文章信息
- 黄和喜, 王岩, 许玉超, 徐玮玮, 刘世拓, 侯喜林, 胡春梅. 2015.
- HUANG Hexi, WANG Yan, XU Yuchao, XU Weiwei, LIU Shituo, HOU Xilin, HU Chunmei. 2015.
- 不结球白菜雄蕊瓣化相关AP3基因的克隆和表达分析
- Clone and expression analysis of stamen patelody-associated AP3 genes from non-heading Chinese cabbage
- 南京农业大学学报, 38(5): 748-756
- Journal of Nanjing Agricultural University, 38(5): 748-756.
- http://dx.doi.org/10.7685/j.issn.1000-2030.2015.05.008
-
文章历史
- 收稿日期:2015-01-30
不结球白菜(Brassica campestris ssp.chinensis Makino)是一种有明显杂种优势的异花授粉作物,原产中国[1]。雄性不育系是不结球白菜优势育种利用的重要材料[2]。细胞质雄性不育(CMS)或细胞核雄性不育(GMS)会导致雄蕊退化,进而造成不能产生花粉或不产生有活性花粉,但其雄性器官仍然残存[3, 4, 5, 6, 7]。而雄蕊变异会导致花器结构发生质的变化,引起的不育性状更稳定、彻底[8]。
花器官发育遗传调控一直是研究的热点。在花的特定区域,器官建成基因受到上游花器官确定性基因的激活,进而特异性地诱导细胞和组织发育成花的萼片、花瓣、雄蕊和雌蕊4种花器官[9]。花形态的突变包括3种类型[10]:花原基突变、花朵对称性突变和器官身份突变。在拟南芥和金鱼草中,4种花器官的形成均由MADS-box基因家族成员控制[11, 12]。B类在该基因家族中研究最为广泛[13, 14, 15],包含3个AP3类家系和1个PI家系,该类基因会导致双子叶植物花中花瓣和雄蕊的特异性改变,AP3基因的突变会引起雄蕊向心皮发生同源异型转变[16]。进化遗传分析表明,在被子植物出现后,首先出现的AP3类基因是paleoAP3 家系,然后经过基因重复事件,演化出TM6和euAP3家系[13],这2个家系可能会亚功能化或非功能化[17, 18]。基因和蛋白功能分析显示,paleoAP3家系和TM6家系基因总是参与雄蕊的调控发育,而euAP3家系基因则主要参与花瓣和雄蕊的形成过程[19, 20]。
前人已经克隆出大量的AP3基因[21, 22, 23],并研究了其在不同花器官上的表达,但大多是研究AP3基因与雄蕊心皮化性状间的关系,例如,Zhang等[23]的研究结果表明:在芜菁中B.AP3.a和B.AP3.b的突变会导致雄蕊心皮化,在甘蓝型油菜中2个B.AP3.a和2个B.AP3.b功能的缺失与雄蕊心皮化相关。在不结球白菜上有关AP3基因的研究鲜有报道,特别是AP3基因与雄蕊瓣化性状间的关系。本研究利用大白菜BrAP3(Bra007067和Bra014822)(BRAD;http://brass-icadb.org/brad/)同源基因设计引物,从不结球白菜雄蕊瓣化系和正常系早期花蕾中分别得到了与花器官发育相关的2个基因完整编码区的cDNA全长,并利用生物信息学方法对其序列进行了分析,同时采用半定量PCR(semi-quantitative RT-PCR),探讨突变系和保持系材料中2个BcAP3在不同花器官和不同发育时期的表达情况,以此推测BcAP3基因的功能,为研究不结球白菜雄蕊变异的分子调控机制提供重要信息。
1 材料与方法 1.1 材料以南京农业大学白菜组提供的不结球白菜突变系(雄蕊瓣化系)及保持系(正常系)的花器官为材料。不结球白菜种子用1 g·L-1的HgCl2消毒10 min,蒸馏水冲洗干净后于28 ℃暗处催芽72 h,移至穴盘。待幼苗长至2叶1心时移至大棚温室,并于成熟期(十叶期)时移至光照培养箱。培养条件:光照/黑暗时间为16 h/8 h,10~15 ℃春化处理2周。之后移至大棚,30 d后取初花期花蕾,-80 ℃保存备用。同时取不同发育时期的花蕾(花蕾直径小于0.5 mm,0.5~1 mm,1~2 mm,大于2 mm)和盛花期的萼片、雄蕊、花瓣和雌蕊,-80 ℃保存备用。
1.2 方法 1.2.1 不结球白菜AP3基因的克隆与测序采用RNA提取试剂盒(TaKaRa RNAiso Reagent,TaKaRa公司)提取花蕾的总RNA。取不结球白菜早期花蕾总RNA 2 μg,使用Prime Script RT reagent Kit(TaKaRa)进行cDNA的合成。扩增PCR总体系20 μL,包括MgCl2(25 mmol·L-1)2 μL,dNTP(2.5 mmol·L-1)2 μL,10×PCR Buffer 2 μL,rTaq酶0.2 μL,引物BcAP3-1-F、BcAP3-2-F、BcAP3-1-R和BcAP3-2 -R(表 1)各1 μL,cDNA模板1 μL。反应程序为:95 ℃ 4 min;95 ℃ 30 s,57 ℃ 30 s,72 ℃ 2 min,35个循环;72 ℃ 10 min。PCR产物采用DNA凝胶回收试剂盒(TaKaRa)回收,将回收的目的片段连接pMD19-T easy载体(TaKaRa),转化大肠杆菌,挑取阳性克隆,送南京金斯瑞生物技术有限公司进行测序。
| 引物名称Primer name | 引物对序列Primer pairs sequence(5′→3′) |
| BcAP3-1-F/R | ATGGCGAGAGGGAAGATCC/TTCGAGAAGGTGGAAGGTAA |
| BcAP3-2-F/R | ATGGCGAGAGGGAAGATC/TTCAAGAAGGTGGAAGGTAATG |
| BcAP3-1-RT-F/R | ATGGAGGCGACTACGATT/GATGTCAGAGGCAGATGG |
| BcAP3-2-RT-F/R | CAAGAAGGTGGAAGGTAA/GTAGACAATGGAGGAGACT |
| actin-F/R | GGAGCTGAGAGATTCCGTTG/GAACCACCACTGAGGACGAT |
核苷酸序列及推测的氨基酸序列用Cluster 1.8进行序列比对,用DNAMAN 6.0进行下一步的结果显示。利用NCBI-Conserved Domain Search进行蛋白保守域预测。利用在线软件SOPMA和Swiss-model分别预测蛋白的二级结构和三级结构。使用Gene Structure Display Server 2.0在线软件分析基因结构。使用MEGA 5.20软件,采用Neighbor-joining方法,自展值设定为1 000,进行物种间进化分析。
1.2.3 BcAP3基因在不结球白菜中的表达分析使用RNA提取试剂盒(TaKaRa)提取各器官材料总RNA,取1 μg RNA反转录(gDNA Eraser,TaKaRa)成cDNA,根据BcAP3基因编码区序列,利用Beacon Designer v 7.9软件进行RT-PCR引物设计,actin基因作为内参。总体系20 μL,包括MgCl2(25 mmol·L-1)2 μL,dNTP(2.5 mmol·L-1)2 μL,10×PCR Buffer 2 μL,rTaq酶0.2 μL,引物BcAP3-1 -RT-F、BcAP3-2 -RT-F、 BcAP3-1-RT-R和BcAP3-2-RT-R(表 1)各1 μL,cDNA模板1 μL。反应程序为:95 ℃ 4 min;95 ℃ 30 s,58 ℃ 30 s,72 ℃ 1 min,25个循环;72 ℃ 10 min。每个反应设3次重复。
2 结果与分析 2.1 不结球白菜突变系和保持系花的表型观察由图 1可见:保持系是雄蕊正常的品系(图 1-A、C),能产生正常的花粉,用于突变系留种;而突变系 的雄蕊表现为瓣状雄蕊(图 1-B、D),似花瓣,不含花粉,但花柱正常,可接受花粉,属于“器官身份突变”类型。
 | 图 1 保持系和突变系的花和花器官比较Fig. 1 Flowers and organs of maintainer and mutant A,C:保持系Maintainer;B,D:突变系Mutant |
分别以花保持系和突变系的早期花蕾的cDNA为模板,克隆得到4条片段,分别命名为保持系BcAP3-1、 BcAP3-2和突变系BcAP3-1、BcAP3-2,长度分别为698、698、698和674 bp(图 2)。保持系和突变系中BcAP3-1片段大小一致,而与保持系BcAP3-2相比,突变系中BcAP3-2缺失了24 bp。
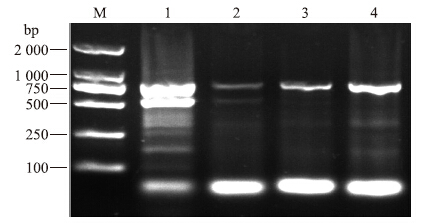 |
图 2 不结球白菜BcAP3-1和BcAP3-2 cDNA编码区域的 RT-PCR扩增结果Fig. 2 The coding region of BcAP3-1 and BcAP3-2 genes in non-heading Chinese cabbage amplified by RT-PCR
M:DNA marker DL2000;1,3:保持系BcAP3-1、BcAP3-2;2,4:突变系BcAP3-1、BcAP3-2 1,3:Maintainer BcAP3-1,BcAP3-2;2,4:Mutant BcAP3-1,BcAP3-2 |
ORF和氨基酸序列比对结果表明:突变系和保持系BcAP3-1的ORF核苷酸序列长度为698 bp,氨基酸序列长度是231个残基;突变系和保持系BcAP3-2的ORF核苷酸序列和氨基酸序列同源性很高,一致性分别为91.99%和94.83%,单碱基置换为33处,其中相应的氨基酸残基改变处为3处,另外缺失24 bp的地方有1处(图 3和图 4)。BcAP3-1和BcAP3-2基因构成相似,全长均由7个外显子和6个内含子组成(图 5),这与黄方等[9]的报道相一致,这类基因属于植物Ⅱ型MADS-box基因(又称MIKC型)。
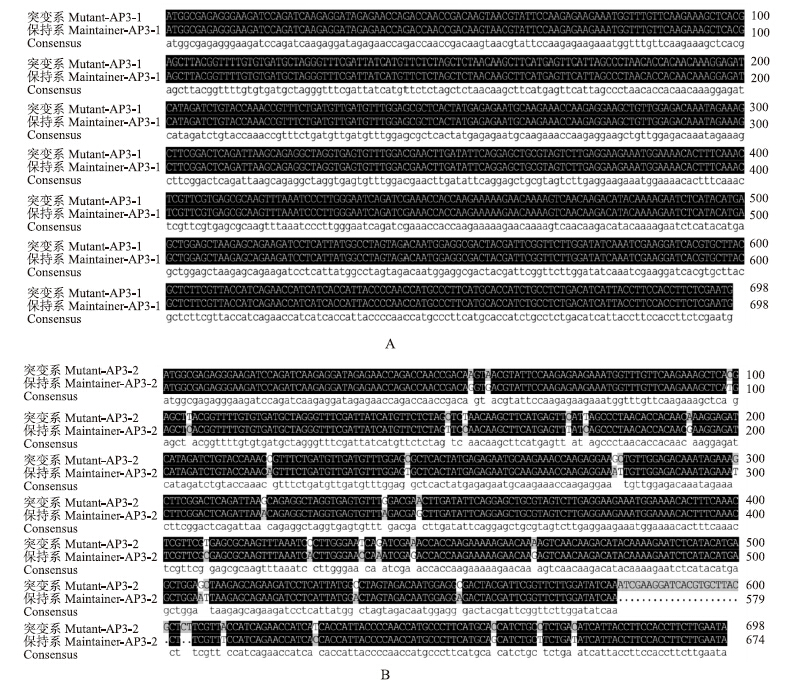 | 图 3 BcAP3-1(A)和BcAP3-2(B)基因核苷酸序列比对结果Fig. 3 Alignment results of BcAP3-1(A)and BcAP3-2(B)gene between two lines |
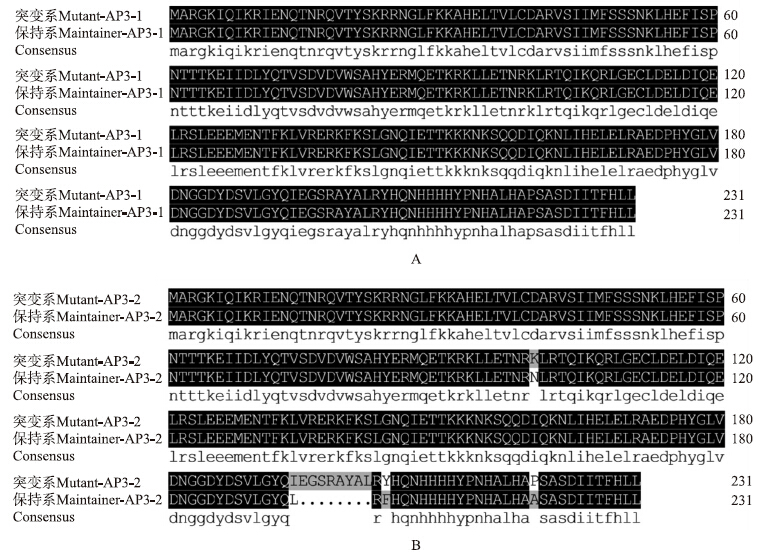 | 图 4 BcAP3-1(A)和BcAP3-2(B)蛋白氨基酸序列比对结果Fig. 4 Alignment results of BcAP3-1(A)and BcAP3-2(B)protein between two lines |
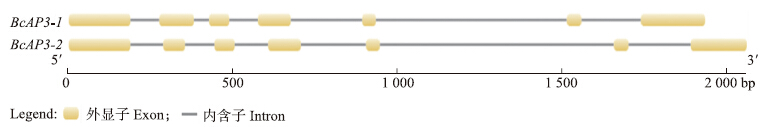 | 图 5 BcAP3-1和BcAP3-2基因的内含子-外显子结构分析Fig. 5 Exon-intron structures for BcAP3-1 and BcAP3-2 gene |
用NCBI软件分析BcAP3-1和BcAP3-2蛋白二级结构中保守的结构域,结果显示2种蛋白保守域一致,都含有1个MADS-box结构域和1个K-box结构域,符合MADS-box家族典型的结构域特征(图 6)。用SOPMA在线软件对不结球白菜BcAP3蛋白的二级结构进行预测,BcAP3-1和BcAP3-2蛋白均具丰富的二级平面结构,其中,2个蛋白分别含46.55%、51.34%的α-螺旋,8.19%、8.93%的β-折叠,21.12%、18.75%的延伸主链,24.14%、20.98%的无规则卷曲。α-螺旋和β-折叠是该蛋白多肽链上的主要元件,延伸主链和无规则卷曲分散分布在整条多肽链中(图 7)。
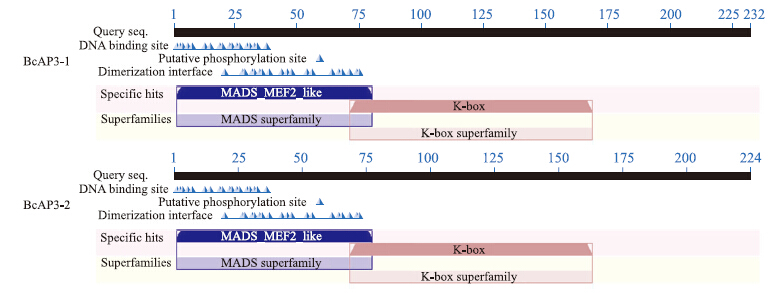 | 图 6 BcAP3蛋白保守域模式图Fig. 6 Conserved domain schematic diagram of BcAP3 protein |
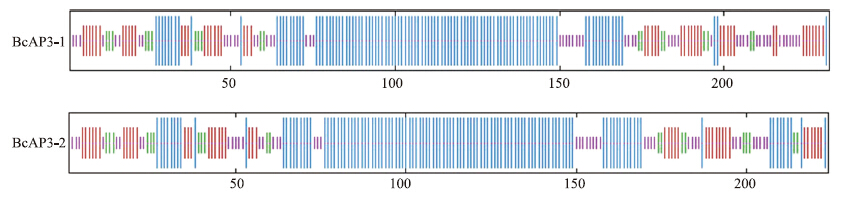 |
图 7 BcAP3蛋白二级结构分析Fig. 7 Secondary structure and disorder prediction of BcAP3
β-折叠用绿色表示,α-螺旋用蓝色表示,延伸主链和无规则卷曲分别用红色和黄色表示。 The green is β-sheet,blue indicates α-helix,the red and yellow represent extended strand and random coil,respectively. |
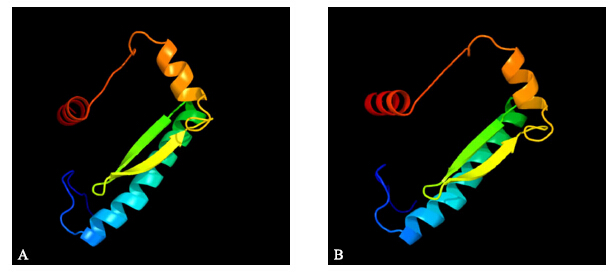 | 图 8 BcAP3-1(A)和BcAP3-2(B)蛋白空间结构示意图Fig. 8 3D-superimposition of the BcAP3-1(A)and BcAP3-2(B)proteins β-折叠用箭头表示,α-螺旋用螺旋表示。The arrow is β-sheet,coils indicate α-helix. |
将不结球白菜的2个AP3蛋白所编码的氨基酸序列和其他已经报道的AP3比对,结果显示,BcAP3 基因属于MADS-box基因家族的Ⅱ类MIKCc-型基因,包含4个不同的区域:1个含DNA结合位点的MADS domain(M)、含亲水残基的I domain(I)、1个含卷曲螺旋结构的Keratine-like domain(K)以及1个富含疏水残基C-terminal domain(C)。BcAP3蛋白在K domain均含有高度保守的(H/Q)YERM保守域。另外,BcAP3-1 和BcAP3-2蛋白的C-terminal domain中都含有PI-motif-derived来源区域和euAP3 motif区域[13]。保持系BcAP3-2、 BcAP3-1和突变系BcAP3-1、BcAP3-2主要在C-terminal domain不同,与已报道的结果[24]一致,且序列相似性为94.83%(图 9)。
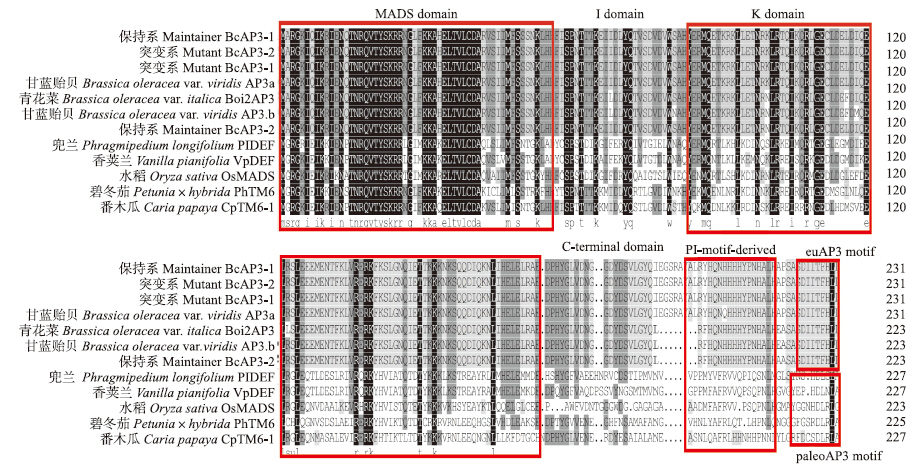 |
图 9 BcAP3和其他物种的代表性AP3蛋白的氨基酸序列一致性比较Fig. 9 Protein alignment of predicted the amino acid sequences of BcAP3 and representatives AP3 from different plants
相同的氨基酸残基用黑色背景表示,灰色背景表明氨基酸残基的一致性超过50%,红色框代表不同的基序。 The identical amino acid residues are indicated with black background,while amino acid grey shade indicate more than 50% identity,red boxes indicate different motifs. |
进化树分析结果表明:BcAP3-1和BcAP3-2属于euAP3家系,而不属于paleoAP3家系[13]。保持系BcAP3-2和甘蓝型油菜对应蛋白最先聚成一类,序列相似性为94%,与其他物种相应蛋白的亲缘关系较远;而突变系BcAP3-1、BcAP3-2及保持系BcAP3-2与甘蓝贻贝有最相近的进化关系,序列相似度为93%(图 10)。
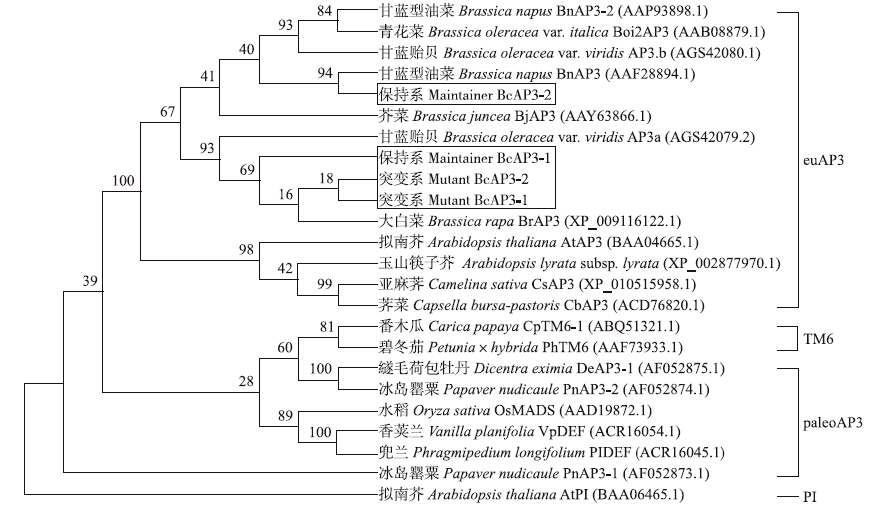 |
图 10 24个代表性AP3蛋白的进化树Fig. 10 Phylogenetic tree of 24 representative AP3 among diverse species
分支上的数字表示Bootstrap验证中基于1 000次重复该节点可信度的百分比。 The numbers for each interior branch indicate the reliability percent of bootstrap values based on 1 000 replications. |
如表 2所示:各物种AP3氨基酸残基数为223~232,蛋白相对分子质量为26 246~27 426,等电点为7.24~8.91,酸性氨基酸含量为14.2%~14.8%,碱性氨基酸残基含量为20.1%~21.1%,蛋白质的不稳定系数均大于40[25],由此可推测:euAP3家系蛋白可能属于不稳定蛋白。
| 植物 Species | 蛋白质 Protein | 氨基酸 残基数 Number of amino acids | 相对分子质量 Relative molecular mass | 理论 等电点 Theoretical pI | 碱性氨基酸 所占比例/% Rate of asic amino acid | 酸性氨基酸 所占比例/% Rate of acidic amino acid | 不稳定 系数 Instability index | 登录号 GenBank accession No. |
| 甘蓝型油菜 B.napus | BnAP3-2 | 224 | 26 457 | 7.85 | 21.0 | 14.7 | 45.69 | AAP93898.1 |
| 青花菜 B.oleracea var.italica | Boi2AP3 | 224 | 26 427 | 7.24 | 20.5 | 14.7 | 42.86 | AAB08879.1 |
| 甘蓝贻贝 B.oleracea var.viridis | BovAP3.b | 224 | 26 515 | 7.85 | 21.0 | 14.7 | 45.90 | AGS42080.1 |
| 甘蓝型油菜 B.napus | BnAP3 | 224 | 26 378 | 8.43 | 21.0 | 14.3 | 44.28 | AAF28894.1 |
| 保持系 Maintainer | BcAP3-2 | 224 | 26 378 | 8.43 | 21.0 | 14.2 | 44.28 | |
| 芥菜B.juncea | BjAP3 | 224 | 26 246 | 8.43 | 20.1 | 14.3 | 44.08 | AAY63866.1 |
| 甘蓝贻贝 B.oleracea var.viridis | BovAP3a | 232 | 27 273 | 8.71 | 20.7 | 14.2 | 47.48 | AGS42079.2 |
| 保持系 Maintainer | BcAP3-1 | 232 | 27 282 | 8.71 | 21.1 | 14.2 | 47.81 | |
| 突变系 Mutant | BcAP3-2 | 232 | 27 282 | 8.71 | 21.1 | 14.2 | 47.81 | |
| 突变系 Mutant | BcAP3-1 | 232 | 27 282 | 8.71 | 21.1 | 14.2 | 47.81 | |
| 大白菜 B.rapa | BrAP3 | 232 | 27 282 | 8.71 | 21.1 | 14.2 | 47.81 | XP_009116122.1 |
| 拟南芥 A.thaliana | AtAP3 | 232 | 27 426 | 8.91 | 20.6 | 14.2 | 45.48 | BAA04665.1 |
| 玉山筷子芥 A.lyrata subsp.lyrate | AllAP3 | 232 | 27 266 | 8.72 | 20.7 | 14.2 | 43.17 | XP_002877970.1 |
| 亚麻荠 C.sativa | CsAP3 | 232 | 27 385 | 8.70 | 21.1 | 14.2 | 47.03 | XP_010515958.1 |
| 荠菜C.bursa-pastoris | CbAP3 | 223 | 26 343 | 7.37 | 20.6 | 14.8 | 47.81 | ACD76820.1 |
组织特异性RT-PCR分析(图 11)显示:BcAP3-1基因在保持系和突变系中的表达模式基本一致,但在花瓣中突变系的表达量明显高于保持系,在保持系的萼片中高表达,在突变系萼片中没有检测到。与BcAP3-1相比,BcAP3-2基因在保持系的花瓣和柱头中表达,而在突变系的花瓣和雄蕊中表达。
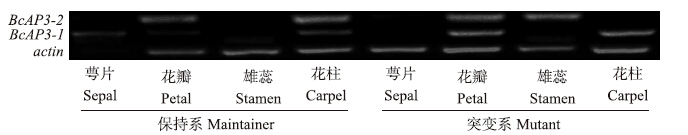 | 图 11 不结球白菜BcAP3基因在不同花器官中的表达Fig. 11 Expression of BcAP3 gene in different floral tissues of non-heading Chinese cabbage |
由图 12可以看出:BcAP3-2基因在保持系和突变系的花发育过程中表达模式相似,前中期表达量随花蕾发育逐渐增加,后期(1 mm<花蕾直径<2 mm)达到最大,之后逐渐下降。而BcAP3-1基因在2种材料中表达存在差异,在保持系中,从前期(小于0.5 mm)到中期(0.5 mm<花蕾直径<1 mm)表达量逐渐增加,然后下降,到末期(大于2 mm)又缓慢增加;而在突变系中,从前期到中期表达量逐渐增加,之后基本不变。
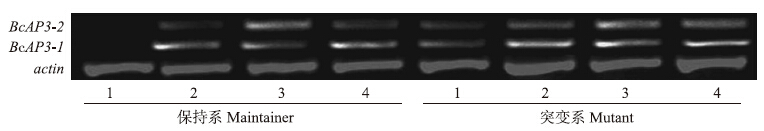 |
图 12 不结球白菜BcAP3基因在花发育不同时期表达Fig. 12 Expression of BcAP3 gene in different flower development of non-heading Chinese cabbage
1~4分别代表花蕾直径小于0.5 mm、0.5~1 mm、1~2 mm、大于2 mm。 1-4 indicate flower bud size of below 0.5 mm,0.5-1 mm,1-2 mm,above 2 mm. |
MADS-box基因家族成员作为转录因子在被子植物花发育调控中起着关键作用。AP3转录因子作为MADS-box基因家族的亚家族,属于花发育相关的B类基因,会引起花器官中的花瓣和雄蕊发生特异性改变。雄性不育系作为一种常规育种材料,对获得优质品种显得十分重要。本试验选取的突变系雄蕊发育不正常,表现为雄蕊瓣化,属于器官身份突变类型;保持系具有正常的花器官,可用于突变系繁种。
本试验在突变系和保持系中分别克隆到2个BcAP3片段,属于AP3亚家族中euAP3家系。测序结果显示,突变系中的BcAP3-2序列比保持系中BcAP3-2少24 bp,氨基酸存在3个残基的差异。氨基酸同源性比较和进化树分析结果表明,BcAP3属于euAP3家系,BcAP3基因编码的氨基酸具有典型的MADS-box家族中Ⅱ类MIKCc-型基因结构域:M区、I区、K区和C区,BcAP3基因结构由7个外显子和6个内含子组成也可以证明这一点;BcAP3蛋白与甘蓝型油菜和甘蓝贻贝具有最相近的进化关系。对BcAP3蛋白进行二级及三级结构的预测表明:BcAP3包含一个由大约56个氨基酸组成的高度保守DNA结合域和一个由70个左右氨基酸构成的中度保守螺旋卷曲,这些特征序列严格符合AP3转录因子三级结构。综上所述,BcAP3 属于MADS-box基因家族。
研究表明,在金鱼草[26]和拟南芥[27]中编码MADS-box转录因子的DEF和AP3对于花瓣和雄蕊的形成是必要的。在本研究中,BcAP3-1在突变系和保持系的雄蕊中表达量基本一致,但在花瓣中存在表达差异;BcAP3-2在突变系的花瓣和雄蕊中表达,但在保持系的雄蕊中无表达。这说明BcAP3-1是典型的euAP3家系基因,而BcAP3-2是一种新型的euAP3家系基因,由于它在保持系中保留了对花瓣的调控,从而失去了对雄蕊形成的控制。从花发育不同时期中BcAP3 mRNA表达量情况来看,在突变系中,BcAP3-2在后期(1 mm<花蕾直径<2 mm)达到最大,与冀瑞琴等[28]发现白菜雄性不育从花蕾直径为1.5 mm开始,然后当花蕾发育到1.75 mm后,雄蕊逐渐出现明显退化现象一致。以上结果说明:BcAP3-2基因可能对不结球白菜雄蕊的变异有重要影响。为进一步了解BcAP3-2基因的功能,下一步准备沉默突变系中的BcAP3-2基因,观察雄蕊是否可以恢复正常,以便了解BcAP3-2在雄蕊瓣化中的具体作用。
| [1] | Liu T K,Li Y,Zhang C W,et al. Overexpression of FLOWERING LOCUS C,isolated from non-heading Chinese cabbage(Brassica campestris ssp.chinensis Makino),influences fertility in Arabidopsis[J]. Plant Molecular Biology Reporter,2012,30(6):1444-1449 |
| [2] | Ai Y,Zhang Q H,Pan C,et al. A study of heterosis,combining ability and heritability between two male sterile lines and ten inbreds of Tagets Patula[J]. Euphytica,2015,203(2):349-366 |
| [3] | 龙欢,姚家玲,涂金星. 3种甘蓝型油菜雄性不育系花药发育的细胞学研究[J]. 华中农业大学学报:自然科学版,2005,24(6):570-575 [Long H,Yao J L,Tu J X. Cytological study on anther development in three types of Brassica napus L.male sterility line[J]. Journal of Huazhong Agricultural University:Natural Science Edition,2005,24(6):570-575(in Chinese with English abstract)] |
| [4] | 危文亮,王汉中,刘贵华. 甘蓝型油菜细胞质雄性不育系NCa花药发育的细胞学观察[J]. 中国农业科学,2005,38(6):1232-1237 [Wei W L,Wang H Z,Liu G H. Anatomical observations of anther development of NCa,a cytoplasmic male sterile line in rapeseed(Brassica napus L.)[J]. Scientia Agricultura Sinica,2005,38(6):1232-1237(in Chinese with English abstract)] |
| [5] | 聂明建,王国槐,朱卫平. 甘蓝型油菜3种类型雄性不育系花药败育的细胞学研究[J]. 中国农业科学,2007,40(7):1543-1549 [Nie M J,Wang G H,Zhu W P. Cytology research on the anther abortion of three male sterility lines in rapeseed(Brassica napus L.)[J]. Scientia Agricultura Sinica,2007,40(7):1543-1549(in Chinese with English abstract)] |
| [6] | 俞咪娜,董媛媛,徐攀峰,等. 油菜胞质不育类型相关基因研究进展[J]. 华北农学报,2008,23(增刊):7-11 [Yu M N,Dong Y Y,Xu P F,et al. Progresses on studying infertility-related genes of cytoplasmic male sterility in Brassica napus L.[J]. Acta Agriculturae Boreali-Sinica,2008,23(Suppl):7-11(in Chinese with English abstract)] |
| [7] | 黄飞,王道杰,黎斌,等. 油菜雄性核不育系及其等位可育系小孢子发育过程的比较研究[J]. 西北植物学报,2006,26(6):1159-1164 [Huang F,Wang D J,Li B,et al. Microsporogenesis comparison of genetic male sterility line and its allele fertile line in Brassica napus L.[J]. Acta Botanica Boreali-Occidentalia Sinica,2006,26(6):1159-1164(in Chinese with English abstract)] |
| [8] | 张彦锋. 不结球白菜同源异型突变雄性不育系HGMS的发现、选育及其研究[D]. 杨凌:西北农林科技大学,2005 [Zhang Y F. The discovery and study on homeotic mutant male sterile line HGMS of non-heading Chinese cabbage[D]. Yangling:Northwest A&F University,2005(in Chinese with English abstract)] |
| [9] | 黄方,迟英俊,喻德跃. 植物 MADS-box 基因研究进展[J]. 南京农业大学学报,2012,35(5):9-18. doi:10.7685/j.issn.1000-2030.2012.05.002 [Huang F,Chi Y J,Yu D Y. Research advances of MADS-box genes in plants[J]. Journal of Nanjing Agricultural University,2012,35(5):9-18(in Chinese with English abstract)] |
| [10] | Schwarz-Sommer Z,Huijser P,Nacken W,et al. Genetic control of flower development by homotic genes in Antirrhinum mojus[J]. Science,1990,250(4983):931-936 |
| [11] | Becker A,Theissen G. The major clades of MADS-box genes and their role in the development and evolution of flowering plants[J]. Molecular Phylogenetics and Evolution,2003,29(3):464-489 |
| [12] | Hileman L C,Sundstrom J F,Litt A,et al. Molecular and phylogenetic analyses of the MADS-box gene family in tomato[J]. Molecular Biology and Evolution,2006,23(11):2245-2258 |
| [13] | Kramer E M,Dorit R L,Irish V F. Molecular evolution of genes controlling petal and stamen development:duplication and divergence within the APETALA3 and PISTILLATA MADS-box gene lineages[J]. Genetics,1998,149(2):765-783 |
| [14] | Aagaard J E,Olmstead R G,Willis J H,et al. Duplication of floral regulatory genes in the Lamiales[J]. American Journal of Botany,2005,92(8):1284-1293 |
| [15] | Janssens S,Geuten K,Viaene T,et al. Phylogenetic utility of the AP3/DEF K-domain and its molecular evolution in Impatiens(Balsaminaceae)[J]. Molecular Phylogenetics and Evolution,2007,43(1):225-239 |
| [16] | Theissen G,Becker A,Di Rosa A,et al. A short history of MADS-box genes in plants[J]. Plant Molecular Biology,2000,42(1):115-149 |
| [17] | Duarte J M,Cui L,Wall P K,et al. Expression pattern shifts following duplication indicative of subfunctionalization and neofunctionlization in regulatory genes of Arabidopsis[J]. Molecular Biology and Evolution,2006,23(2):469-478 |
| [18] | Moore R C,Purugganan M D. The evolutionary dynamics of plant duplicate genes[J]. Current Opinion in Plant Biology,2005,8:122-128 |
| [19] | Kim S,Albert Y M A,Farris J S,et al. Phylogeny and diversification of B-function MADS-box genes in angiosperms:evolutionary and functional implications of a 260-million year-old duplication[J]. American Journal of Botany,2004,91(12):2102-2118 |
| [20] | Vandenbussche M,Theissen G,Peer Y V D,et al. Structural diversification and neo-functionalization during floral MADS-box gene evolution by C-terminal frameshift mutations[J]. Nucleic Acids Research,2003,31(15):4401-4409 |
| [21] | Xiao H,Wang Y,Liu D F,et al. Functional analysis of the rice AP3 homologue OsMADS16 by RNA interference[J]. Plant Molecular Biology,2003,52(5):957-966 |
| [22] | Zhao Y H,Larson-Rabin Z,Li D Z,et al. The expression and phylogenetic analysis of four AP3-like paralogs in the stamens carpels,and single-whorl perianth of the paleoherb Asarum caudigerum[J]. Molecular Biology Reporter,2013,40(8):4691-4699 |
| [23] | Zhang Y F,Wang X F,Zhang W X,et al. Functional analysis of the two Brassica AP3 genes involved in apetalous and stamen carpelloid phenotypes[J]. PLoS ONE,2011,6(6):e20930 |
| [24] | Egea-Cortines M,Saedler H,Sommer H. Ternary complex formation between the MADS-box proteins SQUAMOSA,DEFICIEND and GLOBOSA is involed in the control of floral architecture in Antirrhinum majux[J]. The EMBO Journal,1999,18(19):5370-5379 |
| [25] | Guruprasad K,Bhasker Reddy B V,Pandi M W. Correlation between stability of a protein and its dipeptide composition:a novel approach for predicting in vivo stability of a protein from its primary sequence[J]. Protein Engineering,1990,4(2):155-161 |
| [26] | Davies B,Cartolano M,Schwarz-Sommer Z. Flower development:the Antirrhinum perspective[J]. Advances in Botanical Research,2006,44:279-320 |
| [27] | Zahn L M,Feng B M,Ma H. Beyond the ABC-model:regulation of floral homeotic genes[J]. Advances in Botanical Research,2006,44:164-196 |
| [28] | 冀瑞琴,周雪,宋倩,等. 雄性不育大白菜小孢子败育与花蕾长度的关系[J]. 湖北农业科学,2011,50(17):3552-3555 [Ji R Q,Zhou X,Song Q,et al. The relationship between bud length and microspore abortion in male-sterility Brassica campestris L.ssp.pekinensis[J]. Hubei Agricultural Sciences,2011,50(17):3552-3555(in Chinese with English abstract)] |
 2015, Vol. 38
2015, Vol. 38


