文章信息
- 刘晓东, 李月, 王若仲, 代培红, 刘超, 石书兵
- LIU Xiaodong, LI Yue, WANG Ruozhong, DAI Peihong, LIU Chao, SHI Shubing
- 过表达GH3-5提高拟南芥抗旱的分子机制
- Molecular mechanism of drought tolerance conferred by overexpression of GH3-5
- 南京农业大学学报, 2016, 39(4): 557-562
- Journal of Nanjing Agricultural University, 2016, 39(4): 557-562.
- http://dx.doi.org/10.7685/jnau.201604019
-
文章历史
- 收稿日期:2016-04-07
2. 湖南农业大学植物激素与生长发育湖南省重点实验室, 湖南 长沙 410128
2. Hunan Provincial Key Laboratory of Phytohormones and Growth Development, Hunan Agricultural University, Changsha 410128, China
生长素是植物中一类重要激素,在植物生长发育的许多过程中扮演重要的角色,如胚胎发生、维管组织分化、向光性和植株的形态建成等[1]。其中吲哚-3-乙酸(indole-3-acetic acid,IAA)是植物体内含量最高的一种生长素。近几年研究发现IAA对植物的抗逆性也有重要的调控作用。利用IAA缺失和过量累积的突变体,证明了IAA正向调控了拟南芥和其他植物的抗旱性[2-3]。IAA通过控制下游响应基因的表达调控了植物对环境的反应,其调控表达的基因主要包括Aux/IAA、SAUR(small auxin up RNAs)和GH3三个家族[4]。其中GH3基因家族在拟南芥基因组中包含20个成员,这些基因按GH3-1至GH3-20命名。研究表明一些拟南芥GH3蛋白显示出腺苷酰化植物激素的活性[5],这些激素包括茉莉酸(jasmonic acid,JA),吲哚-3-乙酸和水杨酸(salicylic acid,SA)[6]。根据有无腺苷酰化植物激素的能力及腺苷酰化底物特异性的不同,将19个GH3蛋白(除去只具部分蛋白序列的GH3-20)分为三类:Ⅰ类可腺苷酰化JA,包括2个成员;Ⅱ类基因可腺苷酰化IAA,包括8个成员;Ⅲ类尚未发现它们在体外可腺苷酰化任何植物激素,包括9个成员。其中Ⅱ类基因在植物体内调控IAA的动态平衡[5]。研究发现大多数GH3基因启动子除包含生长素响应元件(AuxRE)外,还含有一些逆境相关响应元件,在不同植物中有多个GH3基因受非生物逆境诱导表达[7, 8, 9, 10]。
SA是在植物抵御病原菌入侵中扮演关键作用的一种激素[11]。除此之外研究还发现SA也能有效提高植物对多种非生物逆境的抗性,如高盐[12]、渗透胁迫[13]、干旱[14]和高温[15]。在SA合成途径中SID2是一个关键酶基因,编码异分枝酸合酶又称ICS1(isochorismate synthase),其对应的突变体sid2中SA的合成显著降低[16]。体内合成的SA通过一系列信号转导途径来行使它的调控功能,而NPR1(non-expresser of pathogenesis-related gene 1)是SA激素信号转导途径的关键调控因子,在SA调控的抗病功能中扮演关键角色[17]。但是在植物体内存在两种SA信号转导途径:NPR1依赖的和NPR1不依赖的信号途径,两者的功能并不相同[18]。
拟南芥GH3-5和GH3-6基因属于Ⅱ类GH3基因家族。体外生化试验中GH3-6只能腺苷酰化IAA,而GH3-5除可腺苷酰化IAA外,同时还可以腺苷酰化SA[5]。前人研究发现GH3-5和GH3-6基因过量表达后,通过腺苷酰化IAA,打破了生长素在体内的动态平衡,抑制了植株正常的生长发育,它们对应的过量表达株系gh3.5-1D和dfl1-D都表现出植株异常矮小,表明GH3-5和GH3-6基因在调控植株生长发育方面具有相似的功能[19, 20]。前期的研究发现GH3-6基因通过调控植物体内生长素的水平,进而调控了植物对干旱的反应过程,dfl1-D植株对干旱非常敏感(文章待发表)。然而另有研究发现GH3-5基因过量表达植株对干旱却表现出较强的抗性[7]。为了解析GH3-5基因过量表达植株抗旱能力发生逆转的机制,本研究从GH3-5与GH3-6腺苷酰化激素的功能差异入手,测定了干旱胁迫后gh3.5-1D和dfl1-D中SA的含量,以揭示GH3-5基因在拟南芥干旱适应过程中与GH3-6基因不同的抗旱机制。
1 材料与方法 1.1 材料dfl1-D(GH3-6基因过表达突变体)及其对应的野生型拟南芥Landsberg erecta生态型(Ler)种子,gh3.5-1D(GH3-5基因过表达植株)、NahG、sid2-2、npr1单突变体和gh3.5-1D/NahG、gh3.5-1D/npr1双突变体以及它们对应的野生型拟南芥Columbia生态型(Col-0)种子,均由中国科学院上海植物生理生态研究所惠赠,各突变体的详细描述见文献[20-21]。拟南芥种子在6%(体积分数)的次氯酸钠中浸泡5 min,之后用无菌水冲洗6次,并播种于1/2 MS固体培养基上,黑暗下4 ℃低温处理4 d,然后转入22 ℃人工气候室(光、暗时间分别为15、9 h)中培养。生长7 d后的幼苗移栽于饱含营养液的人工土壤(蛭石、草炭、珍珠岩体积比为6∶3∶1)中,转入22 ℃人工气候室(光、暗时间分别为15、9 h)中继续生长。
1.2 方法 1.2.1 拟南芥的抗旱性鉴定幼苗移入土壤继续生长20 d后的拟南芥进行断水干旱处理,及时观察突变体和野生型的表型差异,约25 d左右时进行复水处理,复水2 d后拍照,并统计存活率。试验重复3次,每次每个样品干旱处理株数大于15。
1.2.2 SA和ABA含量测定取生长30 d的野生型和突变体完整植株,除去根部多余土壤,然后植株分成2份,一份进行空气干旱处理,另一份将其根部置于湿润的滤纸上,作为对照。每一种处理都进行3个重复,每个重复包括6株拟南芥。3 h后取莲座叶的组织用于激素含量测定,按照Zhang等[20]方法进行SA含量的测定,重复3次。按照王若仲等[22]方法进行ABA含量的测定,重复3次。
1.3 数据统计所有试验结果均为3次重复的平均值±标准差。采用Microsoft Excel 2007软件对数据进行整理和方差分析;采用DPS v7.05软件的LSD最小显著差数法进行差异显著性检验。
2 结果与分析 2.1 干旱胁迫处理后dfl1-D和gh3.5-1D中SA含量GH3-5和GH3-6基因同属于Ⅱ类可腺苷酰化IAA的GH3基因家族,但它们对应的过表达植株gh3.5-1D与dfl1-D在抗旱性上却完全相反。dfl1-D对干旱敏感,而GH3-5基因过表达植株对干旱却有较强的抗性[7]。由于GH3-5和GH3-6基因在腺苷酰化SA的功能上存在差异,而且SA也参与了植物的抗旱反应[23-24]。为了进一步解释上述现象,我们测定了dfl1-D和gh3.5-1D中游离SA的含量。结果(图 1)显示,对于dfl1-D来说,无论是野生型还是突变体,干旱处理3 h后SA的含量都会比干旱处理前0 h样品增加1倍左右。但与野生型(Ler)相比,干旱处理前后dfl1-D中SA的含量并没有明显差别;而对于gh3.5-1D来说,与dfl1-D不同的是,gh3.5-1D突变体干旱处理3 h后体内的SA含量比野生型(Col-0)增加了1倍。推测SA含量的增加可能导致了上述抗旱表型的差异。

|
图 1 干旱胁迫前后dfl1-D和gh3.5-1D中的游离水杨酸(SA)含量
Fig. 1 Free salicylic acid(SA)content after drought treatment in dfl1-D and gh3.5-1D
柱状图上的不同字母表示差异达到5%显著水平。 Different letters on each column are significantly different at 5% level,the same below. |
NahG是一种SA水解酶基因,研究表明NahG转基因植物不能积累SA[25]。如上文所述,SID2是SA合成途径中的一个关键酶,在SID2功能失活突变体sid2-2中SA的合成受阻。为了进一步验证SA的抗旱功能和SA含量的增加是否导致了gh3.5-1D突变体抗旱性的逆转,我们对NahG(NahG转基因拟南芥)和sid2-2单突变体以及gh3.5-1D/NahG双突变体的抗旱性进行了鉴定。结果(图 2)显示,激素SA缺失突变体NahG和 sid2-2复水后,死亡的植株比例显著高于野生型,提示SA正调控了拟南芥的抗旱性(图 2-A和B)。与gh3.5-1D相比,gh3.5-1D/NahG双突变体抗旱性显著下降,其存活率与NahG相似(图 2-C和D),显示出gh3.5-1D突变体较高的抗旱能力依赖于SA。

|
图 2 gh3.5-1D的抗旱性依赖于SA
Fig. 2 Drought tolerance depends on SA in gh3.5-1D
A、B和C:拟南芥干旱胁迫复水2 d后的表型;D:各突变体复水2 d后的存活率 A,B and C:The phenotype of plants 2 days after rewatering;D:Survival rate of plants 2 days after rewatering |
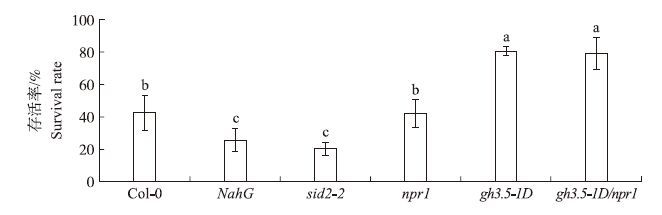
植物体内存在NPR1依赖的和不依赖的两种SA信号途径,究竟是哪种信号途径介导了GH3-5调控的抗旱性?为此我们鉴定了npr1和gh3.5-1D/npr1突变体的抗旱性。结果发现在激素SA积累缺失突变体NahG和sid2-2抗旱性明显下降的情况下,与对照相比,npr1和gh3.5-1D/npr1突变体的抗旱性并没有减弱(图 3),提示NPR1基因不参与拟南芥的干旱适应过程,也可能不参与GH3-5基因过量表达植株gh3.5-1D的抗旱性。
|
图 3 gh3.5-1D的抗旱性不依赖NPR1 Fig. 3 Drought tolerance does not depend on NPR1 in gh3.5-1D |
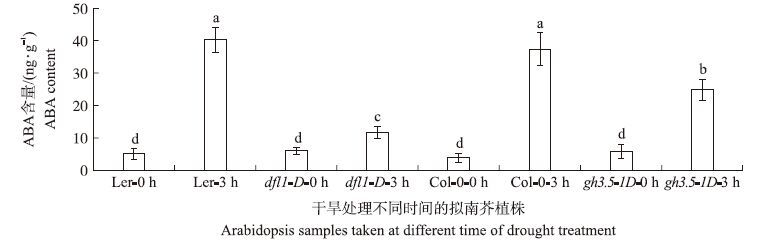
脱落酸(ABA)在植物抗旱中扮演关键角色。有研究表明外源生长素能诱导NCED基因的表达,并进而上调了ABA的水平[26],而SA也能诱导ABA的积累[27]。为此我们同时也测定了突变体中ABA的含量。结果显示,与野生型相比,干旱胁迫3 h后dfl1-D中ABA含量减少了71.2%,而gh3.5-1D只减少了34%(图 4)。
|
图 4 干旱胁迫前后dfl1-D和gh3.5-1D中ABA含量 Fig. 4 ABA content after drought treatment in dfl1-D and gh3.5-1D |
GH3-5和GH3-6基因过表达的突变体gh3.5-1D和dfl1-D都表现出生长素缺失的表型[19-20],显示这2个基因具有相似的功能。另外GH3-5显著受干旱诱导表达[7],而GH3-6基因也同样受干旱诱导表达。然而两者过表达突变体的抗旱能力却完全相反[7]。GH3-5和GH3-6虽然同属于可腺苷酰化IAA的Ⅱ类GH3基因家族,但目前的研究发现两者在活性功能上还是存在一个明显的差别,即GH3-5蛋白还具有腺苷酰化SA的活性[5]。已有研究发现由于GH3-5同时具有腺苷酰化IAA和SA这两种功能,导致其在抗病反应中具有双功能的角色,一方面通过调控IAA的水平减弱了抗病性,另一方面则通过提高SA的含量,增强了抗病性[20]。GH3-5在抗病反应中的双功能角色是否也存在于抗旱反应中?前期对dfl1-D突变体的研究发现,GH3-6基因过量表达后,通过调控IAA抑制了干旱胁迫后体内ABA的合成,进而减弱了植株的抗旱能力(文章待发表)。而目前已经研究证明SA在抗旱反应中扮演正调控的角色[23-24]。GH3-5腺苷酰化SA的功能活性是否改变了gh3.5-1D体内SA的水平,进而产生了与dfl1-D突变体完全相反的抗旱表型?本研究结果发现,干旱胁迫后,植株体内SA的含量都会明显上升,但是gh3.5-1D体内SA的含量要显著高于野生型;而dfl1-D突变体与对应野生型相比SA的含量并没有明显区别。gh3.5-1D体内高水平SA的含量是否就是产生gh3.5-1D抗旱能力增强的原因?即SA减少后gh3.5-1D的抗旱性是否丧失?为此我们鉴定了gh3.5-1D/NahG双突变体的抗旱表型。结果显示,NahG基因的引入,使gh3.5-1D植株获得的抗旱能力丧失,其干旱胁迫存活率与NahG株系相似,表明gh3.5-1D抗旱能力的增强完全依赖于SA水平的增加。
有研究发现IAA可以促进ABA的合成[26],而SA也能诱导ABA的积累[27-28]。这进一步解释了图 4的结果,干旱胁迫后,一方面由于GH3-5和GH3-6的腺苷酰化IAA的活性,导致IAA降解,从而使gh3.5-1D和dfl1-D中ABA的合成受阻;另一方面与dfl1-D不同的是,干旱胁迫后,gh3.5-1D中SA的含量比野生型增加了1倍。可能在另一种途径上又刺激了ABA的合成,进而最终导致gh3.5-1D体内ABA的含量并没有显著减少。上述结果显示出GH3-5在抗旱反应中可能也具有双功能的角色。虽然干旱胁迫后gh3.5-1D中ABA的含量依然低于野生型,但可能由于SA能激活抗氧化酶活性,消除了活性氧对植株的伤害[23],最终使gh3.5-1D的干旱存活率高于野生型。
GH3-5和GH3-6基因在拟南芥抗旱功能上的差异表现,同样也存在于其他植物不同GH3基因中。如过量表达OsGH3-2基因的水稻显著降低了游离IAA的含量,导致植株抗旱能力减弱[29]。但过量表达OsGH3-13基因的水稻虽然也显著降低了IAA的含量,但植株的抗旱能力却明显增强[10],这也暗示着OsGH3-2基因和OsGH3-13基因在水稻抗旱中扮演不同的角色,其中SA可能也是导致这种功能差异的原因。
NPR1是SA信号转导途径中一个关键的信号组分,在SA介导的抗病反应中发挥重要作用[17]。在非生物逆境方面,SA介导的抗盐性依赖于NPR1[30]。然而本研究结果显示SA介导的抗旱性可能并不依赖NPR1,这暗示SA可能通过NPR1依赖的信号转导途径调控了植物的抗盐性,而通过不依赖NPR1的信号转导途径调控了抗旱性。因此有必要对SA介导的抗逆信号转导途径,尤其NPR1不依赖的信号途径进行深入的研究。
| [1] | Wang R,Estelle M. Diversity and specificity:auxin perception and signaling through the TIR1/AFB pathway[J]. Current Opinion in Plant Biology,2014,21:51-58. |
| [2] | Shi H,Chen L,Ye T,et al. Modulation of auxin content in Arabidopsis confers improved drought stress resistance[J]. Plant Physiology and Biochemistry,2014,82:209-217. |
| [3] | Kim J I,Baek D,Park H C,et al. Overexpression of Arabidopsis YUCCA6 in potato results in high-auxin developmental phenotypes and enhanced resistance to water deficit[J]. Molecular Plant,2013,6(2):337-349. |
| [4] | Hagen G,Guilfoyle T. Auxin-responsive gene expression:genes,promoters and regulatory factors[J]. Plant Molecular Biology,2002,49(3/4):373-385. |
| [5] | Staswick P E,Tiryaki I,Rowe M L. Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic,salicylic,and indole-3-acetic acids in an assay for adenylation[J]. The Plant Cell,2002,14(6):1405-1415. |
| [6] | Chang K H,Xiang H,Dunaway-Mariano D. Acyl-adenylate motif of the acyl-adenylate/thioester-forming enzyme superfamily:a site-directed mutagenesis study with the Pseudomonas sp. strain CBS34-chlorobenzoate:coenzyme A ligase[J]. Biochemistry,1997,36(50):15650-15659. |
| [7] | Park J E,Park J Y,Kim Y S,et al. GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis[J]. Journal of Biological Chemistry,2007,282(13):10036-10046. |
| [8] | Singh V K,Jain M,Garg R. Genome-wide analysis and expression profiling suggest diverse roles of GH3 genes during development and abiotic stress responses in legumes[J]. Frontiers in Plant Science,2015,5:789. |
| [9] | Feng S,Yue R,Tao S,et al. Genome-wide identification,expression analysis of auxin-responsive GH3 family genes in maize(Zea mays L.)under abiotic stresses[J]. Journal of Integrative Plant Biology,2015,57(9):783-795. |
| [10] | Zhang S W,Li C H,Cao J,et al. Altered architecture and enhanced drought tolerance in rice via the down-regulation of indole-3-acetic acid by TLD1/OsGH3.13 activation[J]. Plant Physiology,2009,151(4):1889-1901. |
| [11] | Kumar D. Salicylic acid signaling in disease resistance[J]. Plant Science,2014,228:127-134. |
| [12] | Nazar R,Umar S,Khan N A. Exogenous salicylic acid improves photosynthesis and growth through increase in ascorbate-glutathione metabolism and S assimilation in mustard under salt stress[J]. Plant Signaling and Behavior,2015,10(3):e1003751. |
| [13] | Naser Alavi S M,Arvin M J,Kalantari K M. Salicylic acid and nitric oxide alleviate osmotic stress in wheat(Triticum aestivum L.)seedlings[J]. Journal of Plant Interactions,2014,9(1):683-688. |
| [14] | Fayez K A,Bazaid S A. Improving drought and salinity tolerance in barley by application of salicylic acid and potassium nitrate[J]. Journal of the Saudi Society of Agricultural Sciences,2014,13(1):45-55. |
| [15] | Khan M I,Iqbal N,Masood A,et al. Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation[J]. Plant Signaling and Behavior,2013,8(11):e26374. |
| [16] | Wildermuth M C,Dewdney J,Wu G,et al. Isochorismate synthase is required to synthesize salicylic acid for plant defence[J]. Nature,2001,414(6863):562-565. |
| [17] | Gao Q M,Zhu S,Kachroo P,et al. Signal regulators of systemic acquired resistance[J]. Frontiers in Plant Science,2015,6:228. |
| [18] | Janda M,Ruelland E. Magical mystery tour:salicylic acid signalling[J]. Environmental and Experimental Botany,2015,114:117-128. |
| [19] | Nakazawa M,Yabe N,Ichikawa T,et al. DFL1,an auxin-responsive GH3 gene homologue,negatively regulates shoot cell elongation and lateral root formation,and positively regulates the light response of hypocotyl length[J]. The Plant Journal,2001,25(2):213-221. |
| [20] | Zhang Z Q,Li Q,Li Z M,et al. Dual regulation role of GH3.5 in salicylic acid and auxin signaling during Arabidopsis-Pseudomonas syringae interaction[J]. Plant Physiology,2007,145(2):450-464. |
| [21] | Zhang Z,Wang M,Li Z,et al. Arabidopsis GH3.5 regulates salicylic acid-dependent and both NPR1-dependent and independent defense responses[J]. Plant Signaling and Behavior,2008,3(8):537-542. |
| [22] | 王若仲,萧浪涛,蔺万煌,等. 亚种间杂交稻内源激素的高效液相色谱测定法[J]. 色谱,2002,20(2):148-150. Wang R Z,Xiao L T,Lin W H,et al. High performance liquid chromatographic determination of internal hormones in inter-subspecific hybrid rice[J]. Chinese Journal of Chromatography,2002,20(2):148-150(in Chinese with English abstract). |
| [23] | Miura K,Okamoto H,Okuma E,et al. SIZ1 deficiency causes reduced stomatal aperture and enhanced drought tolerance via controlling salicylic acid-induced accumulation of reactive oxygen species in Arabidopsis[J]. The Plant Journal,2013,73(1):91-104. |
| [24] | Okuma E,Nozawa R,Murata Y,et al. Accumulation of endogenous salicylic acid confers drought tolerance to Arabidopsis[J]. Plant Signaling and Behavior,2014,9(3):e28085. |
| [25] | Fragniere C,Serrano M,Abou-Mansour E,et al. Salicylic acid and its location in response to biotic and abiotic stress[J]. FEBS Letters,2011,585(12):1847-1852. |
| [26] | Kraft M,Kuglitsch R,Kwiatkowski J,et al. Indole-3-acetic acid and auxin herbicides up-regulate 9-cis-epoxycarotenoid dioxygenase gene expression and abscisic acid accumulation in cleavers(Galium aparine):interaction with ethylene[J]. Journal of Experimental Botany,2007,58(6):1497-1503. |
| [27] | Bandurska H,Stroinski A. The effect of salicylic acid on barley response to water deficit[J]. Acta Physiologiae Plantarum,2005,27(3):379-386. |
| [28] | Szepesi Á,Csiszar J,Gemes K,et al. Salicylic acid improves acclimation to salt stress by stimulating abscisic aldehyde oxidase activity and abscisic acid accumulation,and increases Na+ content in leaves without toxicity symptoms in Solanum lycopersicum L.[J]. Journal of Plant Physiology,2009,166(9):914-925. |
| [29] | Du H,Wu N,Fu J,et al. A GH3 family member,OsGH3-2,modulates auxin and abscisic acid levels and differentially affects drought and cold tolerance in rice[J]. Journal of Experimental Botany,2012,63(18):6467-6480. |
| [30] | Jayakannan M,Bose J,Babourina O,et al. The NPR1-dependent salicylic acid signalling pathway is pivotal for enhanced salt and oxidative stress tolerance in Arabidopsis[J]. Journal of Experimental Botany,2015,66(7):1865-1875. |




