文章信息
- 叶素银, 徐阳春, 董彩霞. 2015.
- YE Suyin, XU Yangchun, DONG Caixia. 2015.
- 钙和硅叶面肥对“黄冠”梨果实发育期间生理生化变化的影响
- Effect of foliar calcium and silicon fertilizers on the physiological and biochemical changes in ‘Huangguan’ pear fruit during the fruit development
- 南京农业大学学报, 38(5): 824-829
- Journal of Nanjing Agricultural University, 38(5): 824-829.
- http://dx.doi.org/10.7685/j.issn.1000-2030.2015.05.018
-
文章历史
- 收稿日期:2015-01-23
‘黄冠’梨属中早熟品种,肉质细腻,在河北、山西、甘肃等地大面积种植,其成熟季节前后易发生果面褐斑,俗称“鸡爪病”,一般认为与成熟期果实缺钙有关[1],常采用叶面喷施钙肥的方式补充。国内外对果实生育期内喷施钙肥的时期、种类的研究有较多报道[2, 3],研究结果表明喷施钙肥能增加收获期果实中钙含量及果实硬度。在实际生产中,即使在生育期内喷施钙肥,成熟期果面褐斑病仍有发生,表明除果实钙含量之外还存在其他致病因素。硅是植物有益元素,能增强细胞壁强度,改善植株的机械性能,提高抗性[4, 5]。多聚半乳糖醛酸酶(PG)和纤维素酶(Cx)是与细胞壁降解有关的酶[6, 7],其活性高低直接影响细胞壁主要成分原果胶(PP)和纤维素含量[8]。超氧化物歧化酶(SOD)、过氧化物酶(POD)等抗氧化酶活性[9]和丙二醛(MDA)含量[10]是植物体内清除活性氧、降低膜脂过氧化反应的重要指标,常用来表征植物抗性。目前,关于果面褐斑病的研究主要集中在果皮组织中,对果肉研究的较少,张强等[11]指出果肉细胞壁结构的稳定性及抗氧化能力也是果实抗逆性强弱的重要指标。
本试验将盆栽试验与大田试验相结合,在盆栽4年生‘黄冠’梨幼果期和膨大期喷施钙、硅叶面肥,研究其对不同发育时期果肉细胞壁水解酶(PG、Cx)和抗氧化酶(SOD、POD)活性及MDA含量的影响,以及在田间对12年生‘黄冠’梨喷施钙、硅肥,研究其对果实品质及果面褐斑病发生率的影响,旨在揭示钙、硅叶面肥对果实发育过程中果实抗性的影响,为缓减‘黄冠’梨果面褐斑病的发生提供理论依据和实践措施。
1 材料与方法 1.1 试验材料与处理 1.1.1 盆栽试验试验于2014年3—8月在南京农业大学牌楼试验基地网室进行,以4年生盆栽‘黄冠’梨为供试材料。选取生长势、负载量较一致的16株树,每个处理4株,单株为1个重复。共设4个处理:1)对照(Control),喷施清水;2)喷施螯合钙肥(SC);3)喷施硅肥(SS);4)螯合钙肥与硅肥配合喷施(SCS)。螯合钙肥由江苏龙灯化学有限公司提供,喷施浓度为170 mg · L-1;水溶性硅肥由江苏陆富肥料有限公司提供,硅含量大于等于230 g · kg-1,喷施该肥料浓度为3.3 g · L-1。分别在幼果期(花后21、28和36 d)和膨大期(花后80和95 d)晴天傍晚喷施上述叶面肥,先喷施钙肥,2 d后再喷施硅肥,以叶面滴水为止,每株用量约0.75 L。
采样时间从花后70 d起,每15 d采样1次,至花后125 d果实成熟,共采集5次。每次采样时,随机从各处理中4棵树上各采1个果实,每个处理4个果实,用自封袋保存,置于冰盒中,立即带回实验室。用大量去离子水冲洗果实表面,去除果皮和果核,将果肉用液氮速冻,-70 ℃保存备用。
1.1.2 田间试验试验于2014年3—8月在河北省辛集市马庄果园进行,以12年生‘黄冠’梨为材料。选取生长势较一致的36棵树,每个处理9棵,3棵为1个重复。试验处理同1.1.1节。收获时,每棵树采 4个果实带回实验室测定。
1.2 测定指标和方法 1.2.1 果实单果质量测定用电子天平称量各时期所采果实的单果质量。
1.2.2 细胞壁水解酶活性测定参照曹建康等[12]的方法略有改进。分别制作半乳糖醛酸和无水葡萄糖标准曲线,反应完成后于540 nm处测定D值,按标准曲线计算可得果肉的PG和Cx活性。PG活性以每小时每克果肉在37 ℃催化多聚半乳糖醛酸水解生成半乳糖醛酸的质量表示,即μg · h-1 · g-1;Cx活性以每小时每克果肉在37 ℃催化羧甲基纤维素水解形成还原糖的质量表示,即μg · h-1 · g-1。
1.2.3 果胶含量测定PP和可溶性果胶(SP)含量以生成半乳糖醛酸的质量分数(%)表示[12]。
1.2.4 抗氧化酶活性测定SOD活性以每分钟每克果肉的反应体系对氮蓝四唑(NBT)光化还原的抑制为50%作一个SOD活性单位,用U表示;采用愈创木酚法测定POD活性,以每克果肉每分钟吸光度值增加1时为一个POD活性单位,以A470 · min-1 · g-1表示[13]。
1.2.5 MDA含量测定采用硫代巴比妥酸显色法(TBA)测定MDA含量[13],以μmol · g-1表示。
1.2.6 成熟期果实品质的测定果实的纵径和横径用游标卡尺测量(果形指数=果实纵径/果实横径);采用日本ATAGO公司生产的PAL-1型电子折光仪测定可溶性固形物含量;采用意大利BREUZZI公司生产的FT 327型硬度计测定果实硬度。
1.2.7 田间果面褐斑病的统计方法‘黄冠’梨收获时,每棵树随机摘20个果,东西南北方向各5个,每个处理共180个果,统计发病果数。发病率=病果数/总采摘果数。
1.3 数据分析采用SPSS 20.0统计分析软件对数据进行分析,采用Origin 8.5软件制图。
2 结果与分析 2.1 钙、硅叶面肥对梨单果质量及生理代谢的影响 2.1.1 果实单果质量如图 1所示:花后85 d以后,除单施硅肥处理外的其他处理果实单果质量的增加趋势基本一致,对照的增速略低;而花后115 d以后,单施硅肥处理的果实单果质量的增速明显降低,由6.5 g · d-1(花后100~115 d)的速度降至1.1 g · d-1(花后115~125 d)。采收时,SCS处理的单果质量最高,而SS处理最低,显著比对照低13.0%。
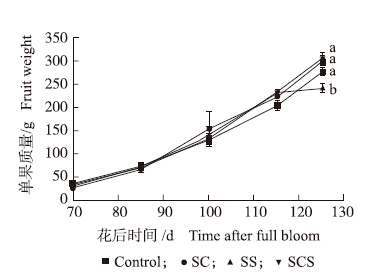 |
图 1 钙、硅叶面肥对果实单果质量的影响
Fig. 1 Effects of foliar calcium and silicon fertilizers on fruit weight
1)Control:对照喷施清水;SC:喷施螯合钙肥;SS:喷施硅肥;SCS:螯合钙肥与硅肥配合喷施;2)同一时期中不同小写字母表示处理间在0.05水平差异显著。 1)Control:Spray water;SC:Separately spray chelated calcium fertilizer;SS:Separately spray silicon fertilizer;SCS:Cooperatively spraying both calcium and silicon fertilizers;2)Value points under different treatments in the same time followed by different letter are significant difference at 0.05 level(Duncan′s Multiple Range test).The same as follows. |
果肉中多聚半乳糖醛酸酶(PG)活性的变化整体呈先上升后下降再上升的趋势(图 2),对照PG活性始终最高,SCS处理的最低,在花后85、100和125 d时,分别比对照显著降低79.7%、75.4%和25.3%。
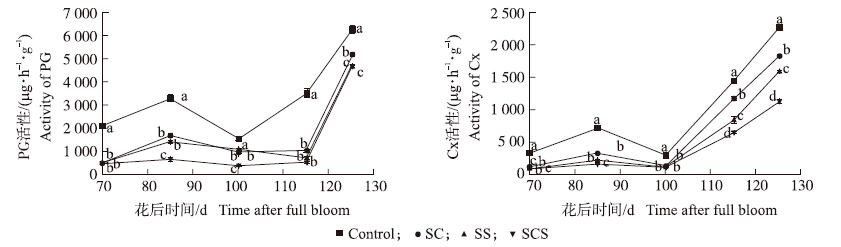 | 图 2 钙、硅叶面肥对果肉中多聚半乳糖醛酸酶和纤维素酶活性的影响 Fig. 2 Effects of foliar calcium and silicon fertilizers on polygalacturonase(PG)and cellulases(Cx)activities in fruit |
各个处理果肉中纤维素酶(Cx)活性的变化趋势整体一致,变化幅度略有不同(图 2)。SCS处理Cx活性最低,对照的最高,且差异显著。在花后115和125 d时,SCS处理Cx活性分别比对照降低了54.9%和50.1%。
2.1.3 果胶含量由图 3可知:在花后115 d以前各个处理果肉中原果胶(PP)含量均不断下降,之后(除SS的处理仍降低外)各处理均趋于平稳或略有上升。喷施叶面肥的处理在各时期均不同程度高于对照,其中SCS处理在花后85、100和125 d时,分别比对照高59.3%、41.4%和40.7%。果肉中可溶性果胶(SP)含量整体呈先下降后上升的趋势(图 3)。其中,对照处理SP含量变化波动不大,而其他3个处理均在花后100 d时SP含量最低,并显著低于对照,SCS处理果实SP含量较对照低60.7%左右。
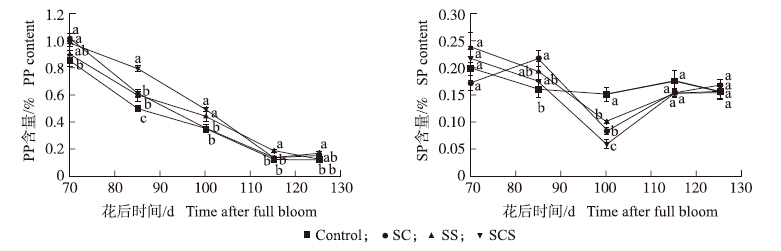 | 图 3 钙、硅叶面肥对果肉中原果胶和可溶性果胶含量的影响 Fig. 3 Effects of foliar calcium and silicon fertilizers on protopectin(PP)and soluble pectin(SP)contents in fruit |
从图 4可知:超氧化物歧化酶(SOD)活性在花后85 d以前增加,之后整体呈下降趋势。自花后85 d起,SCS、SC和SS处理果肉SOD活性均显著高于对照,SCS处理SOD活性始终最高,在花后85、115和125 d时,分别比对照显著增加98.6%、934.6%和244.8%。另外,SS处理果实SOD活性在花后115 d时比SC处理的高,但在125 d时却低于SC处理的。
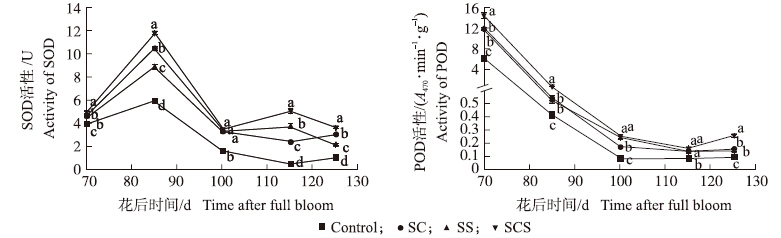 | 图 4 钙、硅叶面肥对果肉中SOD和POD活性的影响 Fig. 4 Effects of foliar calcium and silicon fertilizers on SOD and POD activities in fruit |
如图 4所示:在花后70 d时,SCS处理的果肉中过氧化物酶(POD)活性最高,其次是SS和SC处理,均与对照差异显著,分别高出131.0%、90.6%、89.8%。花后70至85 d,各个处理POD活性急剧下降。花后125 d时,果实中POD活性较低,但SCS处理活性仍比对照显著增加188.9%。
2.1.5 丙二醛含量由图 5可知:在花后115 d以前,果实中MDA含量较低,且波动不大;花后115 d以后,对照MDA含量迅速增加,SC和SS的处理略有上升,而SCS处理略微下降。125 d时,SCS处理的MDA含量比对照显著降低91.1%。
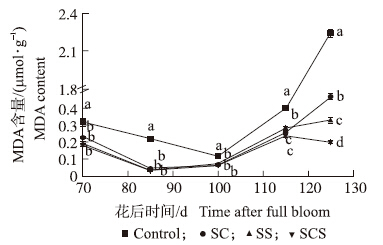 | 图 5 钙、硅叶面肥对果肉中MDA含量的影响 Fig. 5 Effects of foliar calcium and silicon fertilizers on MDA content in fruit |
由表 1可以看出:喷施螯合钙肥、硅肥及其配施处理均降低了梨果面褐斑病发生率,其中SCS处理对果面褐斑病的抑制效果最好,发病率比对照显著降低了49.4%。与对照相比,喷施叶面肥有提高产量及果实硬度的趋势,对单果质量、果形指数和可溶性固形物含量的影响较小。
| 处理 Treatment |
产量/kg Yield of a tree |
单果质量/g Fruit weight |
果形指数 Fruit shape index |
可溶性固形物含量/% Soluble solids content |
硬度/(lb·cm-2) Firmness |
发病率/% Incidence |
| Control | 90.94±8.47 | 287.93±9.79 | 0.95±0.03 | 12.03±0.10 | 7.68±0.09 | 16.67±3.33a |
| SC | 96.64±14.06 | 288.61±10.54 | 0.94±0.02 | 11.39±0.49 | 7.79±0.09 | 12.78±0.96ab |
| SS | 94.23±3.60 | 279.08±3.63 | 0.94±0.05 | 11.60±0.21 | 7.81±0.16 | 11.56±4.29ab |
| SCS | 99.09±2.42 | 304.57±2.96 | 0.96±0.03 | 12.08±0.33 | 7.76±0.15 | 8.44±1.68b |
| 注:1)同列中不同小写字母表示处理间在0.05水平差异显著;2)果形指数=果实纵径/果实横径。 Note: 1)Values within a column followed by different letter are significant difference at 0.05 level(Duncan′s Multiple Range test);2)Fruit shape index=longitudinal diameter/transverse diameter. |
||||||
本研究中,在花后115 d以后,SS处理果实单果质量的增加明显比其他处理缓慢,单果质量显著低于其他处理,田间条件下也表现出施硅肥有降低果实单果质量的趋势,可能与施硅肥有利于果实提早成熟、停止生长有关。卢钢等[14]在早春甜瓜上也发现类似现象。提高果实硬度有利于增强其机械强度[15],与果实中纤维素、原果胶等含量呈极显著正相关[16],本研究中,田间试验下,喷施钙肥、硅肥及其配施有增加果实硬度的趋势,暗示果实细胞壁的稳定性有所增强。
3.2 钙、硅叶面肥对梨果实细胞壁稳定性的影响果胶与纤维素的结合使果胶主要以PP的形式存在,对果实细胞壁的稳定起重要作用,调控果实成熟期的软化[17]。PG和Cx是果实成熟和软化过程中影响细胞壁结构的主要酶[18]。PG催化细胞壁果胶质水解,使PP与纤维素分离[6],同时Cx催化纤维素分解,使其在细胞壁中交联成的网状结构松散[7]。本研究中随着果实发育,果肉中PG和Cx活性整体呈上升趋势,PP含量下降。花后100 d(采前1个月左右,即膨大期)时,PG和Cx活性有一个由降低到升高的转折点,与SP含量先降后升的变化[17]相一致,可能与果实成熟生理的启动有关[18],是果实发育过程中物质代谢发生重大转折的关键时期。SCS处理果肉中PG和Cx活性始终低于对照,在100 d时果肉中PP含量显著高于对照,而SP含量显著低于对照,表明钙、硅配施可以降低膨大期果肉PG和Cx活性,减少细胞壁中果胶和纤维素的水解,避免细胞壁结构的破坏,从而使成熟期果肉中的PP含量也较高,有利于细胞壁的稳定,降低果面褐斑病的发生率。果皮中PP和细胞壁水解相关酶活性的影响是否与果肉中的变化一致,还需要进一步研究。
3.3 钙、硅叶面肥对梨果实活性氧代谢的影响Ho等[19]指出,生理病害的发生一般都伴随着生理代谢系统的紊乱。活性氧伴随着植物生理代谢的进行不断产生,若其产生与清除系统不平衡,积累的活性氧会攻击生物膜。SOD和POD是活性氧清除系统中的主要酶类,能够清除超氧阴离子自由基(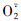 )和过氧化氢,抵御其对细胞膜系统的伤害[9]。有研究表明,随着果实发育,H2O2和
)和过氧化氢,抵御其对细胞膜系统的伤害[9]。有研究表明,随着果实发育,H2O2和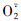 等会大量产生[20],在本研究中SOD和POD活性总体呈下降趋势,这可能是导致活性氧增多的直接原因。在花后85 d时,POD活性较低,而SOD仍维持较高的活性,表明SOD对活性氧代谢平衡有重要作用。喷施硅或钙肥处理果实中SOD、POD活性均相对提高,表明硅和钙提高了梨果活性氧的清除能力[21, 22]。钙肥和硅肥配合施用使这2种活性氧清除酶活性显著高于对照,有利于果肉中的活性氧清除和提高抗氧化能力,其原因可能与果实中硅和钙含量的增加有关。MDA是膜脂过氧化反应的主要产物之一,其含量能反映膜质过氧化程度[10]。成熟期(即花后125 d)钙、硅肥配施处理果肉中MDA含量显著低于对照,表明钙、硅配施能明显减小膜脂过氧化程度,降低活性氧积累对细胞膜的伤害,维持膜的区隔化功能,避免电解质外渗,与上述活性氧清除酶活性变化相对应。
等会大量产生[20],在本研究中SOD和POD活性总体呈下降趋势,这可能是导致活性氧增多的直接原因。在花后85 d时,POD活性较低,而SOD仍维持较高的活性,表明SOD对活性氧代谢平衡有重要作用。喷施硅或钙肥处理果实中SOD、POD活性均相对提高,表明硅和钙提高了梨果活性氧的清除能力[21, 22]。钙肥和硅肥配合施用使这2种活性氧清除酶活性显著高于对照,有利于果肉中的活性氧清除和提高抗氧化能力,其原因可能与果实中硅和钙含量的增加有关。MDA是膜脂过氧化反应的主要产物之一,其含量能反映膜质过氧化程度[10]。成熟期(即花后125 d)钙、硅肥配施处理果肉中MDA含量显著低于对照,表明钙、硅配施能明显减小膜脂过氧化程度,降低活性氧积累对细胞膜的伤害,维持膜的区隔化功能,避免电解质外渗,与上述活性氧清除酶活性变化相对应。
大多数研究认为成熟期‘黄冠’梨果面褐斑病的发生源于果实缺钙[1],但即使在生育期内喷施钙肥,果面褐斑现象仍会发生,尤其是在果实近成熟时遭遇大雨的情况下[23],这说明成熟期果实钙含量低并不是导致发病的单一因素。本研究中,随果实发育,果肉中活性氧清除酶(SOD、POD)活性不断下降,直至成熟时活性微弱,表明成熟期果实内维持生理代谢平衡的能力较低,如何在果实生长期间提高其防御机能,是降低果面褐斑病发生的关键。一般采收前的30~60 d左右(花后65~100 d)是果实膨大期,我们发现,钙、硅配施能显著降低果实细胞壁水解酶(PG、Cx)活性,既维持了膨大期果肉中较高的细胞壁PP含量,又提高了成熟期的PP含量(比对照增加了40.7%),从而有利于维持稳定的细胞壁结构。同时,钙、硅配施显著提高了膨大期和成熟期果肉中SOD和POD活性,有利于增强活性氧清除能力,提高细胞膜的完整性[24]及果实的抗性。因此,在幼果期(花后21、28和36 d)和膨大期(花后80和95 d)喷施钙肥并于每次喷施的2 d后喷施硅肥,可以增强膨大期和成熟期果实抗性,有效降低果面褐斑病发生率。
| [1] | 关军峰,及华,冯云霄. 黄冠梨果皮褐斑病与Ca、Mg、K营养的关系[J]. 华北农学报,2006,21(3):125-128 [Guan J F,Ji H,Feng Y X. The correlation of peel browning spot with nutrition of Ca,Mg,K in Huangguan pears[J]. Agricultural Sciences Journal of Northern China,2006,21(3):125-128(in Chinese with English abstract)] |
| [2] | 王红,张峰,申长卫,等. 不同喷施钙措施对‘库尔勒香梨’顶腐病发生的影响[J]. 南京农业大学学报,2013,36(6):25-29. doi:10.7685/j.issn.1000-2030.2013.06.005 [Wang H,Zhang F,Shen C W,et al. Effects of spraying different calcium fertilizers on the incidence of blossom-end rot of‘Korla Fragrant’pear[J]. Journal of Nanjing Agricultural University,2013,36(6):25-29(in Chinese with English abstract)] |
| [3] | Bhat M Y,Ahsan H,Banday F A,et al. Effect of harvest dates,preharvest calcium sprays and storage period on physico-chemical characteristics of pear[J]. Agric Res Dev,2012,2(4):101-106 |
| [4] | Hayasaka T,Fujii H,Ishiguro K. The role of silicon in preventing appressorial penetration by the rice blast fungus[J]. Phytopathology,2008,98(9):1038-1044 |
| [5] | Chen W,Yao X Q,Cai K Z,et al. Silicon alleviates drought stress of rice plants by improving plant water status,photosynthesis and mineral nutrient absorption[J]. Biol Trace Elem Res,2011,142:67-76 |
| [6] | de Veau E J I,Gross K C,Huber D J,et al. Degradation and solubilization of pectin by β-galactosidases purified from avocadomesocarp[J]. Plant Physiol,1993,87:279-285 |
| [7] | Brummell D A,Cin V D,Crisosto C H,et al. Cell wall metabolism during maturation,ripening and senescence of peach fruit[J]. Journal of Experimental Botany,2004,55:2029-2039 |
| [8] | Showalter A M. Introduction:plant cell wall proteins[J]. Cell Mol Life Sci,2001,58:1361-1362 |
| [9] | Duan X W,Liu T,Zhang D D,et al. Effect of pure oxygen atmosphere on antioxidant enzyme and antioxidant activity of harvested litchi fruit during storage[J]. Food Res Int,2011,44:1905-1911 |
| [10] | Jing G X,Huang H,Yang B,et al. Effect of pyrogallolon the physiology and biochemistry of litchi fruit during storage[J]. Chem Cent J,2013,7:1-11 |
| [11] | 张强,韩冬梅,李建光,等. 贮温对龙眼果肉采后衰老中的抗氧化能力及相关酶的影响[J]. 华南农业大学学报,2012,33(1):18-22 [Zhang Q,Han D M,Li J G,et al. Effects of storage temperature on the oxidation resistance and relevant enzymes during the postharvest senescence of longan aril[J]. Journal of South China Agricultural University,2012,33(1):18-22(in Chinese with English abstract)] |
| [12] | 曹建康,姜微波,赵玉梅. 果蔬釆后生理生化实验指导[M]. 北京:中国轻工业出版社,2007:84-155 [Cao J K,Jiang W B,Zhao Y M. Guidances of Physiological and Biochemical Experiments of Post-harvest Fruits and Vegetables[M]. Beijing:Chinese Light Industry Press,2007:84-155(in Chinese)] |
| [13] | 李合生. 植物生理生化实验原理和技术[M]. 北京:高等教育出版社,2000:184-186,260-261 [Li H S. The Principles and Techniques of Plant Physiology and Biochemistry Experiments[M]. Beijing:Higher Education Press,2000:184-186,260-261(in Chinese)] |
| [14] | 卢钢,曹家树. 硅对甜瓜早熟性及光合特性的影响[J]. 园艺学报,2001,28(5):421-424 [Lu G,Cao J S. Effects of silicon on earliness and photosynthetic characteristics of melon[J]. Acta Horticulturae Sinica,2001,28(5):421-424(in Chinese with English abstract)] |
| [15] | Keegstra K. Plant cell walls[J]. Plant Physiol,2010,154:483-486 |
| [16] | 王玲利,刘超,黄艳花,等. ‘黄冠’梨采后热处理和钙处理对其钙形态及细胞壁物质代谢的影响[J]. 园艺学报,2014,41(2):249-258 [Wang L L,Liu C,Huang Y H,et al. Effects of postharvest heat and calcium treatments on calcium fractions and cell wall metabolism of‘Huangguan’pear fruit[J]. Acta Horticulturae Sinica,2014,41(2):249-258(in Chinese with English abstract)] |
| [17] | 赵树亮,蒋明凤,魏媛媛,等. 梨果实生长过程中细胞壁成分的变化分析[J]. 南方农业学报,2013,44(11):1861-1865 [Zhao S L,Jiang M F,Wei Y Y,et al. Variation of cell wall component during pear growing process[J]. Journal of Southern Agriculture,2013,44(11):1861-1865(in Chinese with English abstract)] |
| [18] | 茅林春,张上隆. 果胶酶和纤维素酶在桃果实成熟和絮败中的作用[J]. 园艺学报,2001,28(2):107-111 [Mao L C,Zhang S L. Role of pectolytic enzymes and cellulase during ripening and woolly breakdown in peaches[J]. Acta Horticulturae Sinica,2001,28(2):107-111(in Chinese with English abstract)] |
| [19] | Ho L,White P J. A cellular hypothesis for the induction of blossom-end rot in tomato fruit[J]. Ann Bot,2005,95:571-581 |
| [20] | Matamoros A M,Jorge L,Dietz K J,et al. Function of antioxidant enzymes and metabolites during maturation of pea fruits[J]. Journal of Experimental Botany,2010,61(1):87-97 |
| [21] |
Liang Y C,Chen Q,Liu Q,et al. Exogenous silicon(Si)increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley( |
| [22] | 刘丽莉,冯涛,向言词. 外源钙对镉胁迫下芥菜型油菜幼苗生长和生理特性的影响[J]. 农业环境科学学报,2009,28(5):978-983 [Liu L L,Feng T,Xiang Y C. Effect of exogenous calcium on seedling growth and physiological characteristics of brassica juncea under cadmium stress[J]. Journal of Agro-Environment Science,2009,28(5):978-983(in Chinese with English abstract)] |
| [23] | 马文会,樊庆耀,黄兰计,等. 黄冠梨鸡爪病发病特点研究[J]. 河北农业科学,2007,11(1):29-31 [Ma W H,Fan Q Y,Huang L J,et al. Investigation on the occurrence of Jizhua disease of Huangguan pear[J]. Journal of Hebei Agricultural Sciences,2007,11(1):29-31(in Chinese with English abstract)] |
| [24] | Kou X H,Wu M S,Li L,et al. Effects of CaCl2 dipping and pullulan coating on the development of brown spot on‘Huangguan’pears during cold storage[J]. Postharvest Biology and Technology,2015,99:63-72 |
 2015, Vol. 38
2015, Vol. 38


