文章信息
- 丁令智, 满秀玲, 肖瑞晗, 蔡体久.
- Ding Lingzhi, Man Xiuling, Xiao Ruihan, Cai Tijiu.
- 寒温带森林根际土壤微生物量碳氮含量生长季内动态变化
- Dynamics of Soil Microbial Biomass Carbon and Nitrogen in the Soil of Rhizosphere during Growing Season in the Cold Temperate Forests
- 林业科学, 2019, 55(7): 178-186.
- Scientia Silvae Sinicae, 2019, 55(7): 178-186.
- DOI: 10.11707/j.1001-7488.20190720
-
文章历史
- 收稿日期:2018-09-30
- 修回日期:2019-03-20
-
作者相关文章
根际是植物细根周围的土壤区域(Fraser et al., 2017),该部分土壤受植物根系生长调控从周围环境聚集养分,被德国微生物生态学家Lorenz Hiltner称为根际土壤(rhizosphere soil)(Balakrishnan et al., 2017;Hartmann et al., 2008)。根际土壤中根际分泌物是土壤有机碳的重要来源之一(Angst et al., 2016),且根际土壤中还有大量的高活性酶,是最活跃的微生物栖息地(Gartner et al., 2012),植物根系通过根际改变植物生长过程中的土壤理化性质以影响土壤微生物的活动和群落结构组成(Bird et al., 2011),该过程能促进氮素养分的循环(Li et al., 2016)。在草原、耕地和林地中,虽然土壤微生物量碳(microbial biomass carbon,MBC)占有机碳的比例低于3.0%,土壤微生物量氮(microbial biomass nitrogen,MBN)占全氮的比例低于5.9%(杨成德等,2007),但土壤MBC和MBN对于土壤养分循环的作用不可忽视(Mukhopadhyay et al., 2016),可作为评价土壤质量和肥力的重要指标(Carter et al., 1999)。研究表明,根际不仅与植物营养、健康和质量关系密切,而且对于微生物驱动的碳固存、生态系统功能和陆地生态系统的养分循环也至关重要(Berg et al., 2010),因此,根际土壤MBC和MBN的研究对于认识植物养分利用方式和土壤质量评价很有必要。
大兴安岭地区拥有我国唯一的寒温带森林,是我国最大的原始林区(段北星等,2018),分布有以兴安落叶松(Larix gmelinii)林为顶级群落的明亮针叶林,还有樟子松(Pinus sylvestris var. mongolica)林、白桦(Betula platyphylla)林、山杨(Populus davidiana)林等森林类型。近年来,国外土壤微生物量研究大多集中于不同土地利用类型、森林类型和演替阶段之间的土壤微生物量变化以及其影响因子分析等。有研究表明,干旱条件下森林土壤微生物量明显高于果园和弃耕地(Moreno et al., 2019);印度喜马拉雅山中部3种森林类型中,混交橡树林的土壤MBC、MBN、微生物活性和底物利用效率较高(Bargali et al., 2018);除了林型的影响外,土壤MBC、MBN会随着演替阶段的推进而增加(Jangid et al., 2011),氮沉降、温度和间伐等也会对土壤微生物量产生影响(Schindlbacher et al., 2011;Simpson et al., 2019;Kim et al., 2018)。我国对森林土壤MBC、MBN的研究主要是比较不同森林类型之间的差异(王风芹等,2015;林尤伟等,2016;何云等,2013;漆良华等,2009),而从林木根际角度分析森林土壤微生物量差异在土壤碳氮养分循环中的作用鲜见报道。鉴于此,本研究以大兴安岭北部针阔混交林为研究对象,探讨立地条件较一致条件下不同树种根际和非根际土壤MBC、MBN含量差异及其生长季动态变化,分析土壤MBC、MBN与土壤养分之间的相关性,以期更加深入揭示寒温带森林不同树种对土壤养分循环的机制和策略,同时也为大兴安岭北部森林的合理经营提供参考。
1 研究区概况与研究方法 1.1 研究区概况试验地设在黑龙江省漠河森林生态系统国家定位观测研究站,该站地处大兴安岭北部漠河县北极村内(122°06′—122°27′E,53°17′—53°30′N),属寒温带大陆性季风气候,年均气温-5 ℃,年降水量约430 mm,降水多集中在夏季,一年间冰雪覆盖时长最高可达200天。森林类型主要是以兴安落叶松为建群种的寒温带明亮针叶林,伴生树种有樟子松、白桦和山杨等。该地区土层薄,生长季短,地带性土壤为棕色针叶林土,也分布有沼泽土、草甸土,是我国多年冻土的主要分布区。
1.2 土样采集为排除立地条件、海拔、草本和灌木等外部环境条件对试验结果的影响,依据大兴安岭北部森林植被类型特点,选择具有代表性的针阔叶混交林(树种组成为5樟2落2白1山),设置调查样地3块,每块样地面积20 m×30 m。2017年5月对样地进行每木检尺,包括树高、胸径、冠幅等,树种生长状况见表 1。
|
|
根据每木检尺结果,计算每一树种的平均树高和平均胸径,按照平均树高和平均胸径在样地内选择标准木,每次调查时每一树种选择3株标准木,采用抖落法(Phillips et al., 2008)采集根际土壤样品。先用铁锹除去表面的枯枝落叶,之后沿树基部挖开土层找到主根,沿着主根挖去上层覆土找到细根和须根,除去不含根系的大块土壤,再抖落紧贴根系的土壤,装入取样袋中,即为根际土壤。同时,在样地内按照“S”形选择5个采样点,样点选择时尽量远离树木,钻取0~20 cm土壤层,轻轻散开,拣出根系及所带土壤,即为非根际土壤(刘顺等,2017)。土壤样品采集时间为2017年5—10月,每月采样1次,间隔30天左右。
1.3 样品测定1) 土壤MBC、MBN测定方法 土壤MBC、MBN采用氯仿熏蒸浸提法(董敏慧等,2017)测定。用新鲜土样进行熏蒸和未熏蒸处理,土壤和溶液比例为1 :2,使用0.5 mol ·L-1的K2SO4溶液浸提,浸提液用德国产multiN/C2100分析仪进行测定。
2) 土壤理化性质测定方法 土壤有机碳的测定首先使用锡纸包裹0.25 g过0.149 mm筛的风干土样,然后采用德国产multiN/C2100分析仪进行分析测定(李红运等,2016);全氮采用5 mL浓硫酸和混合催化剂(硫酸钾-硫酸铜-硒按照100 :10 :1的质量比配置,均匀混合后研磨,过80号筛)消解定溶至100 mL后,用AA3型连续流动分析仪测定(曹伟等,2016);铵态氮、硝态氮采用1 mol ·L-1 KCL浸提,用AA3型连续流动分析仪测定(Chen et al., 2005);pH使用蒸馏水以液土比为2.5 :1浸提,用PHS-3E型pH计测定;含水率采用105 ℃烘干法测定(陈立新,2005)。
1.4 数据统计分析利用“富集率”(enrichment rate,E)表示根际土壤的富集程度,E值大小反映根际效应强弱。E=[(根际土壤含量-非根际土壤含量)/非根际土壤含量]×100%(陈海滨等,2016)。
采用SPSS 22.0软件进行根际和非根际土壤之间差异性分析(t-检验),运用Pearson相关分析探讨土壤MBC、MBN和土壤养分之间的关系。
2 结果与分析 2.1 根际土壤MBC的生长季动态由图 1可知,4个树种根际土壤MBC含量变化因树种自身特性差异而有所不同。樟子松、兴安落叶松根际土壤MBC含量均呈波动式上升,最小值在9月,分别为181.13和114.14 mg ·kg-1,10月均达最大值,分别为367.18和451.05 mg ·kg-1,分别增加1.03和2.95倍。白桦根际土壤MBC含量5月至9月降至最小值175.25 mg ·kg-1,降幅达48.05%,10月升至194.62 mg ·kg-1。山杨根际土壤MBC含量则呈波动式下降,8月达最大值288.65 mg ·kg-1,10月降低至最小值147.34 mg ·kg-1。10月,樟子松、兴安落叶松根际土壤MBC含量均极显著高于白桦、山杨(P<0.01)。4个树种根际土壤MBC含量均极显著高于非根际(P<0.01),且樟子松和山杨根际土壤MBC差异显著(P<0.05)。相对于非根际土壤,樟子松、兴安落叶松、白桦和山杨根际土壤MBC含量分别高出87.99%、73.14%、78.22%和56.96%,根际土壤对MBC的富集效应显著(表 2)。
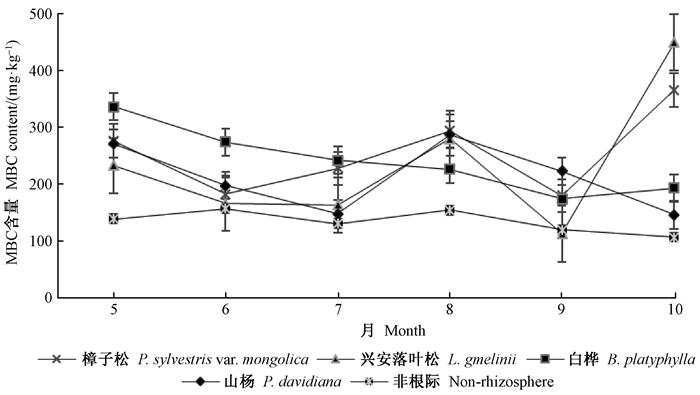
|
图 1 根际与非根际土壤MBC含量动态变化 Fig. 1 Dynamic changes of microbial biomass carbon contents in rhizosphere and non-rhizosphere soils |
|
|
由图 2可知,生长季内4个树种根际土壤MBN含量随月份变化有一定相似性,5—7月下降,8月上升至峰值后下降。樟子松、山杨和白桦根际土壤MBN含量在生长季内呈波动式下降,樟子松、山杨在8月最高,白桦5月最高,生长季变幅分别为76.31~155.49、57.55~185.00和40.38~178.44 mg ·kg-1,最大值分别为最小值的2.04、3.21和4.42倍。兴安落叶松根际土壤MBN含量波动式上升,5—7月降至最小值51.66 mg ·kg-1,降幅32.59%,8月增大1.64倍达最大值136.19 mg ·kg-1,10月降至121.21 mg ·kg-1。10月,樟子松、兴安落叶松根际土壤MBN含量均极显著(P<0.01)高于白桦、山杨,4个树种根际土壤MBN含量均极显著(P<0.01)高于非根际。相对于非根际土壤,樟子松、兴安落叶松、白桦和山杨根际土壤MBN分别高出76.42%、51.40%、77.63%和81.50%(表 2)。
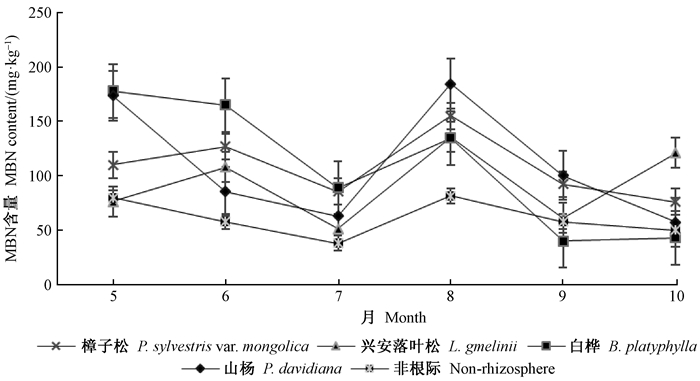
|
图 2 根际与非根际土壤微生物量氮含量的动态变化 Fig. 2 Dynamic changes of microbial biomass nitrogen content in rhizosphere and non-rhizosphere soils |
由表 3可知,4个树种根际和非根际土壤的微生物量碳氮比均有明显动态变化,其中,樟子松、兴安落叶松和白桦的季节性差异远大于山杨和非根际。樟子松、兴安落叶松、白桦、山杨根际和非根际土壤微生物量碳氮比生长季变幅分别为1.42~5.24、1.57~3.79、1.67~4.55、1.55~2.59和1.79~3.53,其生长季均值分别为2.64、2.63、2.81、2.11和2.36。樟子松、兴安落叶松和白桦均值比非根际分别高出11.86%、11.44%和19.07%,而山杨则低于非根际土壤。
|
|
樟子松、兴安落叶松、白桦和山杨根际土壤有机碳含量(26.78~31.76 g ·kg-1)均显著高于非根际土壤,分别比非根际土壤高出58.64%、54.61%、82.95%和54.26%(表 4)。微生物量碳对4个树种根际土壤有机碳库的贡献率在0.83~0.95%之间,均高于非根际土壤(表 5)。
|
|
|
|
樟子松、兴安落叶松、白桦和山杨根际土壤全氮含量(2.41~2.93 g ·kg-1)均显著高于非根际土壤,分别比非根际土壤高出78.52%、80.74%、117.04%和80.00%(表 4)。微生物量氮对4个树种根际土壤全氮的贡献率在3.63~5.08%之间,均低于非根际土壤(表 5)。
2.5 根际和非根际土壤微生物量与土壤理化性质之间的关系由表 6可知,在根际土壤中,MBC与MBN呈极显著正相关(P<0.01),与有机碳相关性显著(P<0.05);MBN与有机碳、含水率呈极显著正相关(P<0.01),与全氮、pH相关性显著(P<0.05)。在非根际土壤中,MBC与MBN、pH达极显著相关水平(P<0.01),与有机碳显著相关(P<0.05);MBN与含水率极显著相关(P<0.01),与pH相关性显著(P<0.05)。这表明,根际、非根际土壤养分与MBC、MBN之间具有复杂的关系。
|
|
土壤MBC、MBN含量生长季动态变化是一个复杂的过程,在森林生态系统中,其受到土层(王宁等,2016)、土壤肥力(Phillips et al., 2008)、土壤碳氮(Yokobe et al., 2018)、土壤温度(Frey et al., 2008)等影响。本研究中,不同树种根际土壤MBC、MBN含量均表现为生长季初期较高,Edwards等(2006)认为,在高纬度寒冷地区,冬季微生物持续活动会积累较高的土壤微生物量,温带的研究结果也表明土壤微生物量在冬季积雪期达到峰值,其随着春季积雪的融化而逐渐降低(Freppaz et al., 2014)。本研究7月根际土壤MBC、MBN含量均出现不同程度下降,可能是因为此时温度、水分条件较为适宜,树木进入迅速生长时期,需要从土壤中吸收大量养分,形成与根际土壤微生物的种间竞争关系,树木生长在此时占据优势,而土壤微生物处于劣势,其生长受到一定抑制。进入8月,土壤温度稳定在较高水平,水分含量充足,树木根系活动增强,根际分泌物增多,因此土壤有机质矿化程度加深,为土壤微生物活动积累了丰富的底物,使其活动增强,微生物量出现峰值。进入9月,根际土壤MBC、MBN含量均出现一定下降,此时研究区进入秋季,温度大幅度降低,土壤微生物活性受到一定抑制,土壤有机质矿化能力减弱,且经过植物生长旺季土壤微生物底物消耗较大,可利用的养分含量减少,因此土壤MBC、MBN含量出现降低。10月,樟子松、兴安落叶松根际土壤MBC、MBN含量出现一定增长,而白桦、山杨则下降,2个针叶树种的土壤微生物量均极显著(P<0.01)高于阔叶树种,说明在寒温带生长季末期,针叶树种的根际效应要强于阔叶树种,这可能是受树种特性和气候条件的影响。
3.2 根际土壤微生物量碳氮比和微生物量贡献率土壤微生物量碳氮比能够反映土壤供应氮素的能力,同时也能在一定程度上指示土壤中微生物的种类,比值越高,表明真菌占微生物比值含量越大(Qiu et al., 2010),这是因为真菌的生物量具有比细菌更高的碳氮比(Harris et al., 2007),其比值在4~15和3~5之间时,分别为真菌和细菌占优势(Paul et al., 1996)。本研究中,根际土壤微生物量碳氮比在1.42~5.24之间,非根际土壤在1.79~3.53之间,表明在研究区土壤微生物以细菌占据优势,且根际土壤的真菌比例高于非根际土壤。白桦根际土壤微生物量碳氮比均值在4个树种中最大,表明白桦根际土壤中真菌菌丝较其他树种更为丰富。樟子松、兴安落叶松和白桦根际土壤微生物量碳氮比的月均值均高于非根际土壤,仅山杨低于非根际土壤,这可能是因为樟子松、兴安落叶松和白桦根际能分泌某些物质促进土壤中真菌繁殖,而山杨根际土壤则相对利于细菌生长。
土壤MBC和MBN分别占有机碳和全氮的百分比即为土壤微生物量对土壤营养库的贡献率,其值越高,表明有越多养分固定在土壤微生物中,可作为潜在的营养源(杨成德等,2007)。本研究中,根际土壤MBC、MBN占有机碳、全氮的比例分别在0.83%~0.95%和3.63%~5.08%之间,非根际为0.80%和5.43%,低于热带森林土壤MBC对土壤有机碳的贡献率(1.5%~5.3%)(Coleman et al., 1989),这表明寒温带森林土壤微生物量固碳能力弱于热带森林土壤,可能是受到气候因子的影响,而土壤MBN对总氮的贡献率与农田土壤(2%~6%)和酸性有机土壤(2.8%~9.8%)一致(Bijayalaxmi et al., 2006)。根际土壤MBC贡献率高于非根际土壤,而MBN却低于非根际土壤,这表明根际土壤较非根际土壤对氮素的吸收率更高。2个针叶树种根际土壤MBC对土壤贡献率均高于阔叶树种,MBN对土壤贡献率也高于白桦,总体表现为针叶树种根际土壤微生物量对土壤营养库的贡献率强于阔叶树种。而在亚热带森林中,阔叶林微生物量对土壤营养库的贡献率高于针叶林(李胜蓝等,2014),这可能是由于温度、湿度、水分和气候条件等的差异导致针阔叶树种的土壤微生物活性不同,进而引起土壤微生物量贡献率的差异。
3.3 根际土壤微生物量和土壤理化性质的关系土壤微生物量受许多因素影响,包括气候、植被、土壤养分、土壤温度和水分(Cui et al., 2018;许淼平等,2018;王宝荣等,2018;Diazravina et al., 1995;Stevenson et al., 2014)。本研究中,无论是根际还是非根际,土壤MBC均与MBN极显著相关,此外,在根际土壤中,MBC还与有机碳极显著相关,MBN与有机碳、全氮、pH和含水率显著或极显著相关;在非根际土壤中,MBC与有机碳、pH显著或极显著相关,MBN与pH、含水率显著或极显著相关,表明MBC、MBN能在一定程度上指示土壤肥力的大小。土壤有机碳主要来源于土壤微生物参与下的地表凋落物和细根的分解(庞圣江等,2018),因此无论是根际还是非根际土壤,MBC均与有机碳显著相关。温带牧场土壤pH变化会对土壤中微生物群落产生影响(Stevenson et al., 2014),在本研究中,根际、非根际土壤MBC、MBN均与pH相关性显著。在非根际土壤中,MBN还与含水率有关;而在根际,除pH和含水率外,还与有机碳、全氮有关,这可能是因为在根际土壤中植物根系分泌物浓度较高,微生物生长所需的底物与其根际分泌物较为密切(Vives-Peris et al., 2018)。
4 结论樟子松兴安落叶松、白桦和山杨4个树种根际土壤MBC、MBN含量均显著高于非根际土壤,根际效应显著,且表现出明显的生长季差异;在生长季末期,针叶树种的根际效应要比阔叶树更为明显;寒温带森林针叶树种根际土壤微生物量对土壤结构和功能的影响高于阔叶树种;根际土壤中,MBC与有机碳关系较为密切,MBN与有机碳、全氮、pH和含水率的关系较为密切。
曹伟, 李露, 赵鹏志, 等. 2016. 坡地黑土团聚体氮库及其分布. 东北林业大学学报, 44(5): 63-66. (Cao W, Li L, Zhao P Z, et al. 2016. Organic nitrogen pool and its distribution of aggregates in sloping black soils. Journal of Northeast Forestry University, 44(5): 63-66. DOI:10.3969/j.issn.1000-5382.2016.05.014 [in Chinese]) |
陈海滨, 马秀丽, 陈志彪, 等. 2016. 南方稀土矿区水土保持植物根际土壤碳氮及pH特征. 土壤学报, 53(5): 1334-1341. (Chen H B, Ma X L, Chen Z B, et al. 2016. Carbon, nitrogen and pH in rhizosphere of soil-water conserving plants in rare earth mining area in south China. Acta Pedologica Sinica, 53(5): 1334-1341. [in Chinese]) |
陈立新. 2005. 土壤实验实习教程. 哈尔滨: 东北林业大学出版社. (Chen L X. 2005. Soil experiment practice course. Harbin: Northeast Forestry University Press. [in Chinese]) |
董敏慧, 张良成, 文丽, 等. 2017. 松树-樟树混交林、纯林土壤微生物量碳、氮及多样性特征研究. 中南林业科技大学学报, 37(11): 146-153. (Dong M H, Zhang L C, Wen L, et al. 2017. Soil microbial biomass C, N and diversity characteristics in pure and mixed forest of Pinus and Cinnamomun. Journal of Central South University of Forestry & Technology, 37(11): 146-153. [in Chinese]) |
段北星, 满秀玲, 宋浩, 等. 2018. 大兴安岭北部不同类型兴安落叶松林土壤呼吸及其组分特征. 北京林业大学学报, 40(2): 40-50. (Duan B X, Man X L, Song H, et al. 2018. Soil respiration and its component characteristics under different types of Larix gmelinii forests in the north of Daxing'an Mountains of northeastern China. Journal of Beijing Forestry University, 40(2): 40-50. [in Chinese]) |
何云, 周义贵, 李贤伟, 等. 2013. 台湾桤木林草复合模式土壤微生物量碳季节动态. 林业科学, 49(7): 26-33. (He Y, Zhou Y G, Li X W, et al. 2013. Seasonal dynamics of soil microbial biomass carbon in Alnus formosana forest-grass compound models. Scientia Silvae Sinicae, 49(7): 26-33. [in Chinese]) |
李红运, 辛颖, 赵雨森. 2016. 火烧迹地不同恢复方式土壤有机碳分布特征. 应用生态学报, 27(9): 2747-2753. (Li H Y, Xin Y, Zhao Y S. 2016. Distribution characteristics of soil organic carbon of burned area under different restorations. Chinese Journal of Applied Ecology, 27(9): 2747-2753. [in Chinese]) |
李胜蓝, 方晰, 项文化, 等. 2014. 湘中丘陵区4种森林类型土壤微生物生物量碳氮含量. 林业科学, 50(5): 8-16. (Li S L, Fang X, Xiang W H, et al. 2014. Soil microbial biomass carbon and nitrogen concentrations in four subtropical forests in hilly region of central Hunan Province, China. Scientia Silvae Sinicae, 50(5): 8-16. [in Chinese]) |
林尤伟, 金光泽. 2016. 冻融期去根处理对小兴安岭6种林型土壤微生物量的影响. 生态学报, 36(19): 6159-6169. (Lin Y W, Jin G Z. 2016. Effects of root resectioning on soil microbial biomass in six forest types in the Xiaoxing'an Mountains during freezing-thawing cycles. Acta Ecologica Sinica, 36(19): 6159-6169. [in Chinese]) |
刘顺, 盛可银, 刘喜帅, 等. 2017. 陈山红心杉根际土壤有机碳、氮含量及根际效应. 生态学杂志, 36(7): 1957-1964. (Liu S, Sheng K Y, Liu X S, et al. 2017. Contents of soil organic carbon and nitrogen forms in rhizosphere soil of Cunninghamia lanceolata and the rhizopshere effect. Chinese Journal of Ecology, 36(7): 1957-1964. [in Chinese]) |
庞圣江, 杨保国, 刘士玲, 等. 2018. 桂西北喀斯特山区4种森林表土土壤有机碳含量及其养分分布特征. 中南林业科技大学学报, 38(4): 60-64, 71. (Pang S J, Yang B G, Liu S L, et al. 2018. The distribution of organic carbon and soil nutrients under four forest types in karst mountain areas of northwest Guangxi, China. Journal of Central South University of Forestry & Technology, 38(4): 60-64, 71. [in Chinese]) |
漆良华, 张旭东, 周金星, 等. 2009. 湘西北小流域不同植被恢复区土壤微生物数量、生物量碳氮及其分形特征. 林业科学, 45(8): 14-20. (Qi L H, Zhang X D, Zhou J X, et al. 2009. Soil microbe quantites, microbial carbon and nitrogen and fractal characteristics under different vegetation restoration patterns in watershed, northwest Hunan. Scientia Silvae Sinicae, 45(8): 14-20. DOI:10.3321/j.issn:1001-7488.2009.08.003 [in Chinese]) |
王宝荣, 杨佳佳, 安韶山, 等. 2018. 黄土丘陵区植被与地形特征对土壤和土壤微生物生物量生态化学计量特征的影响. 应用生态学报, 29(1): 247-259. (Wang B R, Yang J J, An S S, et al. 2018. Effects of vegetation and topography features on ecological stoichiometry of soil and soil microbial biomass in the hilly-gully region of the Loess Plateau, China. Chinese Journal of Applied Ecology, 29(1): 247-259. [in Chinese]) |
王风芹, 田丽青, 宋安东, 等. 2015. 华北刺槐林与自然恢复植被土壤微生物量碳、氮含量四季动态. 林业科学, 51(3): 16-24. (Wang F Q, Tian L Q, Song A D, et al. 2015. Seasonal dynamics of microbial biomass carbon and nitrogen in soil of Robinia pseudoacacia forests and near-naturally restored vegetation in northern China. Scientia Silvae Sinicae, 51(3): 16-24. [in Chinese]) |
王宁, 杨雪, 李世兰, 等. 2016. 不同海拔红松混交林土壤微生物量碳、氮的生长季动态. 林业科学, 52(1): 150-158. (Wang N, Yang X, Li S L, et al. 2016. Seasonal dynamics of soil microbial biomass carbon-nitrogen in the Korean pine mixed forests along elevation gradient. Scientia Silvae Sinicae, 52(1): 150-158. [in Chinese]) |
许淼平, 任成杰, 张伟, 等. 2018. 土壤微生物生物量碳氮磷与土壤酶化学计量对气候变化的响应机制. 应用生态学报, 29(7): 2445-2454. (Xu M P, Ren C J, Zhang W, et al. 2018. Responses mechanism of C:N:P stoichiometry of soil microbial biomass and soil enzymes to climate change. Chinese Journal of Applied Ecology, 29(7): 2445-2454. [in Chinese]) |
杨成德, 龙瑞军, 陈秀蓉, 等. 2007. 东祁连山高寒草甸土壤微生物量及其与土壤物理因子相关性特征. 草业学报, 16(4): 62-68. (Yang C D, Long R J, Chen X R, et al. 2007. Study on microbial biomass and its correlation with the soil physical properties under the alpine grassland of the east of Qilian Mountains. Acta Prataculturae Sinica, 16(4): 62-68. DOI:10.3321/j.issn:1004-5759.2007.04.010 [in Chinese]) |
Angst G, Kögel-Knabner I, Kirfel K, et al. 2016. Spatial distribution and chemical composition of soil organic matter fractions in rhizosphere and non-rhizosphere soil under European beech(Fagus sylvatica L.). Geoderma, 264(part A): 179-187. |
Balakrishnan B, Sahu B K, Lourduraj A V, et al. 2017. Assessment of heavy metal concentrations and associated resistant bacterial communities in bulk and rhizosphere soil of Avicennia marina, of Pichavaram mangrove, India. Environmental Earth Sciences, 76: 58. DOI:10.1007/s12665-016-6378-7 |
Bargali K, Manral V, Padalia K, et al. 2018. Effect of vegetation type and season on microbial biomass carbon in Central Himalayan forest soils, India. Catena, 171: 125-135. DOI:10.1016/j.catena.2018.07.001 |
Berg G, Smalla K. 2010. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. Fems Microbiology Ecology, 68(1): 1-13. |
Bijayalaxmi D N, Yadava P S. 2006. Seasonal dynamics in soil microbial biomass C, N and P in a mixed-oak forest ecosystem of Manipur, North-east India. Applied Soil Ecology, 31(3): 220-227. DOI:10.1016/j.apsoil.2005.05.005 |
Bird J A, Herman D J, Firestone M K. 2011. Rhizosphere priming of soil organic matter by bacterial groups in a grassland soil. Soil Biology & Biochemistry, 43(4): 718-725. |
Carter M R, Gregorich E G, Angers D A, et al. 1999. Interpretation of microbial biomass measurements for soil quality assessment in humid temperate regions. Canadian Journal of Soil Science, 79(4): 507-520. DOI:10.4141/S99-012 |
Chen C R, Xu Z H, Zhang S L, et al. 2005. Soluble organic nitrogen pools in forest soils of subtropical Australia. Plant and Soil, 277(1/2): 285-297. |
Coleman D C, Oades J M, Uehara G. 1989. Dynamics of soil organic matter in tropical ecosystems. Soil Science, 151(2): 184. |
Cui Y, Fang L, Guo X, et al. 2018. Ecoenzymatic stoichiometry and microbial nutrient limitation in rhizosphere soil in the arid area of the northern Loess Plateau, China. Soil Biology & Biochemistry, 116: 11-21. |
Diazravina M, Acea M J, Carballas T. 1995. Seasonal changes in microbial biomass and nutrient flush in forest soils.. Biology and Fertility of Soils, 19(2/3): 220-226. |
Edwards K A, Mcculloch J, Kershaw G P, et al. 2006. Soil microbial and nutrient dynamics in a wet Arctic sedge meadow in late winter and early spring. Soil Biology & Biochemistry, 38(9): 2843-2851. |
Fraser T D, Lynch D H, Gaiero J, et al. 2017. Quantification of bacterial non-specific acid(phoC), and alkaline(phoD)phosphatase genes in bulk and rhizosphere soil from organically managed soybean fields. Applied Soil Ecology, 111: 48-56. DOI:10.1016/j.apsoil.2016.11.013 |
Freppaz M, Said-Pullicino D, Filippa G, et al. 2014. Winter-spring transition induces changes in nutrients and microbial biomass in mid-alpine forest soils. Soil Biology & Biochemistry, 78: 54-57. |
Frey S D, Drijber R, Smith H, et al. 2008. Microbial biomass, functional capacity, and community structure after 12 years of soil warming. Soil Biology and Biochemistry, 40(11): 2904-2907. DOI:10.1016/j.soilbio.2008.07.020 |
Gartner T B, Treseder K K, Malcolm G M, et al. 2012. Extracellular enzyme activity in the mycorrhizospheres of a boreal fire chrono sequence. Pedobiologia-International Journal of Soil Biology, 55(2): 121-127. |
Hartmann A, Rothballer M, Schmid M. 2008. Lorenz Hiltner, a pioneer in rhizosphere microbial ecology and soil bacteriology research. Plant & Soil, 312(1/2): 7-14. |
Harris D, Voroney RP, Paul EA. 2007. Measurement of microbial biomass N:C by chloroform fumigation-incubation. Canadian Journal of Soil Science, 77(4): 507-514. |
Jangid K, Williams M A, Franzluebbers A J, et al. 2011. Land-use history has a stronger impact on soil microbial community composition than aboveground vegetation and soil properties. Soil Biology and Biochemistry, 43(10): 2184-2193. DOI:10.1016/j.soilbio.2011.06.022 |
Kim S, Li G, Han S H, et al. 2018. Thinning affects microbial biomass without changing enzyme activity in the soil of Pinus densiflora, Sieb. et Zucc. forests after 7 years. Annals of Forest Science, 75: 13. DOI:10.1007/s13595-018-0690-1 |
Li H, Yang X, Weng B, et al. 2016. The phenological stage of rice growth determines anaerobic ammonium oxidation activity in rhizosphere soil. Soil Biology & Biochemistry, 100: 59-65. |
Moreno J L, Torres I F, García C, et al. 2019. Land use shapes the resistance of the soil microbial community and the C cycling response to drought in a semi-arid area. Science of the Total Environment, 648: 1018-1030. DOI:10.1016/j.scitotenv.2018.08.214 |
Mukhopadhyay S, Masto R E, Cerdà A, et al. 2016. Rhizosphere soil indicators for carbon sequestration in a reclaimed coal mine spoil. Catena, 141: 100-108. DOI:10.1016/j.catena.2016.02.023 |
Paul E A, Clark F E. 1996. Soil microbiology and biochemistry. San Diego: Academic Press.
|
Phillips R P, Fahey T J. 2008. The influence of soil fertility on rhizosphere effects in northern hardwood forest soils. Soilence Society of America Journal, 72(2): 453-461. DOI:10.2136/sssaj2006.0389 |
Qiu S J, Ju X T, Ingwersen J, et al. 2010. Changes in soil carbon and nitrogen pools after shifting from conventional cereal to greenhouse vegetable production. Soil & Tillage Research, 107(2): 80-87. |
Schindlbacher A, Rodler A, Kuffner M, et al. 2011. Experimental warming effects on the microbial community of a temperate mountain forest soil. Soil Biology & Biochemistry, 43(7): 1417-1425. |
Simpson A C, Zabowski D, Rochefort R M, et al. 2019. Increased microbial uptake and plant nitrogen availability in response to simulated nitrogen deposition in alpine meadows. Geoderma, 336: 68-80. DOI:10.1016/j.geoderma.2018.08.029 |
Stevenson B A, Hunter D W F, Rhodes P L. 2014. Temporal and seasonal change in microbial community structure of an undisturbed, disturbed, and carbon-amended pasture soil. Soil Biology & Biochemistry, 75: 175-185. |
Vives-Peris V, Molina L, Segura A, et al. 2018. Root exudates from citrus plants subjected to abiotic stress conditions have a positive effect on rhizobacteria. Journal of Plant Physiology, 228: 208-217. DOI:10.1016/j.jplph.2018.06.003 |
Yokobe T, Hyodo F, Tokuchi N. 2018. Seasonal effects on microbial community structure and nitrogen dynamics in temperate forest soil. Forests, 9(3): 153. DOI:10.3390/f9030153 |
 2019, Vol. 55
2019, Vol. 55

