文章信息
- 刘晓英, 吴丹, 焦学磊, 刘宇倩, 徐志刚. 2015.
- LIU Xiaoying, WU Dan, JIAO Xuelei, LIU Yuqian, XU Zhigang. 2015.
- 不同光谱能量分布对水稻秧苗生长的影响
- Effect of different spectral energy distribution on growth and development of rice seedlings
- 南京农业大学学报, 38(5): 735-741
- Journal of Nanjing Agricultural University, 38(5): 735-741.
- http://dx.doi.org/10.7685/j.issn.1000-2030.2015.05.006
-
文章历史
- 收稿日期:2015-02-25
光是植物光合作用的能量来源,也是植物某些生命活动的信号[1]。光合色素在红光和蓝光区有最大的光合能效[2]。红光可促进植物茎伸长[3]、叶面积增大[4]、光合产物累积[5]及光合器官的发育[6]等多个生命过程。蓝光可以促进叶绿体发育、气孔开放和蛋白质的合成[7]。前人的研究表明不同植物及同一植物的不同生长阶段或不同器官对同一光质的反应不尽相同,表现出光谱生物学反应的复杂性[8]。但多数研究表明:红蓝复合光对植物的生长效应明显优于单色光,且有效促进植物生物量的积累,可以作为植物生长的光源[9]。
但也有人认为,在红蓝复合光基础上添加其他光谱,更有利于植物的生长。添加黄光、紫光、绿光会缓解樱桃番茄的弱光胁迫[10];添加绿光,促进生菜生长[11];添加白光,促进烟草组培苗的生长[12]。很多研究显示绿光对植物无光合能效,但也有研究指出绿光促进向日葵[13]的光合作用能力强于红光。
水稻(Oryza sativa L.)是世界性主要粮食作物[14],其生产对保证世界粮食安全具有重要意义,而水稻秧苗质量直接影响水稻生产的最终产量和质量。光谱调控的准确性是工厂化水稻育秧的重要保障。前人的光调控试验研究表明:LED灯下的水稻长势优于荧光灯[15],蓝光可促进水稻秧苗生长[16],但红蓝组合光更有利于培育水稻壮苗[15]。前人的研究多集中在单色红光、蓝光或红蓝组合光对水稻秧苗生长的影响,而就不同配比的红蓝光和在红蓝光基础上添加其他光谱对工厂化水稻育秧的研究还鲜见报道。短波红光(主峰波长630 nm)有利于黄瓜的生长[17],长波红光(主峰波长660 nm)可以提高莴苣的品质[18],在光合作用的某些反应中绿光比红光和蓝光更为重要[19],隐花色素和光敏色素容易吸收绿光从而开启光形态发生的响应[20]。鉴于不同波长的红光和绿光的作用,开展以红蓝光为基础光谱添加其他光谱的研究对水稻工厂化育秧中光谱精确调控有重要理论参考。
本研究以660 nm的红光和450 nm的蓝光LED为基础光谱,精确调制这两种光的比例,添加630 nm的红光和510 nm绿光,通过测定水稻秧苗生长指标及生理指标,评判秧苗素质,旨在探讨不同光谱能量分布对水稻工厂化育秧素质的影响。
1 材料与方法 1.1 材料和光处理供试水稻品种为‘南粳5055’,由江苏省农业科学院作物科学研究所水稻组提供。试验于2014年11月在南京农业大学LED植物光源研究中心进行。水稻在32 ℃水中浸种48 h,然后置于28 ℃保温箱中催芽12 h。育苗基质为水稻育秧专用基质,由淮安柴米河农业科技发展有限公司提供,育秧盘为机插秧硬盘(600 mm×280 mm×30 mm),水稻播种覆土后在植物工厂内培养。水稻秧苗培养日温(28±2)℃、夜温(20±2)℃、相对湿度(80±5)%、光暗时间12 h/12 h、光照时间20 d。以LED白光(CK)作为对照,LED光处理为:红蓝1:1(1R1B)、红蓝3:1(2R1B)、红蓝红(R1BR2)、红蓝红绿(R1BR2G),光合光量子通量密度(Photosynthetic photon flux density,简称PPFD)均为320 μmol · m-2 · s-1,各处理的光谱分布如图 1所示,其中1R1B和2R1B的红光的主峰波长为660 nm,蓝光的主峰波长为450 nm,半波宽都为20 nm;R1BR2和R1BR2G的一个红光的主峰波长为660 nm,另一个为630 nm,蓝光的主峰波长为450 nm,R1BR2G的绿光主峰波长510 nm,半波宽均为20 nm。所有试验用光源由南京欧谱润生物科技有限公司提供,LED光谱能量分布设置如表 1所示。
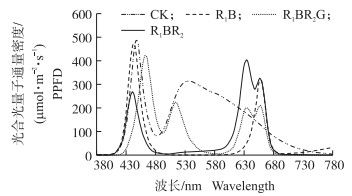 | 图 1 光处理的光谱分布 Fig. 1 Spectral distribution of light treatments |
| μmol · m-2 · s-1 | |||||
| 光处理Light treatment | 红光R660 | 蓝光B450 | 红光R630 | 绿光G510 | 白光CK |
| CK | 320 | ||||
| 1R1B | 160 | 160 | |||
| 2R1B | 240 | 80 | |||
| R1BR2 | 86 | 128 | 106 | ||
| R1BR2G | 64 | 128 | 60 | 68 | |
| 注:光合光量子通量密度均为320 μmol · m-2 · s-1。 Note:Photosynthetic photon flux density(PPFD)was 320 μmol · m-2 · s-1. | |||||
在播种后第20天分别随机选取30株测定形态指标,重复3次。用直尺测量秧苗株高,游标卡尺测量茎粗,用天平测鲜质量,然后将鲜样置于105 ℃烘箱杀青15 min,80 ℃烘干至恒质量测定干质量。叶绿素用80%丙酮提取,采用分光光度计测定[21];参照李合生[22]的方法测定可溶性糖含量;可溶性蛋白含量采用考马斯亮蓝法[23]测定;超氧化物歧化酶(SOD)参照Zhou等[24]的方法测定;抗坏血酸过氧化物酶(APX)按Nakano等[25]的方法测定;谷胱甘肽还原酶(GR)采用Zhu等[26]改进的方法测定。
1.3 数据整理与分析利用Microsoft Excel 2007整理数据,采用SPSS 16.0的Duncan′s法进行差异显著性分析。
2 结果与分析 2.1 不同光谱能量分布对水稻秧苗形态指标的影响如表 2所示:所有LED光处理的水稻株高、叶面积都大于CK处理。1R1B处理的各项形态指标都较2R1B处理低,在R1B基础上添加短波红光的R1BR2处理,茎粗、叶面积和白根数都大于2R1B处理,但与2R1B及R1BR2G处理无显著差异。
| 光处理Light treatment | 株高/cmPlant height | 茎粗/mmStem diameter | 叶面积/cm2Leaf area | 白根数Number of root |
| CK | 12.65d | 1.11b | 1.68c | 6.40ab |
| 1R1B | 14.24c | 1.09b | 2.53bc | 6.13b |
| 2R1B | 16.21a | 1.14ab | 2.82ab | 6.47ab |
| R1BR2 | 15.73ab | 1.22a | 3.11a | 7.27a |
| R1BR2G | 15.30b | 1.18ab | 3.09a | 7.13a |
| 注:不同小写字母表示各处理间在0.05水平差异显著。Note:Different lowercase letters in a column indicate statistically significant differences at 0.05 level. The same as follows. | ||||
从表 3可见:LED光处理的鲜质量、干质量都大于CK处理,各处理的地上部鲜质量、地上部干质量均显著高于对照。2R1B处理的鲜质量、干质量均大于1R1B处理,且地上部鲜质量差异显著。2R1B、R1BR2及R1BR2G处理的全株鲜质量和干质量都大于1R1B、R1BR2和R1BR2G处理的全株鲜质量和干质量无显著差异。根冠比从大到小的处理依次是2R1B、1R1B、R1BR2G、R1BR2和CK。
| 光处理Lighttreatment | 地上部鲜质量/mgShoot freshweight | 地下部鲜质量/mgRoot freshweight | 地上部干质量/mgShoot dryweight | 地下部干质量/mgRoot dryweight | 总鲜质量/mgToial freshweight | 总干质量/mgToial dryweight | 冠根比Crown rootratio |
| CK | 62.75c | 20.85b | 7.54c | 1.55c | 83.60c | 9.09d | 4.88 |
| 1R1B | 69.3b | 21.56ab | 9.55b | 1.61c | 90.91b | 11.16c | 5.98 |
| 2R1B | 79.72a | 23.98ab | 10.31ab | 1.71bc | 103.70a | 12.02bc | 6.05 |
| R1BR2 | 82.92a | 25.23a | 11.03a | 2.02a | 108.15a | 13.05a | 5.48 |
| R1BR2G | 80.17a | 25.32a | 10.55ab | 1.86ab | 105.50a | 12.41ab | 5.70 |
如表 4所示:CK处理的叶绿素a及b含量与LED光处理无显著差异。相较1R1B处理,2R1B处理的叶绿素含量都降低;与2R1B处理相比,R1BR2和R1BR2G的叶绿素a和叶绿素(a+b)的含量都降低,但叶绿素b含量却增加。R1BR2和R1BR2G处理的类胡萝卜素较高,R1BR2的类胡萝卜素含量显著高于其他处理。
| 光处理Light Treatment | 叶绿素a/(mg·g-1)Chlorophyll a | 叶绿素b/(mg·g-1)Chlorophyll b | 叶绿素/(mg·g-1)Chlorophyll | 叶绿素a/bChlorophyll(a/b) | 类胡萝卜素/(mg·g-1)Carotenoid |
| CK | 2.07abc | 0.72ab | 2.79b | 2.88c | 0.39c |
| 1R1B | 2.27a | 0.74ab | 3.00a | 3.07ab | 0.41bc |
| 2R1B | 2.12ab | 0.65b | 2.77b | 3.26a | 0.43bc |
| R1BR2 | 1.86c | 0.83a | 2.71bc | 2.24d | 0.52a |
| R1BR2G | 1.93bc | 0.77ab | 2.70c | 2.54cd | 0.48ab |
如图 2-A所示:LED光处理的秧苗可溶性糖含量都高于CK处理。R1BR2处理的可溶性糖含量最高,显著高于1R1B和CK处理(P<0.05),其他处理间差异不显著。
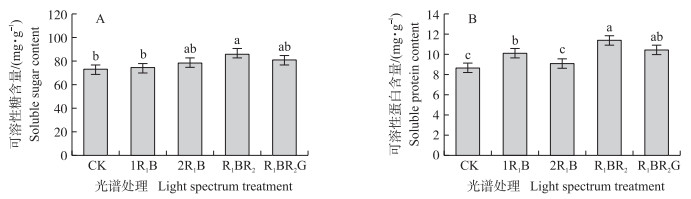 | 图 2 不同光谱能量分布对水稻秧苗可溶性糖(A)和可溶性蛋白(B)含量的影响 Fig. 2 Effect of different spectral energy distribution on soluble sugar(A)and soluble protein(B) contents of rice seedlings |
如图 2-B所示:LED光处理的秧苗可溶性蛋白含量都高于CK处理,与1R1B处理相比,2R1B处理的可溶性蛋白含量显著降低,但当添加了短波红光和绿光后各处理的可溶性蛋白含量显著升高。
2.5 不同光谱能量分布对水稻秧苗叶片酶活性的影响如表 5所示:LED光处理的SOD、APX、GR活性均大于CK处理,2R1B处理的SOD活性显著大于1R1B处理,APX、GR活性无显著差异。R1B基础上添加短波红光或绿光的SOD、APX、GR活性都大于2R1B处理,但R1BR2G处理与2R1B处理间无显著差异。R1BR2处理的SOD、APX、GR活性大于R1BR2G处理,且两处理的SOD活性差异显著。SOD/APX比值表征秧苗清除活性氧的能力,各处理的SOD/APX比值从大到小依次为CK、1R1B、2R1B、R1BR2G、R1BR2。
| 光处理Light treatment | SOD活性/(U·mg-1)SOD activity | APX活性/(U·mg-1)APX activity | GR活性/(U·mg-1)GR activity | SOD/APX |
| CK | 72.44c | 84.87d | 2.38c | 0.86 |
| 1R1B | 75.80c | 90.97cd | 2.49c | 0.84 |
| 2R1B | 81.23b | 104.03bc | 2.99bc | 0.78 |
| R1BR2 | 90.45a | 125.01a | 3.98a | 0.73 |
| R1BR2G | 84.04b | 113.19ab | 3.44ab | 0.75 |
水稻秧苗生长受光谱能量分布的影响。本研究发现,与1R1B处理相比,红光比例较大的2R1B和R1BR2 处理叶面积增大、茎变粗、白根数增多。R1BR2处理的红光总量尽管较2R1B少了48 μmol · m-2 · s-1,但对叶面积和茎粗增加及白根数增多效应更高。闻婧等[18]也指出短波红光比长波红光更有利于莴苣叶面积增大,根系活力和光合速率的提高。波长越短,光子的能量越高[27],短波630 nm的红光相较长波660 nm的红光光子所含的能量高,水稻秧苗喜红光,因此降低红光总量添加短波红光可能是水稻生长增效明显的原因。单色绿光下植物并不是完全不能生长[28, 29],Terashima等[13]认为绿光促进向日葵光合作用的能力比红光强。本研究降低红光的比例,添加绿光后的R1BR2G处理的茎粗、叶面积及白根数并没减少,这可能是由于绿光穿透叶片的能力较红光和蓝光强[20],下层的叶片能够利用的散射绿光进行光合作用,有利于干物质的积累[19]。本研究中2R1B比1R1B处理红光增加了80 μmol · m-2 · s-1,水稻秧苗生物量积累显 著增多;R1BR2处理添加了106 μmol · m-2 · s-1的630 nm的红光,红光总量比2R1B降低了48 μmol · m-2 · s-1,但生物量却与2R1B处理无显著差异,数值上还略有增大,说明增加蓝光量并添加短波630 nm的红光,可以弥补红光总量下降引起水稻生物量积累的减少。与R1BR2处理相比,R1BR2G处理降低了红光的总量,添加68 μmol · m-2 · s-1的510 nm的绿光,生物量与R1BR2处理无显著差异,表明在保持蓝光量不变的条件下,添加510 nm的绿光可同样达到添加630 nm短波红光维持水稻秧苗生物量不变的效果。
在一定范围内,叶绿素含量是光合作用强弱的判断依据[30]。叶绿素a吸收光谱的2个主峰是430和660 nm,叶绿素b吸收光谱的主峰是450和643 nm[31]。蓝光有利于叶绿素积累[6, 8],本研究也发现含有较多蓝光的1R1B处理也积累了较多的叶绿素,同时,2R1B处理也因含有较多的660 nm的红光而积累了较多的叶绿素a。R1BR2和R1BR2G处理因含有630 nm的红光接近叶绿素b红光的吸收主峰积累了较多的叶绿素b,各处理光合色素含量的差异是光谱能量分布差异性的体现。此外,可溶性糖负反馈调节叶绿素的含量[32],本试验在R1BR2处理上体现的最为明显,R1BR2处理的可溶性糖含量最高,但其叶绿素含量却较少。蓝光对光合作用而言,在能量利用率上远不如红光,吸收蓝光后的叶绿素,其贮存的能量虽远大于吸收红光的叶绿素,但超过的部分对光合作用是无用的[31],所以虽然1R1B处理含有较多的叶绿素,光合作用并不旺盛,直接导致在此光照条件的水稻秧苗积累了较少的生物量。Evans[33]发现小麦叶片内CO2同化速率与叶片氮水平呈正相关,虽然我们的研究并没有测定CO2同化速率,但R1BR2和R1BR2G处理具有较高的可溶性蛋白含量和生物量积累,间接反映了添加630 nm的红光和510 nm的绿光都能提升水稻的光合作用。
SOD、APX和GR是抗氧化系统的水-水循环中起关键作用的酶[34],其活性在一定程度上反映了机体的生理活性,间接影响了植株生长。SOD催化氧自由基(O · -2)的歧化反应产生过氧化氢,APX是清除过氧化氢途径的第1个酶,利用抗坏血酸作为还原剂清除过氧化氢[35]。GR是抗坏血酸-谷胱甘肽循环途径中的重要组成部分,将氧化型谷胱甘肽(GSSG)还原成还原型谷胱甘肽(GSH),从而为活性氧的清除提供还原力,保护植物免受伤害[36]。红光显著提高烟草叶片的SOD、APX和GR活性[37],在蓝光下黄瓜的SOD活性高,而绿光抑制黄瓜的SOD、APX活性[38]。本研究发现与1R1B和2R1B处理相比,R1BR2处理的SOD、APX和GR的活性都显著增高,R1BR2处理的蓝光量比1R1B处理减少了32 μmol · m-2 · s-1,而比2R1B处理的蓝光量增加了48 μmol · m-2 · s-1,用106 μmol · m-2 · s-1的630 nm的红光替代了660 nm的红光,表明短波红光(630 nm)可以弥补由于蓝光量增多而使这3种酶的活性下降的潜在可能,并大大提升这3种酶的活性。与R1BR2处理相比,R1BR2G处理由于添加绿光使这3种酶活性略有下降,这可能是由于短波红光(630 nm)量的减少和绿光对SOD、APX活性的抑制作用造成的[38]。SOD/APX反映过氧化氢产生和清除的能力[39],R1BR2和R1BR2G的SOD/APX值较小,再次说明添加短波红光会增加过氧化氢的清除能力,提高机体的活性,有利于水稻秧苗的生长。
综上所述,水稻光形态建成效应对光谱需求量没有精确的界定,但水稻秧苗偏好红光,且在红蓝光的基础上,添加630 nm的红光和510 nm的绿光可以增加光谱宽度,提高干物质的积累,有利于培育壮苗。
| [1] | Fankhauser C. The phytochromes,a family of red/far-red absorbing photoreceptors[J]. Journal of Biological Chemistry,2001,276(15):11453-11456 |
| [2] | McCree K J. The action spectrum,absorptance and quantum yield of photosynthesis in crop plants[J]. Agricultural Meteorology,1972,9:191-216 |
| [3] | Goins G D,Yorio N C,Sanwo M M,et al. Photomorphogenesis,photosynthesis,and seed yield of wheat plants grown under red light-emitting diodes(LEDs)with and without supplemental blue lighting[J]. Journal of Experimental Botany,1997,48(7):1407-1413 |
| [4] | 刘晓英,焦学磊,徐志刚,等. 红蓝LED光对叶用莴苣生长、营养品质和硝态氮含量的影响[J]. 南京农业大学学报,2013,36(5):139-143. doi:10.7685/j.issn.1000-2030.2013.05.024[Liu X Y,Jiao X L,Xu Z G,et al. Effects of red and blue LED on growth,nutritional quality and nitrate nitrogen content of lettuce[J]. Journal of Nanjing Agricultural University,2013,36(5):139-143(in Chinese with English abstract)] |
| [5] | 倪纪恒,陈学好,陈春宏,等. 补充不同光质对温室黄瓜生长发育、和前期产量的影响[J]. 中国农业科学,2009,42(7):2615-2623[Ni J H,Chen X H,Chen C H,et al. Effects of supplemental different light qualities on growth,photosynthesis,biomass partition and early yield of greenhouse cucumber[J]. Scientia Agricultura Sinica,2009,42(7):2615-2623(in Chinese with English abstract)] |
| [6] | Saebo A,Krekling T,Appelgren M. Light quality affects photosynthesis and leaf anatomy of birch plantlets in vitro[J]. Plant Cell,Tissue and Organ Culture,1995,41:177-185 |
| [7] | 邢泽南,张丹,李薇,等. 光质对油葵芽苗菜生长和品质的影响[J]. 南京农业大学学报,2012,35(3):47-51. doi:10.7685/j.issn.1000-2030.2012.03.009[Xing Z N,Zhang D,Li W,et al. Effects of light quality on the growth and quality of Helianthus annuus sprouts[J]. Journal of Nanjing Agricultural University,2012,35(3):47-51(in Chinese with English abstract)] |
| [8] | 车生泉,盛月英. 光质对小苍兰茎尖试管培养的影响[J]. 园艺学报,1997,24(3):269-273[Che S Q,Sheng Y Y. Effects of light quality on freesia shoot tips cultured in vitro[J]. Acta Horticulturae Sinica,1997,24(3):269-273(in Chinese with English abstract)] |
| [9] | Dong C,Hu D W,Fu Y M,et al. Analysis and optimization of the effect of light and nutrient solution on wheat growth and development using an inverse system model strategy[J]. Computers and Electronics in Agriculture,2014,109:221-231 |
| [10] | 刘晓英,徐志刚,常涛涛,等. 不同光质LED弱光对樱桃番茄植株形态和光合性能的影响[J]. 西北植物学报,2010,30(4):725-732[Liu X Y,Xu Z G,Chang T T,et al. Growth and photosynthesis of cherry tomato seedling exposed to different low light of LED light quality[J]. Acta Botanica Boreali-Occidentalia Sinica,2010,30(4):725-732(in Chinese with English abstract)] |
| [11] | Kim H H,Goins G D,Wheeler R M,et al. Green-light supplementation for enhanced lettuce growth under red- and blue-light-emitting diodes[J]. HortScience,2004,39(7):1617-1622 |
| [12] | 苏俊,刘昳雯,杨凡,等. 不同光质对烟草组培苗生长及生理特性的影响[J]. 西北植物学报,2014,34(6):1206-1212[Su J,Liu Y W,Yang F,et al. Effect of different light quality on physiological characteristics and growth of tobacco in vitro under light emitting diodes(LEDs)[J]. Acta Botanica Boreali-Occidentalia Sinica,2014,34(6):1206-1212(in Chinese with English abstract)] |
| [13] | Terashima I,Fujita T,Inoue T,et al. Green light drives leaf photosynthesis more efficiently than red light in strong white light:revisiting the enigmatic question of why leaves are green[J]. Plant and Cell Physiology,2009,50(4):684-697 |
| [14] | van Nguyen N,Ferrero A. Meeting the challenges of global rice production[J]. Paddy and Water Environment,2006,4(1):1-9 |
| [15] | 郭银生,谷艾素,崔瑾. 光质对水稻幼苗生长及生理特性的影响[J]. 应用生态学报,2011,22(6):1485-1492[Guo Y S,Gu A S,Cui J. Effects of light quality on rice seedlings growth and physiological characteristics[J]. Chinese Journal of Applied Ecology,2011,22(6):1485-1492(in Chinese with English abstract)] |
| [16] | 付传明,黄宁珍,赵志国,等. 光质与补光对水稻幼苗生长及光合速率的影响[J]. 广西植物,2007,27(2):255-259[Fu C M,Huang N Z,Zhao Z G,et al. Effects of different light qualities and illumination supplement on growth and photosynthetic rate of rice seedling[J]. Guihaia,2007,27(2):255-259(in Chinese with English abstract)] |
| [17] | 闻婧. LED红蓝光波峰及R/B对密闭植物工厂作物的影响[D]. 北京:中国农业科学院,2009[Wen Q. Influence of wave crest and R/B of red and blue LED in crop growing in the closed plant factory[D]. Beijing:Chinese Academy of Agricultural Sciences,2009(in Chinese with English abstract)] |
| [18] | 闻婧,鲍顺淑,杨其长,等. LED光源R/B对叶用莴苣生理性状及品质的影响[J]. 中国农业气象,2009,30(3):413-416[Wen Q,Bao S S,Yang Q C,et al. Influence of R/B ratio in LED lighting on physiology and quality of lettuce[J]. Chinese Journal of Agrometeorology,2009,30(3):413-416(in Chinese with English abstract)] |
| [19] | Sun J D,Nishio J N,Vogelmann T C. Green light drives CO2 fixation deep within leaves[J]. Plant and Cell Physiology,1998,39:1020-1026 |
| [20] | Klein R M. Effects of green light on biological systems[J]. Biological Reviews,1992,67(2):199-284 |
| [21] | Holm G. Chlorophyll mutations in barley[J]. Acta Agriculturae Scandinavica,1954,4(1):457-471 |
| [22] | 李合生. 植物生理生化实验原理与技术[M]. 北京:高等教育出版社,2000[Li H S. Principles and Techniques of Plant Physiology and Biochemical Experiment[M]. Beijing:Higher Education Press,2000(in Chinese)] |
| [23] | 张以顺,黄霞,陈云凤. 植物生理实验教程[M]. 北京:高等教育出版社,2009[Zhang Y S,Huang X,Chen Y F. Experimental Course of Physiology of Plant[M]. Beijing:Higher Education Press,2009(in Chinese)] |
| [24] | Zhou W,Zhao D,Lin X. Effects of waterlogging on nitrogen accumulation and alleviation of waterlogging damage by application of nitrogen fertilizer and mixtalol in winter rape(Brassica napus L.)[J]. Journal of Plant Growth Regulation,1997,16(1):47-53 |
| [25] | Nakano Y,Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts[J]. Plant and Cell Physiology,1981,22(5):867-880 |
| [26] | Zhu H,Cao Z X,Zhang L,et al. Glutathione and glutathione-linked enzymes in normal human aortic smooth muscle cells:chemical inducibility and protection against reactive oxygen and nitrogen species-induced injury[J]. Molecular and Cellular Biochemistry,2007,301(1/2):47-59 |
| [27] | 潘瑞炽. 植物生理学[M]. 4版. 北京:高等教育出版社,2001[Pan R C. Plant Physiology[M]. 4th ed. Beijing:Higher Education Press,2001(in Chinese)] |
| [28] | 陈文昊,徐志刚,刘晓英,等. LED光源对不同品种生菜生长和品质的影响[J]. 西北植物学报,2011,31(7):1434-1440[Chen W H,Xu Z G,Liu X Y,et al. Effect of LED light source on the growth and quality of different lettuce varieties[J]. Acta Botanica Boreali-Occidentalia Sinica,2011,31(7):1434-1440(in Chinese with English abstract)] |
| [29] | 常涛涛,刘晓英,徐志刚,等. 不同光谱能量分布对番茄幼苗生长发育的影响[J]. 中国农业科学,2010,43(8):1748-1756[Chang T T,Liu X Y,Xu Z G,et al. Effects of light spectral energy distribution on growth and development of tomato seedlings[J]. Scientia Agricultura Sinica,2010,43(8):1748-1756(in Chinese with English abstract)] |
| [30] | 郑洁,胡美君,郭延平. 光质对植物光合作用的调控及其机理[J]. 应用生态学报,2008,19(7):1619-1624[Zheng J,Hu M J,Guo Y P. Regulation of photosynthesis by light quality and its mechanism in plants[J]. Chinese Journal of Applied Ecology,2008,19(7):1619-1624(in Chinese with English abstract)] |
| [31] | 李合生. 现代植物生理学[M]. 北京:高等教育出版社,2006:148-149[Li H S. Modern Plant Physiology[M]. Beijing:Higher Education Press,2006:148-149(in Chinese)] |
| [32] | Lefsrud M G,Kopsell D A,Sams C E. Wavelengths from adjustable light emitting diodes affect secondary metabolites in kale[J]. HortScience,2008,43:2243-2244 |
| [33] | Evans J R. Nitrogen and photosynthesis in the flag leaf of wheat(Triticum aestivum L.)[J]. Plant Physiology,1983,72(2):297-302 |
| [34] | Asada K. The water-water cycle in chloroplasts:scavenging of active oxygens and dissipation of excess photons[J]. Annual Review of Plant Biology,1999,50(1):601-639 |
| [35] | 李泽琴,李静晓,张根发. 植物抗坏血酸过氧化物酶的表达调控以及对非生物胁迫的耐受作用[J]. 遗传,2013,35(1):45-54[Li Z Q,Li J X,Zhang G F. Expression regulation of plant ascorbate peroxidase and its tolerance to abiotic stresses[J]. Hereditas,2013,35(1):45-54(in Chinese with English abstract)] |
| [36] | Gill S S,Anjum N A,Hasanuzzaman M,et al. Glutathione and glutathione reductase:a boon in disguise for plant abiotic stress defense operations[J]. Plant Physiology and Biochemistry,2013,70:204-212 |
| [37] | 文锦芬,柯学,徐超华,等. 光质对烟草叶片生长发育过程中抗氧化系统的影响[J]. 西北植物学报,2012,32(9):1799-1804[Wen J F,Ke X,Xu C H,et al. Effects of light quality on antioxidant defense system during growth and development of tobacco leaves[J]. Acta Botanica Boreali-Occidentalia Sinica,2012,32(9):1799-1804(in Chinese with English abstract)] |
| [38] | 王虹,姜玉萍,师恺,等. 光质对黄瓜叶片衰老与抗氧化酶系统的影响[J]. 中国农业科学,2010,43(3):529-534[Wang H,Jiang Y P,Shi K,et al. Effects of light quality on leaf senescence and activities of antioxidant enzymes in cucumber plants[J]. Scientia Agricultura Sinica,2010,43(3):529-534(in Chinese with English abstract)] |
| [39] | Yamazaki J. Is light quality involved in the regulation of the photosynthetic apparatus in attached rice leaves?[J]. Photosynthesis Research,2010,105(1):63-71 |
 2015, Vol. 38
2015, Vol. 38


