文章信息
- 王曼, 祁克宗, 涂健, 周秀红, 刘红梅, 王惠珂, 尹磊. 2015.
- WANG Man, QI Kezong, TU Jian, ZHOU Xiuhong, LIU Hongmei, WANG Huike, YIN Lei. 2015.
- 禽致病性大肠杆菌PhoP蛋白调控宿主菌DNA检测方法的建立与应用
- Establishment and application of detection method of avian pathogenic E.coli PhoP protein regulating host bacteria DNA
- 南京农业大学学报, 38(4): 645-649
- Journal of Nanjing Agricultural University, 38(4): 645-649.
- http://dx.doi.org/10.7685/j.issn.1000-2030.2015.04.018
-
文章历史
- 收稿日期:2014-10-22
禽致病性大肠杆菌(avian pathogenic Escherichia coli,APEC)不仅给养禽业造成重大经济损失,而且因APEC毒力因子复杂的致病性,对人及禽类公共健康造成巨大的威胁。病原菌对宿主的致病作用,一方面由细菌毒力介导菌体黏附和侵袭细胞,另一方面抑制宿主细胞的防御性反应,如抑制抗菌肽、溶酶体、凋亡等对入侵细菌的清除,因而研究菌体毒力因子对预防禽大肠杆菌病具有重要意义。
PhoP/Q二元调控系统由细菌胞质内的反应调节蛋白(response regulator protein,RR)和跨膜的组氨酸蛋白激酶(histidine protein kinases,HK)组成(RR、HK分别为PhoP和PhoQ的总称),是革兰氏阴性菌中普遍存在的基因表达二元调控系统,其在病原菌毒力基因表达、参与上皮细胞侵袭和抵御抗菌肽(antimicrobial peptides,AMPs)感染等环节中发挥关键的调控作用[1, 2]。PhoP/Q二元系统调控鼠疫耶尔森菌和沙门氏菌的毒力已有详细报道[3],但其对禽大肠杆菌的调控及机制目前还没有深入研究。phoP作为禽大肠杆菌调控基因之一,与宿主菌毒力调控密切相关[4, 5, 6]。由于其自身可以结合基因启动子区来调控基因表达量,利于进行体外功能研究。
本试验通过构建phoP基因的表达载体,诱导表达、纯化得到其蛋白产物,对iss和hlyF基因启动子区进行体外凝胶阻滞试验,测定蛋白的结合活性,探讨PhoP蛋白与APEC毒力基因的调控关系,为后期防治禽大肠杆菌病和靶向阻断其致病途径提供了试验依据。
1 材料与方法 1.1 试验材料与仪器APEC O2型临床分离株AE17(2008年于安徽巢湖分离的鸭源型大肠杆菌,经血清型鉴定为O2型)和基因工程宿主菌DH5α由本实验室保存;APEC O2型分离株、表达宿主菌BL21(DE3)和质粒pET32a均由上海兽医研究所馈赠;EMSA探针生物素标记试剂盒、化学发光法生物素检测试剂盒、正电荷尼龙膜均购自碧云天生物技术有限公司;Ni-NTA亲和层析柱购自上海悦克生物公司。
MyCyclerTM Thermal Cycler PCR扩增仪和ChemiDocXRS化学发光成像仪(美国Bio-Rad公司);BIO-BEST200A凝胶成像仪(美国SIM集团);80-3006-51超微量核酸蛋白分析仪(英国Bio-Drop公司);DYY-12型多用电泳仪(北京市六一仪器厂)。
1.2 APECO2型菌株PhoP基因的克隆与表达 1.2.1 目的基因的获取根据NCBI中报道的APEC O1型phoP基因(GenBank登录号:CP000468.1)编码区序列,利用引物设计软件Prime Premier 5.0设计其引物(表 1),在phoP引物两端加上合适的酶切位点EcoRⅠ和XhoⅠ及相应的保护性碱基后送上海生工生物工程有限公司合成。
| 引物名称Primers | 引物对序列(5′→3′)Primer pairs sequence(5′→3′) |
| phoP F/R | CGGAATTCATGCGCGTACTGGTTGTTGAA/CCCTCGAGTCAGCGCAATTCGAACAGAT |
| pET32a F/R | CGAACGCCAGCACATGGACA/GCTAGTTATTGCTCAGCGG |
| 注:下划线为酶切位点。Restriction sites of EcoRⅠand XhoⅠare underlined. | |
经PCR扩增得到phoP基因片段后,连入pMD19-T载体[7],连接产物转化DH5α感受态细胞,随机挑取单菌落,提取质粒,用EcoRⅠ和XhoⅠ双酶切鉴定。阳性质粒命名为pMD19-T-phoP。将阳性质粒送上海生工生物工程公司测序。
1.2.2 重组质粒pET32a-phoP的构建用EcoRⅠ和XhoⅠ双酶切原核表达载体pET32a和质粒pMD19-T- phoP,分别回收纯化phoP基因和pET32a载体片段,T4 DNA连接酶连接;连接产物转化保存于宿主菌DH5α感受态细胞中,用含氨苄青霉素(Amp)的平板筛选阳性菌落,挑取单菌落用液体LB培养基培养后利用pET32a通用引物(表 1)鉴定目的片段大小并测序。将阳性重组质粒命名为pET32a-phoP[8],其菌株用体积分数为30%的甘油密封后保存于-20 ℃冰箱。
1.2.3 重组PhoP蛋白的表达及纯化将DH5α中测序正确的pET32a-phoP和空质粒转化感受态细胞BL21(DE3),用同样方法鉴定阳性重组质粒。将含pET32a-phoP和空质粒pET32a的BL21(DE3)阳性转化 子分别接种于含Amp的LB液体培养基中,30 ℃、200 r · min-1培养至A600为0.8,加终浓度为0.5 mmol · L-1的IPTG诱导过夜。用120 g · L-1的SDS-PAGE检测PhoP蛋白的表达量[9, 10, 11]。
次日,按照1 ∶ 100(体积比)将重组阳性转化子接种于200 mL LB培养基中,用上述同样方法诱导后收集菌体,用20%PBS缓冲液洗菌3次后重悬菌体,置冰浴中超声粉碎菌体16 min,4 ℃、12 000 r · min-1离心30 min,取上清液缓慢流过Ni-NTA亲和层析柱,然后逐步用高浓度咪唑缓冲液洗脱目的蛋白,以摸索最佳洗脱浓度[12, 13]。将纯化后的蛋白液用截留相对分子质量为10×103的Millipore超滤管4 000 g离心40 min,再用20%PBS缓冲液脱盐浓缩后,4 ℃冰箱保存备用[14, 15]。
1.3 DNA结合检测分析1.3.1 靶基因启动子区的获得和生物素标记
在GenBank上查找iss、hlyF基因启动子区,通过启动子预测网站(http://www.fruitfly.org/seq_tools/promoter.html)和生物信息学分析法对PhoP蛋白与DNA的结合基序进行了预测,选定其对应的启动子序列,设计引物(表 2),PCR扩增iss、hlyF基因启动子区并对产物纯化回收;同时,查找 16 S DNA基因以作为结合试验的阴性对照[16, 17]。对以上3种基因按碧云天生物素标记试剂盒进行标记。
| 引物名称Primers | 引物对序列(5′→3′) Primer pairs sequence(5′→3′) | 产物大小/bp Product size |
| iss F/R | TGTCTGGGAAAATAGTGGC/TAAACGTTTGTTGAGCACATCCT | 392 |
| hlyF F/R | GCCCTGCCACTCTTCATGC/AATTCTTTCCAGTCCCGCT | 408 |
| 16S DNA F/R | CGTTACCCGCAGAAGAAGCA/TCAAGGGCACAACCTCCAAG | 363 |
配制40 g · L-1的非变性聚丙烯酰胺凝胶,电泳缓冲液为0.5×TBE,凝胶凝固后预电泳30 min。取1 μL标记好的 16 S DNA、iss、hlyF探针按4个梯度加重组PhoP蛋白后加结合液混匀,室温孵育20 min,加上样缓冲液进行电泳。电泳完成后,将湿法电转膜装置设置为380 mA后转膜30~60 min。在超净工作台内的紫外灯下完成交联,继而运用化学发光成像仪检测生物素标记的探针迁移位置[18, 19, 20]。
2 结果与分析 a 2.1 APEC O2型菌株PhoP基因的克隆与表达 2.1.1 pET32a-phoP重组质粒的鉴定 将pMD19-T-phoP测序结果与APEC O1 phoP序列进行比对,发现其同源性达99.85%,氨基酸序列与APEC O1型一致,测序结果与上海兽医研究所提供的APEC O2型phoP序列一致。将阳性重组质粒pMD19-T-phoP酶切产物核酸电泳(图 1-A),分别得到约2 692和672 bp的T载体与phoP片段,大小与理论值一致。回收phoP目的片段,构建重组表达质粒pET32a-phoP,用pET32a通用引物进行菌落PCR鉴定。由于含有载体序列174 bp,所以显示其电泳条带约为846 bp(图 1-B),比目的phoP片段672 bp稍大,与理论值一致。其测序结果与上海兽医研究所提供的phoP序列一致,表明重组质粒构建成功。 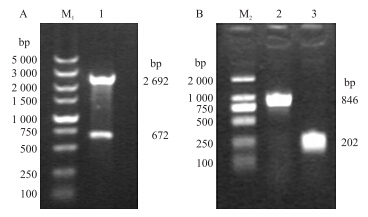 |
图 1 phoP基因的克隆鉴定 Fig. 1 Identification of phoP gene
A.重组质粒pMD19-T-phoP酶切鉴定Identification of recombinant plasmid pMD19-T-phoP by restriction enzyme digestion;B.重组质粒的PCR鉴定Identification of recombinant plasmid by PCR M1.DNA marker(DL5000);M2.DNA marker(DL2000); 1.pMD19-T-phoP经EcoR Ⅰ、XhoⅠ 酶切产物pMD19-T-phoP digested by EcoRⅠand XhoⅠ;2.重组质粒pET32a-phoP Recombinant plasmid pET32a-phoP;3.空质粒pET32a The empty plasmid pET32a |
由于pET32a中T7 promoter端融合表达了相对分子质量约18.37×103的载体标签,所以SDS-PAGE电泳结果显示重组融合蛋白产物大小为42.9×103,比预期目的蛋白理论值(24.53×103)要大(图 2-A),以同样条件诱导的空载体则没有出现这一条带,而是显示出的空载体所表达的整个载体标签蛋白约20.79×103,与理论值一致,确定目标蛋白得到表达。
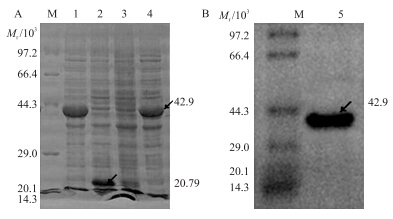 |
图 2 重组PhoP蛋白的原核表达与纯化 Fig. 2 Expression and purification of the recombinant PhoP
A.重组PhoP蛋白的表达鉴定Expression and identify of the recombinant PhoP;B.重组PhoP蛋白的纯化鉴定Purification and identify of the recombinant PhoP M.蛋白标准品Protein marker;1,4.重组pET32a-phoP诱导表达产物Induced expression product of the recombinant pET32a-phoP;2.pET32a的诱导表达产物Induced expression product of the empty plasmid pET32a;3.空宿主菌诱导表达产物Induced expression product of the BL21(DE3);5.纯化的重组PhoP蛋白The purified recombinant PhoP protein |
经过摸索蛋白的咪唑洗脱浓度,最终确定分别用1和20 mmol · L-1、pH 7.5的咪唑溶液洗脱杂蛋白,以200 mmol · L-1的咪唑溶液洗脱目的蛋白,用其洗脱后得到纯度较高的重组PhoP蛋白(图 2-B),经蛋白浓度测定仪测定其质量浓度约为0.5 mg · mL-1。
2.2 重组PhoP蛋白与宿主菌DNA的结合检测分析分别用1 μL的 16 S DNA和iss、hlyF基因启动子区与重组PhoP蛋白梯度混合,其结合液进行非变性聚丙烯酰胺凝胶迁移试验(EMSA)。放射自显影结果表明:重组PhoP蛋白能与iss和hlyF基因结合而与 16 S DNA没有明显的结合(图 3),说明表达的重组PhoP蛋白具有体外活性,而且对基因探针的结合具有选择性。
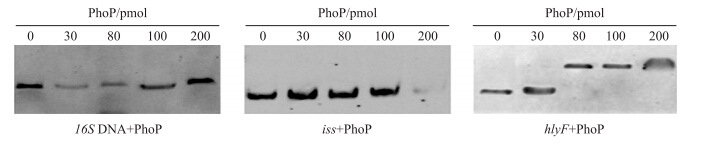 | 图 3 梯度PhoP蛋白与靶基因启动子凝胶迁移试验的放射自显影结果 Fig. 3 Electrophoretic mobility shift assay(EMSA)autoradiography results of gradient PhoP and target DNA promoter |
EMSA试验结果显示:当蛋白量少时,iss和hlyF基因启动子区均未出现迁移阻滞带,而随着蛋白量的增加出现不同程度的滞后带(图 3),但阴性对照 16 S DNA在加入蛋白量为200 pmol时仍没有出现滞后带,从而排除了结合液等外界因素的干扰,保证了试验结果的可靠性。由图中滞后带可以发现,重组PhoP蛋白对不同基因的结合力存在一定差异。放射自显影结果表明:iss基因启动子区与重组PhoP蛋白结合后,在蛋白量为200 pmol时显示出滞后状态;hlyF基因启动子区与重组PhoP蛋白结合后在蛋白量为80 pmol以后显示了滞后状态。这在一定程度上说明PhoP蛋白结合溶血基因hlyF的能力大于结合血清毒力基因iss的能力。
3 讨论大肠杆菌PhoP/Q二元调控系统对菌体自身的致病性起到重要调控作用。目前研究证实PhoP/Q在细菌中的作用就是激活特定的表面蛋白和酶的表达,PhoP/Q基因簇组成一条传感链,PhoQ可探测机体外界环境变化并通过PhoP蛋白磷酸化继而调控下游基因的表达[3, 21],以适应外界环境。大肠杆菌的致病性取决于很多因素,比如控制其毒力的基因和外界环境的变化等。对于大肠杆菌PhoP蛋白由Mg2+介导的基因调控曾有所报道[22, 23],但所能直接调控的与菌体毒力相关的基因至今仍没有明确结论,本试验用凝胶迁移试验验证了PhoP蛋白调控的毒力基因。
本试验中phoP作为调控基因,其蛋白能够结合血清毒力基因iss和溶血基因hlyF,而对稳定遗传的 16 S DNA没有表现出结合作用,这说明大肠杆菌PhoP蛋白可以调控iss和hlyF基因转录,而对 16 S DNA则没有调控作用[16, 21]。iss基因在凝胶迁移过程中出现了探针弥散的现象,可能是因为蛋白在结合探针后电泳时间太长,受阻力和温度影响而使蛋白有所降解,结合力减弱,导致了解离分散[21],但图示能证明此靶基因与PhoP蛋白具有结合特性。因此,在此类试验中,电泳过程应尽量保持低温,并降低电泳时间。由于基因探针与蛋白结合后其结合体相对分子质量变大,后期在电泳过程中的运动速率降低,从而表现出滞后带,尤其是在蛋白量满足基因探针的结合要求后会表现出明显的滞后[3, 20]。本试验根据这一原理通过PhoP蛋白的体外表达纯化及与DNA的凝胶迁移检测初步建立了对PhoP蛋白调控基因的筛选方法,为大肠杆菌PhoP蛋白体外及其体内致病性研究开辟了一条新思路,对后续进一步探究PhoP调控因子奠定了基础,也对大肠杆菌的PhoP/Q二元调控机制有了更进一步的认识。
凝胶迁移试验已成为检测蛋白与其他因子结合活性和作用关系的主要方法[24],也为进一步研究大肠杆菌调控机制奠定了方法基础。为此,针对大肠杆菌筛选出受PhoP/Q系统调控的外膜蛋白、内毒素等相关靶基因,通过控制其毒力基因靶点,阻断其致病过程,发挥靶向拮抗效应,可以达到抗感染的目的。
| [1] | Yang C,Huang T W,Wen S Y,et al. Genome-wide PhoB binding and gene expression profiles reveal the hierarchical gene regulatory network of phosphate starvation in Escherichia coli[J]. PLoS ONE,2012,10(7):e47314 |
| [2] | Prost L R,Miller S I. The Salmonellae PhoQ sensor:mechanisms of detection of phagosome signals[J]. Cellular Microbiology,2008,10(3):576-582 |
| [3] | 秦珑. 整体调控子phop直接调控鼠疫耶尔森氏菌胞内生存能力的研究[D]. 北京:军事医学科学院,2006 [Qin L. Phop,a novel global regulator,directly activates a global regulon contributing to intracellular growth of Yersinia pestis[D]. Beijing:The Academy of Military Medical Sciences,2006(in Chinese with English abstract)] |
| [4] | Alteri C J,Lindner J R,Reiss D J,et al. The broadly conserved regulator PhoP links pathogen virulence and membrane potential in Escherichia coli[J]. Molecular Microbiology,2011,82(1):145-163 |
| [5] | Audrey C,Alisa L,Pierre M,et al. MicA sRNA links the PhoP regulon to cell envelope stress[J]. Molecular Microbiology,2010,76(2):467-479 |
| [6] | Nishino K,Latifi T,Groisman E A. Virulence and drug resistance roles of multidrug efflux systems of Salmonella enterica serovar typhimurium[J]. Molecular Microbiology,2006,59(1):126-141 |
| [7] | 王海霞,缪刘,祁克宗,等. 鸡β防御素2成熟肽基因在毕赤酵母中的表达及其抗菌活性分析[J]. 中国兽医科学,2012,42(7):719-724 [Wang H X,Miao L,Qi K Z,et al. Expression of Gal-2 mature peptide gene in Pichia pastoris and analysis of antimicrobial activity of the recombinant protein[J]. Chinese Veterinary Science,2012,42(7):719-724(in Chinese with English abstract)] |
| [8] | 马春晓,张振超,李祥瑞,等. 捻转血矛线虫精氨酸激酶基因的克隆与表达及酶活性分析[J]. 南京农业大学学报,2014,37(3):100-106. doi:10.7685/j.issn.1000-2030. 2014. 03.015 [Ma C X,Zhang Z C,Li X R,et al. Arginine kinase in Haemonchus contortus:cloning expression and catalytic properties[J]. Journal of Nanjing Agricultural University,2014,37(3):100-106(in Chinese with English abstract)] |
| [9] | Farhat N,Mamarbachi A M,Thorin E,et al. Cloning,expression and purification of functionally active human angiopoietin-like protein 2[J]. Springer Plus,2014,3(1):1-9 |
| [10] | Liu Z Q,Yang P C. Construction of pET-32α(+)vector for protein expression and purification[J]. Molecular Cloning Technique,2012,4(12):651-655 |
| [11] | Reyhaneh M,Jalal B,Samira A,et al. Expression and purification of recombinant ROP1 of Toxoplasma gondii in bacteria[J]. Avicenna Journal of Medical Biotechnology,2013,5(4):227-233 |
| [12] | 王琪炜,陈宝俊,强倩,等. 人转录因子spl在大肠杆菌中的表达与体外纯化[J]. 东南大学学报:医学版,2014,33(1):1-4 [Wang Q W,Chen B J,Qiang Q,et al. Expression in Escherichia coli and purification in vitro of human transcription factor spl[J]. Journal of Southeast University:Medicine Science Edition,2014,33(1):1-4(in Chinese with English abstract)] |
| [13] | Bis R L,Stauffer T M,Singh S M,et al. High yield soluble bacterial expression and streamlined purification of recombinant human interferon α-2a[J]. Protein Expression Purification,2014,99(3):138-146 |
| [14] | Liu F X,Wu X D,Li L,et al. Expression,purification and characterization of two truncated peste des petits ruminants virus matrix proteins in Escherichia coli,and production of polyclonal antibodies against this protein[J]. Protein Expression and Purification,2013,91(1):1-9 |
| [15] | Kumar S,Sharma R K. An improved method and cost effective strategy for soluble expression and purification of human N-myristoyltransferase 1 in Escherichia coli[J]. Molecular and Cellular Biochemistry,2014,392:175-186 |
| [16] | 张义全,高鹤,王丽,等. 鼠疫菌H.NS蛋白的表达与纯化及其DNA结合活性分析[J]. 微生物学报,2011,51(5):615-621 [Zhang Y Q,Gao H,Wang L,et al. Yersinia pestis H.NS protein expression and purified and analysis of DNA binding activity[J]. Acta Microbiologica Sinica,2011,51(5):615-621(in Chinese with English abstract)] |
| [17] | Zhu L H,Qiu L Z,Ren W,et al. Upregulation of a spliced variant of human interferon regulatory factor 3 through binding of the transcription factor Sp1 to the promoter[J]. Biomedical Reports,2014,2(1):142-146 |
| [18] | Mitchell S F,Lorsch J R. Standard in vitro assays for protein-nucleic acid interactions-gel shift assays for RNA and DNA binding[J]. Methods Enzymol,2014,541(15):179-196 |
| [19] | Zhang W,Lü X J,Zhang W K,et al. EMSA and single-molecule force spectroscopy study of interactions between bacillus subtilis single-stranded DNA-binding protein and single-stranded DNA[J]. Langmuir,2011,27(24):15008-15015 |
| [20] | Torres E A,Juárez M D,Demuth D R. Differential transcriptional regulation of Aggregatibacter actinomycetemcomitans lsrACDBFG and lsrRK operons by integration host factor protein[J]. Journal of Bacteriology,2014,196(8):1597-1607 |
| [21] | Zhang Y Q,Wang L,Han Y P,et al. Autoregulation of PhoP/PhoQ and positive regulation of the cyclic AMP receptor protein-cyclic AMP complex by PhoP in Yersinia pestis[J]. Journal of Bacteriology,2013,195(5):1022-1030 |
| [22] | Yamamoto K,Ogasawara H,Fujita N,et al. Novel mode of transcription regulation of divergently overlapping promoters by PhoP,the regulator of two-component system sensing external magnesium availability[J]. Molecular Microbiology,2002,45(2):423-438 |
| [23] | Eguchi Y K,Itou J J,Yamane M T,et al. B1500,a small membrane protein,connects the two-component systems EvgS/EvgA and PhoQ/PhoP in Escherichia coli[J]. Proc Natl Acad Sci USA,2007,104(47):18712-18717 |
| [24] | Hellman L M,Fried M G. Electrophoretic mobility shift assay(EMSA)for detecting protein-nucleic acid interactions[J]. Nature Protocols,2007,2(8):1849-1861 |




