文章信息
- 裴延飞, 刘廷利, 连梓伊, 杨郁文, 陈天子, 王金彦, 张保龙, 柳李旺. 2015.
- PEI Yanfei, LIU Tingli, LIAN Ziyi, YANG Yuwen, CHEN Tianzi, WANG Jinyan, ZHANG Baolong, LIU Liwang. 2015.
- 番茄中受TYLCV诱导上调表达基因tetraspanin3的功能分析
- Functional analysis of tetraspanin3 gene up-regulated by Tomato yellow leaf curl virus(TYLCV)in tomato
- 南京农业大学学报, 38(6): 896-900
- Journal of Nanjing Agricultural University, 38(6): 896-900.
- http://dx.doi.org/10.7685/j.issn.1000-2030.2015.06.004
-
文章历史
- 收稿日期: 2015-03-12
2. 江苏省农业科学院江苏省农业生物学重点实验室, 江苏 南京 210014
2. Provincial Key Laboratory of Agrobiology, Jiangsu Academy of Agricultural Sciences, Nanjing 210014, China
番茄黄化曲叶病(Tomato yellow leaf curl virus,TYLCV)是番茄最为严重的病害之一,感病植株明显矮化,叶缘黄化,叶片变小并卷曲,其严重危害番茄生长、开花和座果,导致毁灭性绝产[1]。近年来随着烟粉虱在世界各地的爆发,TYLCV在世界许多国家和地区发生[2, 3, 4, 5, 6],在中国许多省市也大面积爆发,并有逐渐扩张的趋势[7, 8, 9, 10]。通过基因工程来提高番茄的抗病性,在番茄育种中日益受到重视。通过克隆与抗TYLCV有关基因并将之导入植物体内,或者抑制利于TYLCV侵染植物的基因的表达,可以提高植物抗病性[11]。
Tetraspanin蛋白是具有高度保守的氨基酸序列和特殊结构的膜蛋白,由4个跨膜结构域和3个串联这4个跨膜结构域的环状结构组成,其中细胞膜外侧有2个环状结构,细胞膜内侧有1个环状结构,Tetraspanin蛋白的高度保守性表明tetraspanin基因家族具有相似或者相近的功能[12]。tetraspanin有很长的进化史,从酵母到人等多种多样的生物体中均被发现[13]。对tetraspanin基因家族的研究起源于对动物医学的研究,在动物中对tetraspanin基因的功能和作用机制研究相当深入,Tetraspanin蛋白已经被证实有利于多种病毒侵染动物,并且当tetraspanin基因表达受到抑制后,生物体对一些病毒有更好的抵抗能力,在医学上这一理论已被用于许多病毒引起的病症防治,如人乳头癌病毒(HPV)、猫免疫缺陷病毒(FIV)、犬热病病毒(CDV)[14, 15, 16]等。tetraspanin对动物的繁殖、抗病性及生长发育有着重要作用[12, 17, 18],然而在植物中研究很少,如拟南芥tetraspanin 1/tornado2/ekeko(tet1/tet2 )[19, 20, 21, 22]。tetraspanin基因家族有17个成员,tetraspanin 1~tetraspanin17 [22],而根据TYLCV诱导后番茄转录组数据[23]分析,发现tetraspanin基因家族中仅tetraspanin 3在被TYLCV诱导后显著上调表达,且tetraspanin 3在拟南芥中的同源基因对生长发育有重要作用[19, 20, 21, 22],而在番茄中tetraspanin 3 的功能尚未见报道。本研究通过生物信息学软件分析tetraspanin 3进化,利用RT-qPCR探索番茄tetraspanin 3组织表达及对TYLCV诱导响应表达模式,并通过病毒诱导的基因沉默(VIGS)将番茄中tetraspanin 3沉默,研究该基因对番茄抗TYLCV能力和生长发育的影响,为tetraspanin 3运用于抗TYLCV番茄遗传改良工作提供理论依据。
1 材料与方法 1.1 材料含有Ty- 2 抗性基因的抗番茄黄化曲叶病番茄材料CLN2777A由江苏省农业科学院蔬菜研究所提供。农杆菌菌株EHA105由江苏省农业科学院生物技术所功能基因项目组提供。
总RNA提取试剂盒购自TIANGEN生物公司,质粒提取、DNA片段纯化/回收试剂盒购于AXYGEN公司。实时荧光定量PCR采用德国耶拿公司qTOWER2.2六通道荧光定量PCR仪。病毒诱导的基因沉默(virus-induced gene sliencing,VIGS)载体:pTRV2(空载体,用于携带目的基因)、pTRV1(辅助载体)和pTRV2-PDS(阳性对照)由清华大学刘玉乐教授提供。引物由Primer Premier 5.0软件评价生成,并由上海Invitrogen公司合成。
1.2 方法 1.2.1 tetraspanin 3基因家族进化树的构建利用番茄tetraspanin 3(Solyc11g072480.1.1)序列在NCBI蛋白数据库(http://blast.ncbi.nlm.nih.gov/Blast.cgi)中进行对比,选择同源性较高的基因,利用软件MEG 5.0构建进化树。
1.2.2 TYLCV接种及tetraspanin 3在侵染过程中的表达选择生长状况良好并且长势相近的具有3片真叶的番茄苗60株,分为2组,每组30株,分别放置于具有带TYLCV病毒烟粉虱和不带病毒烟粉虱的防虫网箱中,然后均置于25 ℃,每天光照16 h,黑暗8 h,水分、营养适宜且相同的环境中培养,3 d后,消灭烟粉虱。分别取接种3和7 d后的番茄样品,通过荧光定量PCR方法测定番茄中tetraspanin 3表达量。
1.2.3 tetraspanin 3在番茄各器官中表达量挑选3株长势一致的番茄植株,分别提取根、茎、新叶、老叶、花和未成熟果实的RNA;将RNA反转录成cDNA(TaKaRa),然后通过荧光定量检测方法,分析tetraspanin 3在番茄各器官中表达量。
1.2.4 载体构建根据本实验室番茄RNA-seq数据中上调表达的基因tetraspanin 3(Solyc11g072480.1.1)序列的特异区段设计引物TET-F(GGAATTCCATATGATGAGAACCAGTAATCACTT)和TET-R(CGCGGATCCTTAGAAGTGTATCCTGCTAG)。以番茄自交系CLN2777A的cDNA为模板扩增出858 bp片段,然后通过XbaⅠ和BamHⅠ将该片段插入到pTRV2载体中,转化大肠杆菌DH5α,通过菌落PCR筛选阳性克隆并通过测序验证质粒pTRV2.tetraspanin 3 。通过电转化法转化农杆菌菌株EHA105,利用VIGS技术将番茄中tetraspanin 3沉默,通过子叶注射法将载体导入番茄幼苗中,注射pTRV2空载体番茄作为对照组,用番茄八氢番茄红素脱氢酶基因(pds)作为试验阳性对照[24]。观测沉默植株表型并检测沉默植株抗病性。通过观测pds基因的沉默效果初步判定VIGS沉默效果。在沉默处理2周后,选择沉默植株与非沉默植株各50株,接种TYLCV病毒。接种TYLCV病毒的方法与1.2.2相同。提取接种TYLCV 10、15和20 d后的番茄总DNA,通过荧光定量PCR测定tetraspanin 3沉默后番茄和对照组番茄的病毒量[11]。
1.2.5 荧光定量PCR引物采用Primer Premier 5.0软件,按照RT-qPCR要求设计番茄actin内参基因、tetraspanin 3基因和病毒衣壳蛋白的编码基因v2 的引物对。actin扩增引物对F/R:TGGTCGGAATGGGACAGAAG/CTCAGTCAGGAGAACAGGGT;tetraspanin 3扩增引物对F/R:AAGTTGTGGTAAG-ATGAGAAGGATT/GCAGCAACCAGACTCAATAGGAC;v2 扩增引物对F/R:GGATTTCGTTGTATGTTAGC/ATGATTATATCGC-CTGGTC。采用SYBR PrimeScriptTM RT-PCR试剂盒进行测定,每个样品设3个重复。RT-qPCR实验数据用Sequence detection software version 1.3.1软件处理,并参照Schefe等[25]的方法进行效率校正,图表数据采用SPSS 16.0软件处理。
2 结果与分析 2.1 番茄基因tetraspanin 3的进化分析利用NCBI数据库及软件MEG 5.0构建tetraspanin 3 的进化树,结果(图 1)显示:番茄tetraspanin 3 基因与葡萄中同源基因在一个独立的分支上,与其他物种亲缘关系较远。
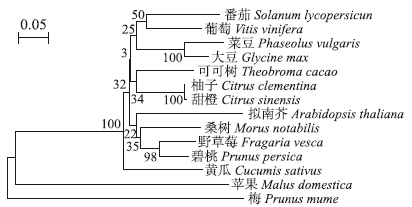 | 图 1 番茄tetraspanin 3的进化树Fig. 1 Phylogenetic tree of tetraspanin 3 in tomato |
由图 2可见:tetraspanin 3在番茄各个器官中均有表达。在茎、老叶、花中的相对表达量较高,在根和果实中的相对表达量较低,而在新生叶中相对表达量处于中间水平。
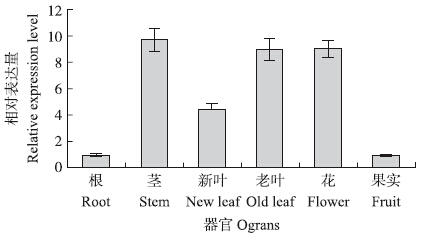 | 图 2 番茄不同器官中tetraspanin 3的相对表达量Fig. 2 Relative expression level of tetraspanin 3 in different organs of tomato |
由图 3可以看出:当病毒侵染番茄后,tetraspanin 3出现上调表达,与接种TYLCY后番茄RNA-seq得到的数据[23]一致,表明番茄基因tetraspanin 3与TYLCV侵染有关。
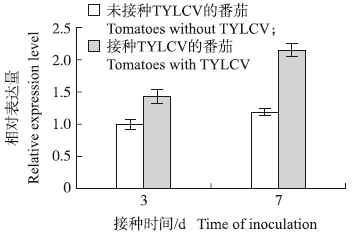 | 图 3 tetraspanin 3在TYLCV侵染过程中的表达Fig. 3 The expression of tetraspanin 3 in the process of the infection of TYLCV |
番茄植株注射pds基因2~3周后,叶片表现出白化表型(图 4-A),表明pds基因已被沉默。此时,检测tetraspanin 3表达量,沉默植株tetraspanin 3表达量约为对照组的35%,达到显著沉默效果。
 |
图 4 tetraspanin 3沉默番茄植株的表型Fig. 4 The phenotype of tetraspanin 3-silenced tomato plantA.沉默pds基因表型,左侧为pds基因沉默植株,右侧为对照植株;B.tetraspanin 3沉默后番茄植株表型,左侧为对照植株,右侧为tetraspanin 3基因沉默植株;C.tetraspanin 3沉默后番茄叶片表型,左侧为对照植株叶片,右侧为沉默植株叶片 A.The phenotype of pds gene-silenced plant,the left was pds gene-silenced plant,the right was the control;B.The phenotype of tetraspanin 3-silenced tomato,the left was the control,the right was the gene-silenced tomato;C.The phenotype of the leaf of the tetraspanin 3-silenced tomato,the left was the leaf of the control,the right was the leaf of the gene-silenced tomato. |
tetraspanin 3被沉默后的50株番茄中,33株出现与对照组有显著差异的表型(图 4-B、C),叶片出现严重扭曲,表明该基因与番茄叶片生长发育有关,这与其同源基因tornado 1和tornado2影响拟南芥根部径向分化和平周分化相似[21],对植物生长发育都有影响。
2.5 tetraspanin 3沉默植株对TYLCV的抗性将tetraspanin 3沉默植株接种TYLCV。对接种后TYLCV病毒量检测发现,沉默植株中TYLCV病毒量比对照组(没有沉默tetraspanin 3)低50%以上(图 5),表明tetraspanin 3表达被抑制后,降低了TYLCV对番茄植株的侵染程度,番茄对TYLCV抗性增强。
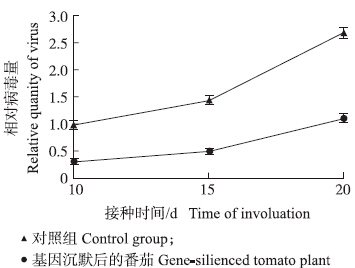 | 图 5 tetraspanin 3沉默番茄植株对TYLCV的抗性Fig. 5 The resistance to TYLCV of tetraspanin 3- silenced tomato plant |
目前,对植物中tetraspanin基因家族的研究相对较少,其作用机制和生化途径更是鲜有报道[19, 20, 21, 22]。tetraspanin 3基因是tetraspanin基因家族的一员,Tetraspanin蛋白具有高度保守的氨基酸序列,动物中tetraspanin已经被证实与许多病毒侵染有关[12, 14, 15, 16, 17, 18],从理论上推断在番茄中tetraspanin 3 可能与病毒侵染植物体有关系,而番茄中tetraspanin 3的功能未见报道。本试验对TYLCV诱导后番茄的RNA-seq数据[23]及实时荧光定量表达结果分析得出,经TYLCV诱导后番茄的tetraspanin 3 上调表达,表明tetraspanin 3与病毒感染植物确实存在着某种关系。进一步利用VIGS技术将tetraspanin 3沉默后并进行抗TYLCV能力检测,研究tetraspanin 3与TYLCV侵染番茄的关系。从tetraspanin 3 沉默后的番茄植株抗病性鉴定结果可以看出,tetraspanin 3沉默后的番茄,TYLCV病毒量比对照组番茄低50%以上,这与Tetraspanin3蛋白在虾中的同源蛋白(FCTetraspanin3蛋白)的细胞膜外较大环状结构缺失后,使白斑综合病毒(WSSV)对虾的感染程度显著下降相似[26],表明tetraspanin 3被沉默后,TYLCV对番茄植株侵染受到抑制,即番茄对TYLCV抗性增强,反之,tetraspanin 3表达利于TYLCV对番茄侵染。tetraspanin 3利于病毒侵染生物体,可能与 Tetraspanin蛋白结构有密切关系,Tetraspanin蛋白有4个保守跨膜结构域,能为病毒侵染细胞提供途径[13]。
本研究中,当tetraspanin 3 被沉默后,番茄叶片出现扭曲,表明tetraspanin 3 与番茄叶片生长发育有关,这与tetraspanin 3 在拟南芥中的同源基因tornado 1和tornado2的功能相似。当tornado1和tornado2 突变后,拟南芥根发育不正常[22],2个试验共同表明不同物种的tetraspanin基因缺失,影响的组织器官可能不同。tetraspanin 3影响番茄叶片生长发育,可能是因为Tetraspanin3蛋白作为一种细胞膜上的跨膜蛋白,具有信号识别和物质运输的功能,当tetraspanin 3被沉默后,影响了植物细胞及组织、器官间信号传递及物质交流[13, 14, 15, 16, 17],进而影响了番茄叶片生长发育。本研究中,tetraspanin 3 在番茄各器官中均有表达,但是当tetraspanin 3 表达被抑制后,只有叶片出现扭曲,说明tetraspanin 3在各器官均有作用,但是对叶片生长发育影响较大。
基因工程育种已经成为培育生物新品种的有效途径[11]。tetraspanin 3被沉默后能够有效抑制TYLCV对番茄侵染,因此,理论上可将tetraspanin 3基因用于抗TYLCV番茄种质创新,但是由于tetraspanin 3对TYLCV侵染植物起重要作用的生化途径及机制尚未阐明,且沉默tetraspanin 3 对叶片生长发育有一定影响,因此需对tetraspanin 3及其基因家族功能进行深入研究,以期早日将该基因运用于抗TYLCV番茄新品种选育工作中。
| [1] | 余文贵,赵统敏,杨玛丽,等. 黄化曲叶病及其抗病育中的研究进展[J]. 江苏农业学报,2009,25(4):925-930 [Yu W G,Zhao T M,Yang M L,et al. Research progress on Tomato yellow leaf curl disease and resistance breeding[J]. Jiangsu Journal of Agricultural Sciences,2009,25(4):925-930(in Chinese with English abstract)] |
| [2] | Lee H,Song W,Kwak H R,et al. Phylogenetic analysis and inflow route of Tomato yellow leaf curl virus(TYLCV)and Bemisia tabaco in Korea[J]. Molecules and Cells,2011,30(5):467-476 |
| [3] | Huanssen I M,Lapidot M,Thomma B P H J. Emerging viral diseases of tomato crops[J]. Molecular Plant-Microbe Interactions,2010,23(5):539-548 |
| [4] | Klan A J,Idris A M,Al-Saady N A,et al. A divergent isolate of Tomato yellow leaf curl virus from Oman with an associated DNA beta satellite an evolutionary link between Asian and the Middle Eastren virus-satellite complexes[J]. Virus Genes,2008,36:169-176 |
| [5] | Banuelos-Hernandez B,Mauricio-Castillo J A,Cardenas-Conejo Y,et al. A new strain of tomato severe leaf curl virus and a unique variant of Tomato yellow leaf curl virus from Mexico[J]. Arch Virol,2012,157:1835-1841 |
| [6] | Fazeli R,Heydarnejad J,Massumi H,et al. Genetic diversity and distribution of tomato-infecting begomoviruses in Iran[J]. Virus Genes,2009,38:311-319 |
| [7] | 王冬生,匡开源,袁永达,等. 番茄黄化曲叶病毒在上海发生流行初步观察[J]. 上海蔬菜,2007(4):61-62 [Wang D S,Kuang K Y,Yuan Y D,et al. Research progress on Tomato yellow leaf curl virus in Shanghai[J]. Shanghai Vegetables,2007(4):61-62(in Chinese with English abstract)] |
| [8] | 何自福,虞皓,毛明杰,等. 中国台湾番茄黄化曲叶病毒侵染引起广东番茄黄化曲叶病毒病[J]. 农业生物技术学报,2007,15(1):119-123 [He Z F,Yu H,Mao M J,et al. Tomato yellow leaf curl disease in Guangdong caused by tomato leaf curl Taiwan virus[J]. Journal of Agricultural Biotechnology,2007,15(1):119-123(in Chinese with English abstract)] |
| [9] | 岳宁,丁铭,董家红,等. 中国番茄黄化曲叶病毒在云南的发生分布及其遗传多样性[J]. 云南大学学报:自然科学版,2008,30(S1):57-62 [Yue N,Ding M,Dong J H,et al. Genetic diversity and distribution of tomato yellow leaf curl China virus in Yunnan Province[J]. Journal of Yunnan University:Natural Sciences Edition,2008,30:57-62(in Chinese with English abstract)] |
| [10] | 余文贵,赵统敏,杨玛丽,等. 山东、安徽两省栽培番茄烟粉虱传双生病毒的PCR检测及序列分析[J]. 江苏农业学报,2009,25(4):747-751 [Yu W G,Zhao T M,Yang M L,et al. PCR detection and sequence analysis of white fly transmitted geminivirus in tomato from Anhui and Shandong Provinces[J]. Jiangsu Journal of Agricultural Sciences,2009,25(4):747-751(in Chinese with English abstract)] |
| [11] | 李楠,杨玛丽,陈天子,等. 番茄抗黄化曲叶病毒相关基因CLNLR的功能分析[J]. 园艺学报,2014,41(5):889-897 [Li N,Yang M L,Chen T Z,et al. Functional characterization of CLNLR gene involved in tomato defense against tomato yellow leaf curl disease[J]. Acta Horticulturae Sinica,2014,41(5):889-897(in Chinese with English abstract)] |
| [12] | Huang S,Tian H,Chen Z,et al. The evolution of vertebrate tetraspanins:gene loss,retention,and massive positive selection after whole genome duplications[J]. BMC Evolution Biology,2012,10:306 |
| [13] | Peter N,Lynda J. Tetraspanins-gateways for infection[J]. Infectious Disorders-Drug Targets,2102,12:4-17 |
| [14] | Richards K F,Mukherjee S,Bienkowska-Haba M,et al. Human papillomavirus species-specific interaction with the basement membrane-resident non-heparan sulfate receptor[J]. Viruses,2014,6:4856-4879 |
| [15] | Hoffmann G,Thum J,Ackley C,et al. Decline in CD4+cell numbers in cats with naturally acquired feline immunodeficiency virus infection[J]. Journal of Virology,1992,66(3):1484-1488 |
| [16] | Kurzeder C,Koppold B,Suer G,et al. CD9 promotes adeno-associated virus type 2 infection of mammary carcinoma cells with low cell surface expression of heparin sulphate proteoglycans[J]. International Joural of Molecular Medicine,2007,19(2):325-330 |
| [17] | Davis C,Harris H J,Ke H,et al. In silico directed mutagenesis identifies the CD81/claudin-1 hepatitis C virus receptor interface[J]. Cellular Microbiology,2012,14(12):1892-1903 |
| [18] | Michael T,Karin S,Gunnar H,et al. Production of human tetraspanin proteins in Escherichia coli[J]. Protein Expression and Purification,2012,82:373-379 |
| [19] | Ernesto O,Bernd R,Koen D. The ekeko mutant demonstrates a role for tetraspanins-like protein in plant development[J]. Biochemical and Biophysical Research Communications,2003,310:1054-1061 |
| [20] | Cnops G,Neyt P,Raes J,et al. The tornado1 and tornado2 genes function in several patterning processes during early leaf development in Arabidopsis thaliana[J]. The Plant Cell,2006,18:852-866 |
| [21] | Cnops G,Wang X,Linstead P,et al. Tornado1 and tornado2 are required for the specification of radial and circumferential pattern in the Arabidopsis root[J]. Development,2000,127:3385-3394 |
| [22] | Feng W,Klaas V,Mieke L. Tetraspanin genes in plants[J]. Plant Science,2012,190:9-15 |
| [23] | Chen T Z,Lv Y D,Zhao T M,et al. Comparative transcriptome profiling of a resistant vs.susceptible tomato(Solanum lycopersicum)cultivar in response to infection by Tomato yellow leaf curl virus[J]. PLoS ONE,2008,8(11):810-816 |
| [24] | 刘廷利,杨玛丽,刘小双,等. 一个受TYLCV诱导上调表达的番茄基因LeFLS2的功能分析[J]. 江苏农业学报,2014,30(2):376-380 [Liu T L,Yang M L,Liu X S,et al. Functional annalysis of gene LeFLS2 of tomato infected by Tomato yellow leaf curl virus(TYLCV)[J]. Jiangsu Journal of Agricultural Sciences,2014,30(2):376-380(in Chinese with English abstract)] |
| [25] | Schefe J H,Lehmann E,Buschmann R,et al. Quantitative real-time RT-PCR data analysis:current concepts and the novel""gene expression's CT difference""formula[J]. Journal of Molecular Medicine,2006,84:901-910 |
| [26] | Gu L,Wang B,Li F H,et al. Blcoking the large extracellular loop(LEL)domain of FcTetraspanin-3 could inhibit the infection of white spot syndrome virus(WSSV)in Chinese shrimp,Fenneropenaeus chinensis[J]. Fish and Shellfish Immunology,2012,doi:10.1016/j.fsi.2012.02.022 |
 2015, Vol. 38
2015, Vol. 38


