×L | 3.57a | 13.48a | HNZK×L | 3.64b | 9.77b | |
| SXXA×R | 3.62a | 12.44a | HNZK×R | 3.55b | 9.10b | |
| QHXN | 3.03a | 11.83a | ZJJH | 4.16a | 14.80a | |
| QHXN×L | 3.50a | 14.82a | ZJJH×L | 4.17a | 13.58a | |
| QHXN×R | 3.46a | 13.51a | ZJJH×R | 3.88a | 12.64a | |
| QHHL | 3.32a | 12.11a | HNYY | 3.98a | 14.71a | |
| QHHL×L | 3.06a | 12.46a | HNYY×L | 3.52b | 11.14a | |
| QHHL×R | 3.16a | 12.98a | HNYY×R | 3.53b | 11.43a | |
| HBSY | 3.31a | 12.47a | GZGY | 4.41a | 12.28a | |
| HBSY×L | 3.02a | 11.06a | GZGY×L | 4.07a | 9.10b | |
| HBSY×R | 3.23a | 11.94a | GZGY×R | 3.93a | 8.19b | |
| HBJZ | 4.23a | 17.93a | ||||
| HBJZ×L | 3.61b | 13.84b | ||||
| HBJZ×R | 3.81ab | 15.38ab | ||||
| 注:1)×L 表示以抗草丁膦转基因油菜为父本,×R表示以抗草甘膦油菜为父本;2)相同母本不同小写字母表示差异显著(P<0.05)。 | ||||||
| Note:1)×L means glufosinate-resistant transgenic oilseed rape as male parent,×R means glyphosate-resistant transgenic oilseed rape as male parent.2)The data followed by different lowercase letter in the same femal parent are significantly different(P<0.05). The same as follows. | ||||||
温室种植后,各F1都能正常出苗,出苗率和野芥菜无显著差异,达到90%以上(数据未列出)。经过2次草甘膦筛选后,授抗草甘膦油菜花粉的F1植株都表现为抗性,作为阳性对照的抗草甘膦油菜生长良好,作为阴性对照的野芥菜呈现药害并死亡。药害症状表现为1周左右心叶基部开始黄化,并渐渐向叶上部扩展,直至整株变黄死亡。经过2次草丁膦筛选后,授抗草丁膦油菜花粉的F1植株都表现为抗性,作为阳性对照的抗草丁膦油菜生长良好,作为阴性对照的野芥菜药害症状在3 d后就很明显,首先老叶边缘失水、失绿、卷曲,然后逐渐向叶中央和心叶扩展,直至整株死亡。
根据设计的特异性引物对挑选的各F1的总DNA进行PCR扩增,分别得到了epsps基因的约527 bp的扩增条带和pat基因的约390 bp的扩增条带(图 1,以部分野芥菜种群为例),与相应的阳性对照扩增条带一致,阴性对照无扩增产物。这说明试验所用的F1为携带抗性基因的F1。
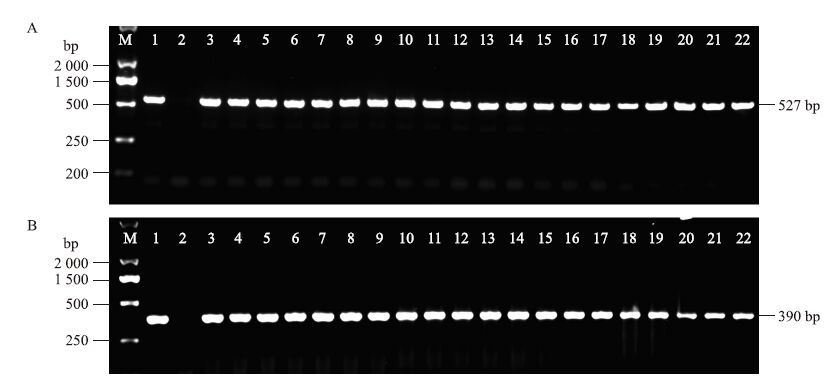
|
图 1 F1中epsps和pat基因的分子检测
Fig. 1 Detection of the epsps gene and pat gene in F1
A:M.DL2000 marker;1.野芥菜 Wild B.juncea;2.抗草甘膦转基因油菜Glyphosate-resistant transgenic oilseed rape;3~6.F1(JSMS×R);7~10.F1(SCMS×R);11~14.F1(QHHL×R);15~18.F1(HBEZ×R);19~22.F1(GZGY×R). B:M.DL2000 marker;1.野芥菜Wild B.juncea;2.抗草丁膦转基因油菜Glufosinate-resistant transgenic oilseed rape;3~6.F1(JSMS×L);7~10.F1(SCMS×L);11~14.F1(QHHL×L); 15~18.F1(HBEZ×L);19~22.F1(GZGY×L). |
所有供试F1的茎粗和单株生物量与作为母本的野芥菜无显著差异,因此数据未列出。从株高来看:以8个种群野芥菜(SCMS、SXXA、HBSY、HBEZ、AHCH、ANCZ、HNZK和ZJJH)为母本的2种F1的株高显著高于各自的野芥菜,JSNT与抗草甘膦转基因油菜的F1的株高显著高于野芥菜,其余各F1的株高与各自的野芥菜无显著差异(表 3)。所有的F1套袋后都没有产生有效角果,也没有种子。各种群野芥菜都能正常结实,每株有效角果数为133~523,每角饱粒数为12.15~17.28(数据未列出)。这说明供试的F1自交都不能产生种子。因此,在温室套袋自交的情况下,虽然F1都能进行很好的营养生长,但不能产生后代,因而适合度为零。
| 野芥菜或F1 Wild B.juncea or F1 | 株高/cm Plant height | 野芥菜或F1 Wild B.juncea or F1 | 株高/cm Plant height | |
| JSMS | 118.20a | HBEZ | 89.00b | |
| F1(JSMS×L) | 111.40a | F1(HBEZ×L) | 108.40a | |
| F1(JSMS×R) | 125.25a | F1(HBEZ×R) | 123.20a | |
| JSNT | 90.60b | AHCH | 116.00b | |
| F1(JSNT×L) | 103.75ab | AHCH×L | 141.20a | |
| F1(JSNT×R) | 108.80a | AHCH×R | 138.60a | |
| SCMS | 75.94b | AHCZ | 92.60b | |
| F1(SCMS×L) | 126.80a | F1(AHCZ×L) | 109.20a | |
| F1(SCMS×R) | 133.00a | F1(AHCZ×R) | 108.80a | |
| SXHZ | 122.00a | HNLY | 116.33a | |
| F1(SXHZ×L) | 118.60a | F1(HNLY×L) | 118.20a | |
| F1(SXHZ×R) | 136.80a | F1(HNLY×R) | 124.60a | |
| SXXA | 105.92b | HNZK | 107.60b | |
| F1(SXXA×L) | 125.50a | F1(HNZK×L) | 133.80a | |
| F1(SXXA×R) | 120.17a | F1(HNZK×R) | 144.80a | |
| QHXN | 136.60a | ZJJH | 115.27b | |
| F1(QHXN×L) | 126.00a | F1(ZJJH×L) | 156.66a | |
| F1(QHXN×R) | 123.60a | F1(ZJJH×R) | 157.80a | |
| QHHL | 133.20a | HNYY | 137.00a | |
| F1(QHHL×L) | 130.60a | F1(HNYY×L) | 122.75a | |
| F1(QHHL×R) | 131.80a | F1(HNYY×R) | 137.00a | |
| HBSY | 100.00b | GZGY | 117.75a | |
| F1(HBSY×L) | 119.00a | F1(GZGY×L) | 111.40a | |
| F1(HBSY×R) | 123.20a | F1(GZGY×R) | 119.00a | |
| HBJZ | 117.80a | |||
| F1(HBJZ×L) | 121.00a | |||
| F1(HBJZ×R) | 127.20a |
从表 4可见:各种群F1田间种植后生长良好。7个种群(SCMS、SXXA、HBEZ、AHCH、AHCZ、HNZK和ZJJH)中的2种F1的株高显著高于各自的野芥菜,2个种群(JSNT和SXHZ)野芥菜与抗草甘膦转基因油菜杂交获得的F1的株高显著高于亲本野芥菜,其他F1都和亲本野芥菜的株高相似。供试F1 单株有效角果数显著低于亲本野芥菜,但都能形成35.42~147.00个有效角果。大多数F1的单株干质量与亲本野芥菜相当,只有JSNT和SXHZ与抗草丁膦转基因油菜杂交的F1显著低于亲本野芥菜;SCMS、HBEZ和HNZK的2种F1的单株干质量显著高于亲本野芥菜。供试的F1 的角果长都显著短于亲本野芥菜,每角饱粒数只有0.19~0.78,显著低于各自的野芥菜,但单株都能结出几粒到近100粒饱满种子。总适合度除GZGY的2种F1的后代及QHHL授抗草甘膦转基因油菜花粉的F1显著低于母本外,其他的F1后代的总适合度都和各自的亲本野芥菜无显著差异,介于0.54~1.14之间。
| 野芥菜或F1Wild B.juncea or F1 | 株高/cmPlant height | 有效角果数Silique number per plant | 单株干质量/gDry aboveground biomass | 角果长/cmSilique length | 每角饱粒数Seeds number per silique | 总适合度Composite fitness |
| JSMS | 124.08a | 202.67a | 12.05a | 3.54a | 16.37a | 1.00a |
| F1(JSMS×L) | 128.08a | 57.50b | 11.09a | 1.91b | 0.53b | 0.56a |
| F1(JSMS×R) | 120.50a | 48.00b | 12.48a | 2.17b | 0.42b | 0.58a |
| JSNT | 92.62b | 207.35a | 19.67a | 3.59a | 16.80a | 1.00a |
| F1(JSNT×L) | 106.31ab | 44.33b | 13.39b | 2.53b | 0.35b | 0.55a |
| F1(JSNT×R) | 118.00a | 59.33b | 17.67ab | 2.70b | 0.61b | 0.65a |
| SCMS | 58.75b | 55.58a | 4.19b | 3.22a | 11.49a | 1.00a |
| F1(SCMS×L) | 106.05a | 45.25a | 8.52a | 2.28c | 0.57b | 1.08a |
| F1(SCMS×R) | 101.53a | 54.97a | 8.69a | 2.71b | 0.54b | 1.14a |
| SXHZ | 102.00b | 207.08a | 18.80a | 3.52a | 15.55a | 1.00a |
| F1(SXHZ×L) | 109.25ab | 119.58b | 11.77b | 2.55b | 0.78b | 0.61a |
| F1(SXHZ×R) | 113.30a | 93.60b | 14.83ab | 2.67b | 0.57b | 0.63a |
| SXXA | 105.92b | 249.44a | 19.09a | 3.77a | 15.46a | 1.00a |
| F1(SXXA×L) | 125.50a | 72.08b | 20.72a | 2.61b | 0.81b | 0.66a |
| F1(SXXA×R) | 120.17a | 80.92b | 17.48a | 2.82b | 0.51b | 0.63a |
| QHXN | 93.35a | 162.63a | 10.92a | 3.25a | 15.28a | 1.00a |
| F1(QHXN×L) | 103.67a | 40.33b | 9.26a | 2.36b | 0.53b | 0.59a |
| F1(QHXN×R) | 106.30a | 42.67b | 8.06a | 2.65b | 0.31b | 0.60a |
| QHHL | 123.33a | 129.33a | 11.86a | 3.45a | 14.97a | 1.00a |
| F1(QHHL×L) | 119.58a | 35.42b | 11.17a | 2.23b | 0.38b | 0.57ab |
| F1(QHHL×R) | 109.00a | 40.00b | 7.50a | 2.42b | 0.23b | 0.51b |
| HBSY | 108.08a | 193.83a | 16.78a | 3.49a | 17.56a | 1.00a |
| F1(HBSY×L) | 113.44a | 70.33b | 13.42a | 2.05b | 0.74b | 0.57a |
| F1(HBSY×R) | 113.67a | 69.22b | 12.03a | 2.53b | 0.68b | 0.58a |
| HBJZ | 73.00a | 168.13a | 8.80a | 3.64a | 17.85a | 1.00a |
| F1(HBJZ×L) | 91.67a | 48.67b | 8.21a | 2.24c | 0.26b | 0.62a |
| F1(HBJZ×R) | 105.88a | 46.03b | 12.12a | 2.61b | 0.29b | 0.77a |
| HBEZ | 58.38b | 111.75a | 4.99b | 3.21a | 12.21a | 1.00a |
| F1(HBEZ×L) | 90.00a | 58.50b | 11.58a | 2.08c | 0.58b | 1.02a |
| F1(HBEZ×R) | 102.87a | 55.52b | 12.16a | 2.50b | 0.32b | 1.10a |
| AHCH | 80.67b | 184.67a | 14.04a | 3.68a | 13.22a | 1.00a |
| F1(AHCH×L) | 106.00a | 48.75b | 13.27a | 1.89b | 0.31b | 0.61a |
| F1(AHCH×R) | 107.00a | 68.58b | 12.66a | 2.34b | 0.19b | 0.65a |
| AHCZ | 74.33b | 227.00a | 10.98a | 2.73a | 14.04a | 1.00a |
| F1(AHCZ×L) | 117.22a | 43.22b | 13.85a | 1.71c | 0.41b | 0.74a |
| F1(AHCZ×R) | 108.56a | 68.44b | 12.70a | 2.26b | 0.42b | 0.76a |
| HNLY | 102.83a | 179.83a | 15.39a | 3.04a | 15.39a | 1.00a |
| F1(HNLY×L) | 99.50a | 45.39b | 13.43a | 2.18b | 0.31b | 0.57a |
| F1(HNLY×R) | 116.13a | 83.60b | 12.96a | 2.46b | 0.75b | 0.66a |
| HNZK | 101.08b | 259.25a | 15.56b | 3.33a | 16.82a | 1.00a |
| F1(HNZK×L) | 131.00a | 106.61b | 24.08a | 2.10c | 0.61b | 0.78a |
| F1(HNZK×R) | 140.94a | 100.70b | 25.57a | 2.44b | 0.59b | 0.84a |
| ZJJH | 107.90b | 272.60a | 19.95a | 3.62a | 17.98a | 1.00a |
| F1(ZJJH×L) | 158.00a | 147.00b | 17.96a | 2.43b | 0.76b | 0.72a |
| F1(ZJJH×R) | 150.33a | 125.08b | 16.13a | 2.44b | 0.63b | 0.67a |
| HNYY | 107.29a | 232.05a | 20.48a | 3.95a | 15.38a | 1.00a |
| F1(HNYY×L) | 125.94a | 44.06b | 17.02a | 2.32b | 0.47b | 0.56a |
| F1(HNYY×R) | 118.73a | 46.10b | 15.24a | 2.48b | 0.41b | 0.54a |
| GZGY | 127.17a | 253.34a | 18.98a | 4.06a | 14.92a | 1.00a |
| F1(GZGY×L) | 105.44a | 85.67b | 13.14a | 2.35b | 0.30b | 0.49b |
| F1(GZGY×R) | 101.11a | 89.39b | 13.92a | 2.65b | 0.27b | 0.51b |
从表 5可知:绝大多数F1后代的出苗率显著低于各自的母本野芥菜,但各F1后代仍具有较高的出苗能力,除GZGY的2种F1后代的出苗率只有20%~24%外,其余出苗率均高于48%。HBJZ和ZJJH与2种转基因油菜的F1后代出苗率与亲本野芥菜相当,JSMS与抗草丁膦转基因油菜的F1后代及SXHZ、HBSY、HBEZ、AHCH、 HNZK和HNYY与抗草甘膦转基因油菜的F1后代的出苗率与各自的野芥菜无显著差异。
| 野芥菜或F1Wild B.juncea or F1 | 出苗率/%Emergence rate | 存活率/%Surviving rate | 野芥菜或F1Wild B.juncea or F1 | 出苗率/%Emergence rate | 存活率/%Surviving rate | |
| JSMS | 85a | HBEZ | 94a | |||
| PF1(JSMS×L) | 84a | 39.29 | PF1(HBEZ×L) | 56b | 53.57 | |
| PF1(JSMS×R) | 59b | 14.48 | PF1(HBEZ×R) | 82a | 32.94 | |
| JSNT | 98a | AHCH | 95a | |||
| PF1(JSNT×L) | 74b | 43.24 | PF1(AHCH×L) | 76b | 41.36 | |
| PF1(JSNT×R) | 48c | 21.43 | PF1(AHCH×R) | 86ab | 42.31 | |
| SCMS | 88a | AHCZ | 91a | |||
| PF1(SCMS×L) | 54b | 77.78 | PF1(AHCZ×L) | 73b | 42.11 | |
| PF1(SCMS×R) | 50b | 76.00 | PF1(AHCZ×R) | 65b | 32.35 | |
| SXHZ | 91a | HNLY | 84a | |||
| PF1(SXHZ×L) | 48b | 41.67 | PF1(HNLY×L) | 70b | 60.00 | |
| PF1(SXHZ×R) | 84a | 28.40 | PF1(HNLY×R) | 59b | 36.67 | |
| SXXA | 93a | HNZK | 91a | |||
| PF1(SXXA×L) | 70b | 12.86 | PF1(HNZK×L) | 58b | 48.28 | |
| PF1(SXXA×R) | 59b | 8.62 | PF1(HNZK×R) | 93a | 45.98 | |
| QHXN | 90a | ZJJH | 83a | |||
| PF1(QHXN×L) | 64b | 71.88 | PF1(ZJJH×L) | 82a | 43.00 | |
| PF1(QHXN×R) | 51b | 60.00 | PF1(ZJJH×R) | 73a | 18.75 | |
| QHHL | 89a | HNYY | 92a | |||
| PF1(QHHL×L) | 62b | 70.97 | PF1(HNYY×L) | 72b | 8.33 | |
| PF1(QHHL×R) | 62b | 30.43 | PF1(HNYY×R) | 78ab | 31.40 | |
| HBSY | 98a | GZGY | 78a | |||
| PF1(HBSY×L) | 73b | 62.50 | PF1(GZGY×L) | 20b | 75.00 | |
| PF1(HBSY×R) | 98a | 55.05 | PF1(GZGY×R) | 24b | 74.29 | |
| HBJZ | 84a | |||||
| PF1(HBJZ×L) | 82a | 46.67 | ||||
| PF1(HBJZ×R) | 73a | 38.89 | ||||
| 注:P 表示后代。P means progeny. | ||||||
用相应的除草剂抗性筛选后,各种群的F1后代都有不同比例的植株存活下来,其中4个种群(SCMS、QHXN、HBSY和GZGY)的2种F1后代的存活率都在50%以上;QHHL、HBEZ和HNLY与抗草丁膦油菜的F1后代的存活率高于50%,其他F1后代的存活率为8.33%~48.28%。根据设计的特异性引物对挑选的各回交后代的总DNA进行PCR扩增,分别得到了epsps基因的约527 bp的扩增条带和pat基因的约390 bp的扩增条带,与相应的阳性对照抗除草剂油菜扩增条带一致,阴性对照野芥菜无扩增产物(图略)。因此,经过除草剂筛选后存活的植株都携带抗性基因。这说明2种抗性基因都能在绝大多数供试的F1开放授粉后代中以不同频率传递下去。
3 讨论亲和性是发生基因流动的首要条件。基因组的同源程度越高,亲缘关系越近,亲和性也越好[3]。油菜(AACC)和野芥菜(AABB)都是同源四倍体,它们有一组共同的基因组,因此亲和性较好。前人研究已经证实转基因油菜(B.napus)和芥菜或野芥菜(B.juncea)能杂交产生后代[23-26],但同时成功的种间或属间杂交还依赖基因型[8-9]。本试验以不同省份采集的17个种群野芥菜与2种转基因油菜人工授粉的结果说明,供试的野芥菜授2种抗除草剂转基因油菜的花粉后都能大量结实,因此2种抗除草剂转基因油菜和各种群野芥菜的亲和性都很好,存在向野芥菜流动的可能性,且可能性相似。这提示我们转基因油菜在我国释放和各地的野芥菜都有发生基因漂移的潜在生态风险。野芥菜种群及转基因油菜的基因型对杂交亲和性影响不明显。
转基因作物中的抗性基因特别是抗除草剂基因能在杂种中长期持续传递下去是转基因渗入到杂草中的重要因子,否则杂种在除草剂选择压下就不能存活,因而也不会对农田生态系统和杂草防治造成很大影响[4, 29-30]。在本研究中,2种抗除草剂转基因油菜的抗性基因都能以100%的频率传递给供试的17个种群的野芥菜,并能以不同频率传递给F1的后代。这说明2种抗除草剂转基因油菜的抗性基因都能很容易通过花粉漂移进行扩散,因此,种植2种抗性转基因油菜时务必防范抗性基因的漂移。
从理论上讲,F1获得的后代属于回交1代,抗性分离比应该符合1∶1的孟德尔遗传定律。然而,在试验中发现不同F1的后代携带抗性基因植株的比例不同,造成这一结果的原因之一可能是由于检测的植株数量有限,样本量小;其次,不同的F1植株在田间环境下接受的花粉种类不一样,有的可能是来自亲本野芥菜的花粉,也可能来自不同种群的野芥菜花粉。由于野芥菜遗传结构的多样性及在减数分裂时可能发生A和C染色体的同源重组,导致抗性分离比不符合孟德尔遗传规律。已有研究表明:A基因组与C基因组具有部分同源性,二者间能够发生基因重组[31-33]。另外,基因插入位点和抗性基因启动子甲基化对抗性基因在后代中的传递和表达也有显著影响[34-35]。
适合度的考查在植物的整个生活史过程中都需要进行,包括营养生长阶段的竞争能力、生殖生长阶段的繁殖能力、种子的休眠和萌发能力等[5]。在本试验中,人工杂交获得F1的萌发率都达到90%以上,且和各自亲本野芥菜的萌发率相当,这说明F1种子的萌发已不是限制抗性基因在野外扩散的因素。尽管F1开放授粉获得的后代的出苗率绝大多数都显著低于亲本野芥菜,但大多数出苗率为48%~84%,因此,大多数F1开放授粉的后代都有在野外扩散的可能性。
温室种植各F1后,在营养生长方面各F1并不具有劣势,但生殖生长上劣势明显。这是因为转基因油菜和野芥菜的染色体分别为AACC=38和AABB=36条,杂交产生F1的染色体是AABC,2n=37,由于B 和C基因组在F1 减数分裂时不能正常配对造成的。不能产生后代就不能在自然界中生存定植,阻断了抗性基因的逃逸。在开放授粉条件下,各种群F1都具有良好的营养生长能力,都能形成有效角果并能结出饱满种子,总适合度上只有3种F1低于亲本,其他F1都和亲本野芥菜无显著差异。各F1后代的部分种子能正常出苗,且出苗的植株中有8%~78%的后代携带抗性基因。这些后代在田间自然情况下有可能继续和野芥菜或其他近缘种如栽培油菜回交,不断提高适合度,完成抗性基因的渗入,因此在田间环境下,转基因油菜的抗性基因渗入到野芥菜的可能性不容忽视。已有研究表明,野芥菜与抗除草剂转基因油菜的F1通过和野芥菜不断回交,后代的育性得以恢复[28]。
深入了解作物和野生近缘种的杂交后代在不同生态环境条件下的适应性,有助于从深层次开展转基因作物释放给环境造成的潜在危害[36]。Warwick等[37]证实携带抗草甘膦基因的转基因油菜和近缘种芜菁(B.rapa)的杂种即使在无除草剂选择压力的田间环境条件下也能持续生存6年;Londo 等[1]报道路边喷施草甘膦的雾滴漂移也能导致抗草甘膦油菜和芜菁后代适合度的提高;Hovich 等[38]报道在竞争环境条件下,野萝卜和栽培萝卜的杂交后代能产生更多的种子,提高了杂种定植成功的可能性。因此,还有待于进一步在田间不同条件下继续研究抗除草剂转基因油菜与不同种群野芥菜杂交后代的适合度。
| [1] | Londo J P,Bautista N S,Sagers C L,et al. Glyphosate drift promotes changes in fitness and transgene gene flow in canola(Brassica napus)and hybrids[J]. Annals of Botany,2010,106(6):957-965. |
| [2] | Dale P J. Spread of engineered genes to wild relatives[J]. Plant Physiology,1992,100(1):13-15. |
| [3] | Scheffler J A,Dale P J. Opportunities for gene transfer from transgenic oilseed rape(Brassica napus)to related species[J]. Transgenic Research,1994,3(5):263-278. |
| [4] | Devos Y,de Schrijver A,Reheul D. Quantifying the introgressive hybridisation propensity between transgenic oilseed rape and its wild/weedy relatives[J]. Environmental Monitoring and Assessment,2009,149(1/2/3/4):303-322. |
| [5] | Jenczewski E,Ronfort J,Chèvre A M. Crop-to-wild gene flow,introgression and possible fitness effects of transgenes[J]. Environmental Biosafety Research,2003,2(1):9-24. |
| [6] | Rieger M A,Lamond M,Preston C,et al. Pollen-mediated movement of herbicide resistance between commercial canola fields[J]. Science,2002,296(5577):2386-2388. |
| [7] | 宋小玲,强胜. 三种类型油菜和野油菜亲和性及F1代的适合度*——潜在基因漂移研究[J]. 应用与环境生物学报,2003,9(4):375-361. Song X L,Qiang S. Sexual compatibility of three species of oilseed rape(Brassica spp.)with wild rapes(B.juncea var.gracilis Tsen et Lee)and the fitness of F1*:potential for gene transfer[J]. Chinese Journal of Applied Environmental Biology,2003,9(4):357-361(in Chinese with English abstract). |
| [8] | Baranger A,Chèvre A M,Eber F,et al. Effect of oilseed rape genotype on the spontaneous hybridization rate with a weedy species:an assessment of transgene dispersal[J]. Theoretical and Applied Genetics,1995,91(6/7):956-963. |
| [9] | Guéritaine G,Bonavent J F,Darmency H. Variation of prezygotic barriers in the interspecific hybridization between oilseed rape and wild radish[J]. Euphytica,2003(3):349-353. |
| [10] | 戴林建,李栒,张四伟,等.芸芥与芸薹属属间杂交亲和性研究[J]. 作物研究,2002,16(3):123-125. Dai L J,Li X,Zhang S W,et al. Crossabilities between Brassica spp.and Eruca sativa[J]. Crop Research,2002,16(3):123-125(in Chinese with English abstract). |
| [11] | 戴兴临,程春明,潘斌,等.油菜与蔊菜远缘杂交亲和性研究初报[J]. 江西农业学报,2001,13(1):60-61. Dai X L,Cheng C M,Pan B,et al. Preliminary study on compatibility of distant hybridization between rape(Brassica napus)and Rorippa indica[J]. Acta Agriculturae Jiangxi,2001,13(1):60-61(in Chinese with English abstract). |
| [12] | Darmency H,Fleury A. Mating system in Hirschfeldia incana and hybridization to oilseed rape[J]. Weed Research,2000,40(2),231-238. |
| [13] | Chèvre A M,Eber F,Baranger A,et al. Gene flow from transgenic crops[J]. Nature,1997,389(6654):924. |
| [14] | Chèvre A M,Eber F,Baranger A,et al. Characterisation of backcross generations obtained under field conditions from oilseed rape-wild radish F1 interspecific hybrids:an assesment of transgene dispersal[J]. Theoretical and Applied Genetics,1998,97(1):80-98. |
| [15] | Gueritaine G,Sester M,Eber F,et al. Fitness of backcross six of hybrids between transgenic oilseed rape(Brassica napus)and wild radish(Raphanus raphamistrum)[J]. Molecular Ecology,2002,11(8),1419-1426. |
| [16] | 郑爱琴,强胜,宋小玲. 抗除草剂转基因油菜与野芥菜的杂交1代与5种常规栽培油菜回交后代的适合度[J]. 应用与环境生物学报,2014,20(3):337-344. Zheng A Q,Qiang S,Song X L. Fitness of backcross between F1(wild B.juncea×herbicide-resistant transgenic oilseed rape)and 5 conventional cultivate varieties[J]. Chinese Journal of Applied Environmental Biology,2014,20(3):337-344(in Chinese with English abstract). |
| [17] | Hauser T P,Damgaard C,Jørgensen R B. Frequency-dependent fitness of hybrids between oilseed rape(Brassica napus)and weedy B.rapa(Brassicaceae)[J]. American Journal of Botany,2003,90(4):571-578. |
| [18] | Campbell L G,Snow A A. Competition alters life history and increases the relative fecundity of crop-wild radish hybrids(Raphanus spp.)[J]. New Phytologist,2007,173(3):648-660. |
| [19] | 郭青云,涂鹤龄,邱学林,等. 野芥菜田间发生规律与防除技术研究[J]. 青海农林科技,1998(4):38-41. Guo Q Y,Tu H L,Qiu X L,et al. Study on the occurrance and control technology of wild B.juncea in field[J]. Science and Technology of Agriculture and Forestry in Qinghai,1998(4):38-41(in Chinese). |
| [20] | Huangfu C H,Song X L,Qiang S. ISSR variation within and among wild Brassica juncea populations:implication for herbicide resistance evolution[J]. Genetic Resources and Crop Evolution,2009,56(7):913-924. |
| [21] | Huangfu C H,Song X L,Qiang S. Morphological disparities in the epidermal and anatomical features of the leaf among wild Brassica juncea populations[J]. Weed Biology and Management,2009,9(3):234-242. |
| [22] | Huangfu C H,Song X L,Qiang S,et al. Response of wild Brassica juncea populations to glyphosate[J]. Pest Managment Science,2007,63(11):1133-1140. |
| [23] | Jørgensen R B,Andersen B,Landbo L,et al.Spontaneous hybridization between oilseed rape(Brassica napus)and weedy relatives[J]. Acta Horticulturae,1996(407):193-200. |
| [24] | Liu Y B,Wei W,Ma K P,et al. Backcrosses to Brassica napus of hybrids between B.juncea and B.napus as a source of herbicide-resistant volunteer-like feral populations[J]. Plant Science,2010,179:459-465. |
| [25] | 浦惠明,戚存扣,张洁夫,等.转基因抗除草剂油菜对十字花科杂草的基因漂移[J]. 生态学报,2005,25(4):910-916. Pu H M,Qi C K,Zhang J F,et al. The studies on gene flow from herbicide-tolerant rapeseed to cruciferous weeds[J]. Acta Ecologica Sinica,2005,25(4):910-916(in Chinese with English abstract). |
| [26] | 宋小玲,皇甫超河,强胜. 抗草丁膦和抗草甘膦转基因油菜的抗性基因向野芥菜的流动[J]. 植物生态学报,2007,31(4):729-737. Song X L,Huangfu C H,Qiang S. Gene flow from transgenic glufosinate- or glyphosate-tolerant oilseed rape to wild rape[J]. Journal of Plant Ecology,2007,31(4):729-737(in Chinese with English abstract). |
| [27] | Di K,Stewart CN,Jr,Wei W,et al. Fitness and maternal effects in hybrids formed between transgenic oilseed rape(Brassica napus L.)and wild brown mustard[B.juncea(L.)Czern et Coss.]in the field[J]. Pest Management Science,2009,65(7):753-760. |
| [28] | Song X L,Wang Z,Zuo J,et al. Potential gene flow of two herbieide-tolerant transgenes from oilseed rape to wild B.juncea var.gracilis[J]. Theoretical and Applied Genetics,2010,120(8):1501-1510. |
| [29] | Chèvre A M,Adamczyk K,Eber F,et al. Modelling gene flow between oilseed rape and wild radish:Ⅰ. Evolution of chromosome structure[J]. Theoretical Applied Genetics,2007,114(2):209-221. |
| [30] | Jørgensen R B,Hauser T,D'Hertefeldt T,et al. The variability of processes involved in transgene dispersal-casestudies from Brassica and related genera[J]. Environmental Science and Pollution Research,2009,16(4):389-395. |
| [31] | Hansen L B,Siegismund H R,Jørgensen R B. Progressive introgression between Brassica napus(oilseed rape)and B.rapa[J]. Heredity,2003(3):276-283. |
| [32] | Leflon M,Eber F,Letanneur J C,et al. Pairing and recombination at meiosis of Brassica rapa(AA)×Brassica napus(AACC)hybrids[J]. Theoretical and Applied Genetics,2006,113(8):1467-1480. |
| [33] | Tsuda M,Okuzaki A,Kaneko Y,et al. Persistent C genome chromosome regions identified by SSR analysis in backcross progenies between Brassica juncea and B.napus[J]. Breeding Science,2012,62(4):328-333. |
| [34] | Zhu B,Lawrence J R,Warwick S I,et al. Inheritance of GFP-Bt transgenes from Brassica napus in backcrosses with three wild B.rapa accessions[J]. Environmental Biosafety Research,2004,3(1):45-54. |
| [35] | Lee G H,Park E Y,Park Y D. Transgene instability due to promoter hypermethylation and deletion intransgenic Nicotiana benthamiana[J]. Horticulture Environment and Biotechnology,2014,55(1):42-49. |
| [36] | Campbell L G,Snow A A. Can feral weeds evolve from cultivated radish(Raphanus sativus,Brassicaceae)?[J]. American Journal of Botany,2009,96(2):498-506. |
| [37] | Warwick S I,Légère A,Sinard M J,et al. Do escaped transgenes persist in nature? The case of an herbicide resistance transgene in a weedy Brassica rapa population[J]. Molecular Ecololgy,2008,17(5):1387-1395. |
| [38] | Hovick S M,Campbell L G,Snow A A,et al. Hybridization alters early life-hostory traits and increases plant colonization success in a novel region[J]. The Ametican Naturalist,2012,179(2):192-203. |




