文章信息
- 王丹阳, 高付永, 孙炜, 芮伟康, Khanizadeh Shahrokh, 张绍铃, 陶书田. 2015.
- WANG Danyang, GAO Fuyong, SUN Wei, RUI Weikang, Khanizadeh Shahrokh, ZHANG Shaoling, TAO Shutian. 2015.
- ‘砀山酥梨’果实CCoAOMT基因的克隆与表达分析
- Cloning and expression analysis of CCoAOMT gene in fruit of ‘Dangshansuli’
- 南京农业大学学报, 38(1): 33-40
- Journal of Nanjing Agricultural University, 38(1): 33-40.
- http://dx.doi.org/10.7685/j.issn.1000-2030.2015.01.006
-
文章历史
- 收稿日期:2014-05-25
2. 江苏省丰县农林局, 江苏 丰县 221700;
3. Eastern Cereals and Oilseeds Research Centre, Agriculture and Agri-Food Canada, Ottawa, Ontario, K1A 0C6
2. Fengxian Agriculture and Forestry Bureau of Jiangsu Province, Fengxian 221700, China;
3. Eastern Cereals and Oilseeds Research Centre, Agriculture and Agri-Food Canada, Ottawa, Ontario, K1A 0C6
‘砀山酥梨’(Pyrus bretschneideri‘Dangshansuli’)在我国已有超过500年的栽培历史,分布区域较广,为我国产量第一的梨品种。其果实不仅风味好,营养价值高,而且有药用价值,深受人们喜爱。近年来由于品种退化和管理不善等因素,‘砀山酥梨’石细胞含量增加,果肉口感粗糙多渣,影响了梨果实的品质[1, 2, 3, 4]。研究表明,在‘砀山酥梨’的石细胞中,木质素的含量高达29.8%[5, 6]。由于石细胞的形成是木质素在细胞壁沉积的结果,因此其主要成分是木质素。在其他植物中,大部分参与木质素生物合成途径的酶类及编码基因都已得到纯化和分离[7, 8],其中咖啡酰-辅酶AO-甲基转移酶(CCoAOMT)被认为是木质素生物合成途径中的关键酶之一[9, 10, 11]。研究发现其在多种植物中存在基因家族现象,研究者已从多种植物中分离得到了CCoAOMT基因[12, 13, 14, 15, 16, 17]。但是关于梨果实中CCoAOMT基因的研究,特别是结合石细胞形成、木质素合成开展的相关研究却未见报道。
本研究以‘砀山酥梨’果实为试材,通过RT-PCR技术克隆获得梨PbCCoAOMT基因全长,并对其进行生物信息学分析;进一步利用荧光定量PCR技术对梨果实发育不同时期的PbCCoAOMT基因表达进行了分析,结合对梨果实发育过程中石细胞含量变化趋势的研究,探讨PbCCoAOMT基因在梨果实木质素合成中的作用,以期为通过调控石细胞的合成而改良梨果实品质奠定基础。
1 材料与方法 1.1 试验材料与处理
试验于2012年4月至10月在江苏省徐州市丰县大沙河镇梨园进行。选择同一果园内树势健壮、管理水平一致,树龄为15年的‘砀山酥梨’(P.bretschneideri‘Dangshansuli’)20株,于2012年4月初,分别在树冠的中层选择花蕾发育期及大小基本一致的枝条挂牌。依据陶书田等[18]的方法对梨果实发育过程中石细胞团发育规律进行研究,从梨盛花期正常授粉后21 d至果实膨大期,每隔6 d采集1次样品,果实膨大期至果实成熟,分别每隔16、17、15和31 d采集1次样品。每次采集果实20个,并于当天用生物冰袋带回实验室,果实清洗去皮并采用四分法将果肉切碎混匀,部分直接用于RNA提取,其余经液氮速冻后于-80 ℃冰箱中保存备用。
1.2 试剂
大肠杆菌(Escherichia coli)菌株DH5α由南京农业大学梨工程技术研究中心保存;Rever Tre AceTM Kit购自TOYOBO公司;DNaseⅠ、LA Taq DNA聚合酶、pMD19-T载体、dNTPs、SYBR染料和DNA marker均购自TaKaRa公司;凝胶回收试剂盒购自Axygen公司。PCR引物的合成和测序均由上海英骏生物技术有限公司合成。
1.3 总RNA的提取及cDNA的合成
采用改良的CTAB法[19]提取梨果肉的RNA;用12 g · L-1琼脂糖凝胶电泳检测RNA质量,并用NanoDrop 2000检测浓度后,保存于-80 ℃备用。将提取的RNA按照如下体系进行DNA消化,10×reaction Buffer(Mg2+)1 μL,DNaseⅠ1 μL,RNase-Free 1 μL,DEPC水7 μL,RNA 1 μg,37 ℃ 30 min;加入1 μL 50 mmol · L-1 EDTA后65 ℃ 10 min,按照Rever Tre AceTM Kit说明书合成cDNA第1链,于-20 ℃保存备用。
1.4 PbCCoAOMT基因的克隆与生物信息学分析
根据从NCBI数据库中搜索得到的关于植物木质化的CCoAOMT基因序列与梨基因组数据库调取的序列比对,采用软件Primer Premier 5.0设计基因扩增的特异性引物。F0:5′-TTTCTGGTGGCAGGTCG-3′;R0:5′-CCGACATGAACGTTGACG-3′,应用PCR仪对其进行扩增。25 μL PCR反应体系为:10×LA PCR Buffer 2.5 μL,MgCl2 2.5 μL,dNTP Mixture 4 μL,正、反向引物各0.5 μL,LA Taq DNA酶0.25 μL,ddH2O 13.25 μL,模板cDNA 1.5 μL。PCR反应程序为:94 ℃ 3 min;94 ℃ 30 s,60 ℃ 40 s,72 ℃ 90 s,共40个循环;72 ℃ 10 min,10 ℃ 10 min。取5 μL PCR产物用12 g · L-1琼脂糖凝胶电泳检测扩增结果。采用凝胶回收试剂盒回收纯化目的条带,与pMD19-T载体连接,转化大肠杆菌DH5α,挑取白斑单菌落在液体培养基中振荡培养,取1 μL菌液进行PCR扩增鉴定,选取菌液PCR产物经电泳检测与RT-PCR扩增产物大小一致的阳性克隆送上海英骏生物技术有限公司测序。
测序完成后,3次测序结果中的2次共同核苷酸合并为基因片段最终序列。结合梨基因组数据库利用GSDS在线对基因结构分析。利用DNAMAN V6和Primer Premier 5.0软件进行序列比对和氨基酸序列的推导,并通过NCBI的BLAST工具与GenBank中的已知序列比较,进行蛋白序列的相似性分析。利用ProtParam软件在线分析蛋白质的分子结构和理化性质;利用MEGA 6.06软件对氨基酸序列进行系统进化树分析;通过ProtScale分析软件在线对其进行亲疏水性分析;通过BioXM 2.6分析软件对Signal peptide进行预测;采用TMPRED在线分析软件预测蛋白质的跨膜结构域,蛋白质的二级结构和三级结构预测分别使用在线分析软件SOPMA和SWISS-MODEL完成。
1.5 石细胞含量的测定
石细胞的分离采用冷冻分离[20]和盐酸处理[6]相结合的方法。取大小基本一致的3个果实(幼果适当多采),去皮,取食用部分,按四分法取样,准确称取100 g,置于-20 ℃低温冰箱冷冻24 h。样品取出室温解冻后,加入200 mL蒸馏水,并用组织捣碎机(1 000~1 500 r · min-1)捣碎3 min。将匀浆转移到1 000 mL的烧杯中,加入700 mL蒸馏水,用玻璃棒搅拌1 min,静置3 min,使石细胞充分沉降,然后倾出上层悬浮液,将沉淀物悬浮于0.5 mol · L-1盐酸中30 min,弃去漂浮物,用蒸馏水洗涤。漂洗5~6次,同时收集前两次漂洗倾出的上层悬浮液,再次漂洗。合并所得的石细胞,用粗滤纸过滤,最后分离得到纯净的石细胞,烘干至恒质量,称量。
1.6 PbCCoAOMT基因实时荧光定量表达分析
根据获得的PbCCoAOMT基因全长序列,设计荧光定量PCR特异性引物F1:5′-AGCAACAAACTCAGGCAGGG-3′和R1:5′-GGAGGGAGTAGCCGGTGTAA-3′,以Pyrus EFα 1 (AY338250)作为内参[21]。实时定量PCR采用SYBR Green Master Mix(TaKaRa)试剂盒,在IQ2(Bio-Rad,USA)实时荧光定量PCR仪上进行。以‘砀山酥梨’不同发育时期的果肉cDNA(稀释至300 ng · μL-1)为模板,对CCoAOMT进行特异性表达分析。扩增条件:95 ℃ 5 min;94 ℃ 10 s,60 ℃ 30 s,72 ℃ 30 s,45个循环;72 ℃ 3 min。重复3次,采用Light Cycler 480荧光定量软件进行采集与分析处理数据。采用2-ΔΔCT法[21]分析基因相对表达量的变化。
2 结果与分析 2.1 ‘砀山酥梨’果实PbCCoAOMT基因全长cDNA克隆
以‘砀山酥梨’幼果果肉中RNA为模板,利用F0/R0引物进行RT-PCR扩增,获得了预期大小的cDNA片段(图 1)。将目的片段进行回收纯化,与pMD19-T载体连接转化大肠杆菌DH5α感受态细胞,经过PCR阳性克隆验证并送上海英骏生物技术有限公司测序,获得PbCCoAOMT基因的全长为1 133 bp,包括1个744 bp(228~971 bp)的开放阅读框,起始密码子为ATG,终止密码子为TAG,G+C含量为50.13%,共编码247个氨基酸,GenBank登录号为KJ577544。
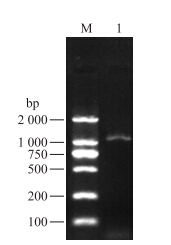 | 图 1 ‘砀山酥梨’果实PbCCoAOMT 基因全长的PCR扩增 Fig. 1 cDNA full-length PCR amplification of PbCCoAOMT from‘Dangshansuli’ M:DNA marker(DL2000);1:PbCCoAOMT |
从梨基因组数据库调取相对应的基因全长发现,克隆得到的PbCCoAOMT基因包括5个外显子和4个内含子。外显子长度依次为33、80、145、132和294 bp。第1个内含子的长度为206 bp,位于第11和12个氨基酸之间;第2个内含子长度为140 bp,插入第38个氨基酸之中;第3个内含子长度为97 bp,插入第133个氨基酸之中;第4个内含子长度为192 bp,位于第177和178个氨基酸之间。GT-AG结构为4个内含子的左右边界。
用在线网站(http://web.expasy.org/protparam/)预测PbCCoAOMT编码蛋白质的分子式为C1251H1977N329O369S9,总原子数为3 935,预测相对分子质量为27.81×103。Leu、Ala和Asp在氨基酸组成中出现频率较高,分别占总氨基酸的11.7%、8.1%和7.3%,而Cys、Trp和His出现的频率较低,仅占0.8%、0.8%和2.0%,理论pI为5.32,其中带正电荷(Arg+Lys)和负电荷(Asp+Glu)残基总数分别为28和35,不稳定系数为36.97,脂肪族系数为99.92,亲水性平均系数为-0.231,半衰期为30 h。
对PbCCoAOMT蛋白的疏水性预测显示,多肽链具有最高分值2.256,疏水性最强;最低分值-2.367,亲水性最强,整体表现亲水性。TMHMM软件预测PbCCoAOMT蛋白不存在跨膜结构域。采用分析软件BioXM 2.6将获得的CCoAOMT氨基酸序列的信号肽序列进行预测,分析得知,其没有信号肽序列,为分泌蛋白的可能性为0.037。
通过SOPMA预测PbCCoAOMT氨基酸序列的二级结构,发现其多肽链中主要结构元件是α螺旋和随机卷曲,分别占42.51%和31.17%,延伸链占17.41%,β转角占8.91%;运用SWISS-MODEL工具对梨CCoAOMT的氨基酸序列进行同源三维建模(图 2),其与数据库所供模板的序列一致性为91.63%,建模范围是从第21个到第247个氨基酸。
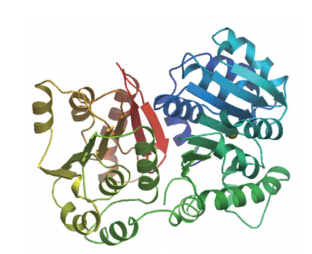 | 图 2 ‘砀山酥梨’PbCCoAOMT蛋白的三维建模 Fig. 2 The three-dimensional modeling of PbCCOMT protein in‘Dangshansuli’ |
将本研究获得的PbCCoAOMT(KJ577544)编码的氨基酸序列与枇杷(AFZ76980.1)、毛白杨(AFZ78548.1)和陆地棉(ACQ59095.1)编码的CCoAOMT氨基酸序列进行多重比对分析,同 源性分别达到98.79%、91.90%和90.40%(图 3)。
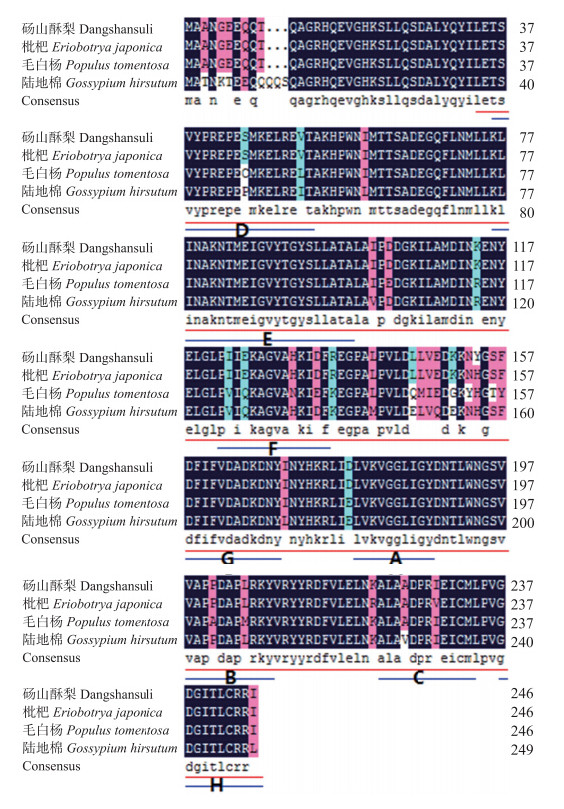 |
图 3 PbCCoAOMT与其他植物同源基因编码氨基酸序列比对 Fig. 3 Comparison of amino acids sequence of PbCCoAOMT and homologous proteins of other plants 蓝线标注的A、B和C代表植物甲基酶所共有的保守序列元件,蓝线标注的D、E、F、G和H代表CCoAOMT基因家族中所特有的标签序列,红线标注的代表CCoAOMT基因家族中保守结构域。 A,B,and C marked with blue line represent conservative sequence elements of plant-methyltransferase;D,E,F,G and H marked with blue line represent specific tag sequence of CCoAOMT gene family;The region maked with red line represent conserved domains of CCoAOMT gene family. |
在Pfam(http://pfam.xfam.org/)上对其进行保守结构域分析,表明咖啡酰-辅酶AO-甲基转移酶属于PfamPF01596,Methyltransf_3家族,且与其他植物的CCoAOMT酶蛋白序列有高度保守性,具有植物甲基酶所共有的A、B和C保守序列元件及D、E、F、G和H 5个保守序列元件,它们为CCoAOMT基因家族中所特有的标签序列。此家族包括3大类甲基化酶,它们分别是CCoAOMT、儿茶酚氧甲基化酶(COMT)、与细菌来源的抗生素合成相关的氧甲基化酶[22]。
利用MEGA 6.06软件构建PbCCoAOMT与已报道的部分植物CCoAOMT蛋白序列之间的进化树,结果(图 4)表明:PbCCoAOMT与枇杷(Eriobotrya japonica)、毛白杨(Populus tomentosa)、陆地棉(Gossypium hirsutum)的亲缘关系很近,而与龙眼(Dimocarpus longan)的亲缘关系最远。
 | 图 4 UPGMA法构建PbCCoAOMT与其他植物CCoAOMT蛋白的系统进化树 Fig. 4 Phylogenetic tree of PbCCoAOMT and other CCoAOMT proteins by UPGMA method |
由图 5可以看出:随着‘砀山酥梨’果实的生长发育石细胞含量呈先上升后下降的趋势。自盛花后21 d开始,石细胞含量急剧增长,至盛花后40 d其维持在一定水平,并在盛花后46 d达到高峰(15.45%),对比本课题组陶书田[6]的研究结果,确认这是木质素代谢中的一个关键时期。盛花后46 d之后,由于果实的迅速膨大,果肉中石细胞含量逐渐下降,至果实成熟时含量极低。
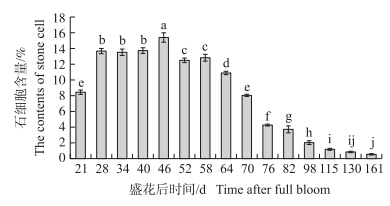 |
图 5 ‘砀山酥梨’果实不同发育时期石细胞含量的变化 Fig. 5 Changes of stone cell contents at different developmental stages of‘Dangshansuli’ 不同小写字母表示差异显著(P<0.05)。 Different small letters indicate significant difference at 0.05 level. The same as follows. |
如图 6所示:在‘砀山酥梨’果实发育过程中,PbCCoAOMT基因相对表达量呈先上升后下降的趋势。幼果期其相对表达量较高,且逐渐升高,并在盛花后40 d达到最大值2.23,之后快速下降,盛花后82 d之后基因的相对表达量一直保持极低水平,至果实接近成熟时,已经检测不到基因的表达。相关性分析表明,‘砀山酥梨’果实不同发育时期PbCCoAOMT的表达量与石细胞含量呈极显著正相关(r=0.734,P<0.01)。
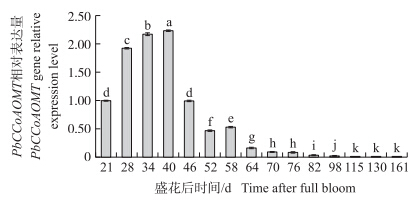 | 图 6 ‘砀山酥梨’果实PbCCoAOMT基因 在不同发育时期的表达 Fig. 6 Expression of PbCCoAOMT gene at different developmental stages of‘Dangshansuli’fruit |
本研究从‘砀山酥梨’果肉中克隆了1个编码CCoAOMT的基因PbCCoAOMT全长,其基因组序列分别由5个外显子和4个内含子组成,外显子与内含子长度有所差异,而内含子左右边界GT-AG结构便于RNA加工过程中内含子被正确切除。倪志勇等[23, 24]对棉花GhCCoAOMT 1和GhCCoAOMT2 基因的研究发现其分别具有5个外显子和4个外显子,Do等[14]对拟南芥AtCCoAOMT 1 基因组序列分析发现其是由4个外显子和3个内含子组成,不同植物CCoAOMT基因结构中内含子剪切位点保持一致,说明CCoAOMT基因在不同的植物中有一定的保守性。对PbCCoAOMT编码的氨基酸序列进行多重比对并构建进化树发现,其具有CCoAOMT类基因家族的共同保守结构域,且A、B、C、E和H 5个序列元件保守性较高,说明其是CCoAOMT基因家族的一个成员,D、F和G序列元件则与所选基因编码氨基酸有所差异,这也许是不同基因编码蛋白的特异性所致,但与枇杷的CCoAOMT基因编码的氨基酸相比,只有在第155个氨基酸位点处发生变异(H→Y)。蛋白系统发生树分析表明其与蔷薇科其他植物亲缘关系较近,且同源性与其他物种较高,说明其在进化过程中较保守。
咖啡酰-辅酶AO-甲基转移酶(CCoAOMT)催化咖啡酰CoA甲基化为阿魏酰CoA,是木质素形成过程中一个重要的酶。关于CCoAOMT基因在木质素生物合成中的功能研究取得了一定进展,例如反义RNA能够选择性的关闭特定基因,而在转基因烟草[11]、杨树[25]和辐射松[9]中,通过反义抑制CCoAOMT基因均可降低木质素含量,S/G比值增加进而改变木质素组成,且不影响植物的正常生长。本试验对PbCCoAOMT基因在‘砀山酥梨’果实发育过程中的表达模式进行了研究,发现其在幼果期有较高的表达量,果实膨大期至成熟表达量较低。此外,有研究发现‘砀山酥梨’果实中石细胞在盛花后一周已开始形成,在盛花后15~67 d即梨果实的细胞分裂期是‘砀山酥梨’果实石细胞合成的旺盛时期,木质素与石细胞含量随着果实发育呈先上升后下降的趋势[3, 4, 6, 20, 26, 27],本试验结果与之相一致,表明梨果实PbCCoAOMT基因的相对表达量与果实中石细胞含量的变化规律有一定的相关性。本试验结果表明:PbCCoAOMT基因表达量变化和石细胞含量变化呈正相关,结合本课题组Wu等[1]之前发现的G木质素和S木质素被证明在梨石细胞中有较高的含量,且有学者推测CCoAOMT对G木质素的积累有促进作用[1, 28, 29, 30, 31],这基本说明CCoAOMT参与梨石细胞木质素的生物合成,也从侧面说明了本研究中克隆得到的PbCCoAOMT基因可能是参与梨果实中木质素代谢途径的基因之一,而这与李晓峰等[32]对‘砀山酥梨’果皮中CCoAOMT在果实不同发育阶段均有表达量的分析有一定不同,可能是因为木质素的合成在果肉与果皮部位差异所造成,具体功能有待进一步验证,梨中其他CCoAOMT基因也有待分离与鉴定。这也为以后深入研究PbCCoAOMT具体功能及通过基因调控技术来改变果实中石细胞含量从而改善梨果实品质提供了一定的理论依据。
| [1] | Wu J,Wang Z W,Shi Z B,et al. The genome of the pear(Pyrus bretschneideri Rehd.)[J]. Genome Res,2013,23(2):396-408 |
| [2] | Tao S T,Khanizadeh S,Zhang H,et al. Anatomy,ultrastructure and lignin distribution of stone cells in two Pyrus species[J]. Plant Science,2009,176(3):413-419 |
| [3] | Cai Y P,Li G Q,Nie J Q,et al. Study of the structure and biosynthetic pathway of lignin in stone cells of pear[J]. Scientia Horticulturae,2010,125(3):374-379 |
| [4] | 刘小阳,高贵珍,李红霞,等. 砀山酥梨果实发育与石细胞形成的动态研究[J]. 淮北煤炭师范学院学报,2006,27(1):49-53 [Liu X Y,Gao G Z,Li H X,et al. The dynamic research Dangshan pear fruit development and stone cell formation[J]. Journal of Huaibei Coal Industry Teachers College,2006,27(1):49-53(in Chinese with English abstract)] |
| [5] | 张振铭,张绍铃,乔勇进,等. 不同果袋对砀山酥梨果实品质的影响[J]. 果树学报,2006,23(4):510-514 [Zhang Z M,Zhang S L,Qiao Y J,et al. Effect of bagging with different types of bags on fruit quality of Dangshansu pear cultivar[J]. Journal of Fruit Science,2006,23(4):510-514(in Chinese with English abstract)] |
| [6] | 陶书田. 梨(Pyrus)果实石细胞的结构成分分析及相关酶基因的克隆[D]. 南京:南京农业大学,2009:16-18 [Tao S T. Characterization of sclereid structure and composition and cloning of sclereid related enzyme genes in pear(Pyrus)fruit[D]. Nanjing:Nanjing Agricultural University,2009:16-18(in Chinese with English abstract)] |
| [7] | Anterola A M,Lewis N G. Trends in lignin modification:a comprehensive analysis of the effects of genetic manipulations/mutations on lignification and vascular integrity[J]. Phytochemistry,2002,61(3):221-294 |
| [8] | 周,王玉锋,娄来清,等. 甜高粱4-香豆酸辅酶A连接酶基因(4CL)的克隆与鉴定及时空表达分析[J]. 南京农业大学学报,2014,37(3):9-19. doi:10.7685/j.issn.1000-2030.2014.03.002 [Zhou Y,Wang Y F,Lou L Q,et al. Cloning,characterization and spatio-temporal expression analysis of 4-coumarate:CoA ligase genes(4CL)in sweet sorghum[J]. Journal of Nanjing Agricultural University,2014,37(3):9-19(in Chinese with English abstract)] |
| [9] | Wagner A,Tobimatsu Y,Phillips L,et al. CCoAOMT suppression modifies lignin composition in Pinus radiata[J]. The Plant Journal,2011,67(1):119-129 |
| [10] | 赵华燕,魏建华,路静,等. 利用反义CCoAOMT基因培育低木质素含量毛白杨的研究[J]. 自然科学进展,2004,14(9):1067-1071 [Zhao H Y,Wei J H,Lu J,et al. Research on fostering Populus tomentosa with low lignin content by antisensenucleic CCoAOMT gene[J]. Progress in Natural Science,2004,14(9):1067-1071(in Chinese)] |
| [11] | 黄春琼,刘国道,郭安平. 反义CCoAOMT基因调控烟草木质素的生物合成[J]. 安徽农业科学,2008,36(19):8026-8027 [Huang C Q,Liu G D,Guo A P. Lignin biosynthesis regulated by antisense CCoAOMT gene in tobacco[J]. Journal of Anhui Agricultural Sciences,2008,36(19):8026-8027(in Chinese with English abstract)] |
| [12] | Ye Z H,Kneusel R E,Matern U,et al. An alternative methylation pathway in lignin biosynthesis in Zinnia[J]. The Plant Cell,1994,6(10):1427-1439 |
| [13] | Martz F,Maury S,Pinçon G,et al. cDNA cloning,substrate specificity and expression study of tobacco caffeoyl-CoA 3-O-methyltransferase,a lignin biosynthetic enzyme[J]. Plant Molecular Biology,1998,36(3):427-437 |
| [14] | Do C T,Pollet B,Thevenin J,et al. Both caffeoyl coenzyme A 3-O-methyltransferase 1 and caffeic acid O-methyltransferase 1 are involved in redundant functions for lignin,flavonoids and sinapoyl malate biosynthesis in Arabidopsis[J]. Planta,2007,226(5):1117-1129 |
| [15] | 陈建荣,郭清泉,张学文. 苎麻CCoAOMT基因全长cDNA克隆与序列分析[J]. 中国农业科学,2006,39(5):1058-1063 [Chen J R,Guo Q Q,Zhang X W. Cloning full length of ramie caffeoyl-CoA 3-O-methyltransferase cDNA and sequence analysis[J]. Scientia Agricultura Sinica,2006,39(5):1058-1063(in Chinese with English abstract)] |
| [16] | 赵华燕,沈庆喜,吕世友,等. 水稻咖啡酰辅酶A-O-甲基转移酶基因(CCoAOMT)表达特性分析[J]. 科学通报,2004,49(14):1390-1394 [Zhao H Y,Shen Q X,Lü S Y,et al. Expression analysis of caffeoyl-CoA 3-O-methyltransferase gene of Oryza sativa L.ssp.japonica[J]. Chinese Science Bulletin,2004,49(14):1390-1394(in Chinese)] |
| [17] | 陈虎,何新华,罗聪,等. 龙眼咖啡酰辅A-O-甲基转移酶(DLCCoAOMT)基因的克隆和表达分析[J]. 中国农业科学,2012,45(1):118-126 [Chen H,He X H,Luo C,et al. Molecular cloning of longan caffeoyl-CoA O-methyltransferase(DLCCoAOMT)and its expression analysis[J]. Scientia Agricultura Sinica,2012,45(1):118-126(in Chinese with English abstract)] |
| [18] | 陶书田,张绍铃,乔勇进,等. 梨果实发育过程中石细胞团及几种相关酶活性变化的研究[J]. 果树学报,2004,21(6):516-520 [Tao S T,Zhang S L,Qiao Y J,et al. Study on sclereids and activities of several related enzymes during the development of pear fruit[J]. Journal of Fruit Science,2004,21(6):516-520(in Chinese with English abstract)] |
| [19] | Gasic K,Hernandez A,Korban S S. RNA extraction from different apple tissues rich in polyphenols and polysaccharides for cDNA library construction[J]. Plant Molecular Biology Reporter,2004,22(4):437-438 |
| [20] | 王斌,张楠,闫冲冲,等. 套袋对砀山酥梨果实石细胞发育及木质素代谢的影响[J]. 园艺学报,2013,40(3):531-539 [Wang B,Zhang N,Yan C C,et al. Bagging for the development of stone cell and metabolism of lignin in Pyrus bretschneideri‘Dangshansuli’[J]. Acta Horticulturase Sinica,2013,40(3):531-539(in Chinese with English abstract)] |
| [21] | Wu J,Zhao G,Yang Y N,et al. Identification of differentially expressed genes related to coloration in red/green mutant pear(Pyrus communis L.)[J]. Tree Genetics and Genomes,2013,9(1):75-83 |
| [22] | 侯晓玮. 钩端螺旋体腺苷甲硫氨酸依赖型氧甲基化酶和人线粒体外膜蛋白mitoNEET的结构生物学研究[D]. 合肥:中国科学技术大学,2008:7-8 [Hou X W. Struetural biology researeh on SAM-dependent O-methyltransferase from leptospira interrogans and human mitoNEET[D]. Hefei:University of Science and Technology of China,2008:7-8(in Chinese with English abstract)] |
| [23] | 倪志勇,吕萌,马文静,等. 棉花咖啡酰辅酶A-O-甲基转移酶基因的克隆及表达[J]. 西北植物学报,2009,29(10):1946-1953 [Ni Z Y,Lü M,Ma W J,et al. Cloning and expression analysis of CCoAOMT gene from Gossypium hirsutum L.[J]. Acta Botanica Boreali-Occidentalia Sinica,2009,29(10):1946-1953(in Chinese with English abstract)] |
| [24] | 倪志勇,吕萌,范玲. 棉花咖啡酸-O-甲基转移酶基因的克隆及特征分析[J]. 中国农业科学,2010,43(6):1117-1126 [Ni Z Y,Lü M,Fan L. Cloning and characteriz ation of CCoAOMT gene from Gossypium hirsutum L.[J]. Scientia Agricultura Sinica,2010,43(6):1117-1126(in Chinese with English abstract)] |
| [25] | Zhong R Q,Morrison W H,Himmelsbach D S,et al. Essential role of caffeoyl coenzyme A-O-methyltransferase in lignin biosynthesis in woody poplar plants[J]. Plant Physiology,2000,124(2):563-578 |
| [26] | 阿拉木萨,李宝江. 梨果实石细胞团的发育、分布及其对果实品质的影响[J]. 北方果树,1999(4):4-6 [Ala M S,Li B J. Development,distribution of grit in pear fruit and its effects on ddible qualities[J]. Northern Fruits,1999(4):4-6(in Chinese with English abstract)] |
| [27] | 聂敬全,蔡永萍,张士鸿,等. 砀山酥梨果实石细胞与薄壁细胞发育关系的解剖学研究[J]. 园艺学报,2009,36(8):1209-1214 [Nie J Q,Cai Y P,Zhang S H,et al. The anatomic study on relationship of stone cells and parenchyma cellsduring fruit development of Pyrus bretschneideri[J]. Acta Horticulturase Sinica,2009,36(8):1209-1214(in Chinese with English abstract)] |
| [28] | Chen C Y,Meyermans H,Burggraeve B,et al. Cell-specific and conditional expression of caffeoyl-coenzyme A-3-O-methyltransferase in poplar[J]. Plant Physiology,2000,123(3):853-868 |
| [29] | Huang J L,Gu M,Lai Z B,et al. Functional analysis of the Arabidopsis PAL gene family in plant growth,development,and response to environmental stress[J]. Plant Physiology,2010,153(4):1526-1538 |
| [30] | Chen S Y,Cai Y Y,Zhang L X,et al. Transcriptome analysis reveals common and distinct mechanisms for sheepgrass(Leymus chinensis)responses to defoliation compared to mechanical wounding[J]. PLoS ONE,2014,9(2):e89495 |
| [31] | Sengupta S,Lahiri Majumder A. Physiological and genomic basis of mechanical-functional trade-off in plant vasculature[J]. Frontiers in Plant Science,2014,5:224 |
| [32] | 李晓峰,李雪,贾兵,等. '砀山酥梨’褐皮芽变木质素含量及相关酶活性与CCoAOMT表达量分析[J]. 园艺学报,2012,39(5):828-836 [Li X F,Li X,Jia B,et al. Analysis of enzyme activity and lignin content and expression of CCoAOMT gene in the pericarp of'Dangshansuli’and its russet mutant[J]. Acta Horticulturase Sinica,2012,39(5):828-836(in Chinese with English abstract)] |




