文章信息
- Zhang Bingyu, Su Xiaohua, Huang Qinjun, Zhang Xianghua, Hu Zanmin
- 张冰玉, 苏晓华, 黄秦军, 张香华, 胡赞民
- Regeneration of Transgenic Poplar(Populus alba×P.glandulosa) Expressing Levansucrase from Bacillus subtilis
- 转果聚糖蔗糖转移酶基因银腺杨的获得
- Scientia Silvae Sinicae, 2005, 41(3): 48-53.
- 林业科学, 2005, 41(3): 48-53.
-
文章历史
Received date: 2004-08-24
-
作者相关文章
2. 中国科学院遗传发育生物学研究所 北京 100101
2. Institute of Genetics and Developmental Biology, CAS Beijing 100101
Poplar(Populus L.) is an important forest genus globally because of its rapid growth and suitability for cultivation on short rotations. Commercial poplar plantations have expanded rapidly in recent years due to increasing demand for fiber for paper and boards. Unfortunately most poplars are sensitive to drought, which can slow down growth and development, reduce productivity and, in extreme cases, cause mortality. To date, the most constantly successful approach to improve the drought tolerance of poplar has been traditional breeding.
In addition to traditional breeding, the direct introduction of genes by genetic transformation is proved to be an attractive and rapid technique in poplars, which are model plants for forest biotechnology due to their small genome and ease of vegetative propagation. A lot of genes have been transferred to poplar via genetic engineering, such as the genes for herbicide resistance (Confalonieri et al., 2000), insect resistance (Delledonne et al., 2001), disease resistance (Liang et al., 2001), lignin metabolism (Franke et al., 2000), growth and wood quality (Olsen et al., 1997), abiotic stress resistance (Strohm et al., 1999) and flower development (Rottmann et al., 2000).And the corresponding agronomic traits have been improved in some transgenic poplar, for example, an insect-resistant transgenic poplar clone (Populus nigra) expressing the Bt protein (Hu et al., 2001) was commercialized in China in 2001. Improving drought tolerance via genetic transformation has also been attempted in poplar, and increased water and salt stress tolerance in transgenic aspen has been achieved by inserting a gene encoding a boiling stable protein (BspA) (Wang et al., 1998).
Up to now, the most common genes used for improving the drought resistance in plants by gene transfer is the genes encoding enzymes that catalyse the conversion of a naturally occurring substrate into a product with osmoprotective properties, such as codA(Sawahel, 2003), p5cs (Zhang et al., 1997), TPS1 (Yeo et al., 2000) and SacB (Ebskamp et al., 1994). SacB gene of Bacillus subtilis is the structural gene of levansucrase(EC 2.4.1.10), a bacterial fructosyltransferase enzyme, which catalyzes transfructosylation reactions and uses sucrose as a substrate (Steinmetz et al., 1985). The main reaction for fructan biosynthesis is: nGF (sucrose)→ G-Fn (fructan with DPn)+n-1 G (glucose). Fructans are polymers of fructose and were implicated in protecting plants against water deficit caused by drought or low temperatures (Hendry et al., 1993). Fructan accumulation has been reported in several plant species transformed with bacterial levansucrase gene, including tobacco(Nicotiana tabacum) (Caimi et al., 1997; Turk et al., 1997; Tatyana et al., 2002), potato(Solanum tuberosum) (van der Meer et al., 1994; Röber et al., 1996), maize(Zea mays)(Caimi et al., 1996), sugar beet (Beta vulgaris) (Pilon-Smits et al., 1999), Italian ryegrass(Lolium multiflorum) (Ye et al., 2001) and white clover(Trifolium repens)(Colin et al., 2002). Fructan-producing tobacco plants (Pilon-Smits et al., 1999) and sugar beet (Pilon-Smits et al., 1999) performed significantly better under drought conditions than did wild-type plants.
The aim of this study was to introduce the Bacillus subtilis SacB gene, coding for accumulation of fructan to commercially planted poplar cultivar P. alba × P. glandulosa in order to improve its drought tolerance. This paper reports results of the transformation work and preliminary observations on growth of transformed lines in greenhouse.
1 Materials and methods 1.1 Plant material and growth conditionsA poplar clone(P. alba×P. glandulosa) was used for transformation. Young shoot cuttings were surface sterilized and grown on MS medium. Plantlets were propagated on MS medium containing 0.02 mg·L-1 NAA through subculture of stem segments every 3~4 weeks in a growth chamber at (25±2) ℃, with a 16/8 h light/dark cycle.
1.2 Bacteria strains and genes used for transformationAgrobacterium tumefaciens strain LBA4404, carrying binary plasmid pKP, kindly provided by Prof. Yin-Mei Zhang and Dr Zan-Min Hu, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, was used for plant transformation. The pKP plasmid contains the SacB gene from Bacillus subtilis, coding for the enzyme levansucrase fused to the carboxypetidase Y vacuolar sorting signal from yeast (Fig. 1).

|
Fig.1 Schematic representation of the binary plasmid pKP containing SacB gene 35 S: CaMV 35 S promoter; AIMV: alfalfa mosaic virus RNA 4 translational enhancer; cpy: carboxypeptidase Y; Nos: nopaline synthase terminator sequence. |
P. alba×P. glandulosa was transformed by a leaf disc procedure, based on the method described by Confalonieri et al. (1998). Agrobacterium treatment of the leaf discs from fully expanded young leaves grown in vitro was as follows: the single clone of Agrobacterium tumefaciens strain LBA4404 was grown overnight on a shaker (200 r·min) at 28 ℃ in YEP liquid medium (Sambrook et al., 1989) containing 100 mg·L-1 Km and 100 mg·L-1 rifampicin (Sigma).Bacteria were collected by centrifuging for 10 min at 5 000 r·min-1 and re-suspending in liquid MS to a final density of 0.4~0.6 (OD600), and the leaf discs (0.5~1.0 cm2) were immersed in the suspension for 30 min, shaking constantly during the treatment. Then the leaf discs were dried on sterile filter paper and co-cultivated on MS medium containing 1.0 mg·L-1 6-BA and 0.05 mg·L-1 NAA at 26 ℃ for 2 days in darkness. Following co-cultivation, the leaf explants were rinsed with sterile water 3 times, dried on sterile filter paper and placed on the same MS medium as above but supplemented with 300 mg·L-1 cefotaxime and 60 mg·L-1 Km for selection. Green explants were transferred onto fresh selection medium every two weeks. Regenerating shoots (1.0~1.5 cm) were excised and transferred to a rooting MS medium containing 0.02 mg·L-1 NAA and 50 mg·L-1 Km. The Km-resistant plantlets obtained were propagated in vitro by subculture of the stem segments for further studies. The regeneration of the transformed plants and routine in vitro propagation were all carried out in a growth chamber at (25±2) ℃, under a 16 h photoperiod of cool-white fluorescent light.
1.4 Verification of SacB-transgenic plants 1.4.1 PCR analysisGenomic DNA was extracted from leaf of the individual transformants and untransformed plants grown in tissue culture by the CTAB method as described by Csaikl et al. (1998). PCR reactions (final volume 20 μL) were carried out using the following primers: forward, 5′-AAGAAACGAACCAAAAGCCATA-3′; reverse, 5′- CCTTTGATGTTCAGCAGGAAG-3′.These primers amplify a DNA fragment of about 1 270 base pairs (bp) from the SacB gene.Samples were heated to 94 ℃ for 3 min, followed by 25 cycles of 94 ℃ for 30 s, 56 ℃ for 30 s, and 72 ℃ for 1 min with a final extension step of 72 ℃ for 10 min. The amplified DNA was subjected to electrophoresis on a 1.2% agarose gel using the plasmid pKP as a positive control.
1.4.2 Southern dot hybridizationSacB coding region (PCR-amplified and gel-purified) was used for hybridization probes. ECL Direct Nucleic Acid Labelling and Detection System (Amersham Biosciences) was used to perform non-radioactive hybridization. Genomic DNA (10 μg) was denatured in boiling water for 5 min, immediately transferred onto ice, and then spotted onto Hybond-N+ membrane (Amersham-pharmacia Biotech, Bucks, UK). After air-drying, the genomic DNA was cross-linked to the nylon membrane by UV radiation. Prehybridization, hybridization and washes were conducted under the stringent conditions suggested by the manufacturer.
1.4.3 RT-PCR analysisTotal RNA from individual transformants and untransformed plants grown in tissue culture was isolated using the TRIzol reagent (Invitrogen, USA). The RNA samples were treated with RQ1 RNase-Free DNase (1 U·μL-1, Promega, USA) at 37 ℃ for 45 min and then purified using the RNeasy Kit (QIAGEN, USA). The SacB 5′ primer(5′-GACGGCACTGTCGCAAACTATCACG-3′) and 3′primer (5′-TCTACGTAGTGAGGATCTCTCAGCG-3′) were used to amplify an internal 477 bp SacB coding sequence. The reverse transcription reaction was carried out using SuperScript TM ⅡRNase H-Reverse Transcriptase according to the instructions given by the manufacturer (GIBCO-BRL, Life Technologies). The RT-PCR products were checked by gel electrophoresis as describedabove.
1.5 Growth conditions in the greenhouseYoung, well-developed plants (4~5 leaf stage) of SacB-transgenic lines and control plants were grown in a greenhouse with natural light and heating (28 ℃/21 ℃) in April in Beijing, 3 plants per pot at first and one plant one month later. All the plants were maintained under a normal watering regime throughout the growing season. The transgenic and control plants were observed.
2 Results 2.1 Recovery of the SacB-transgenic poplarAgrobacterium-mediated transformation was performed and the SacB gene from Bacillus subtilis was incorporated into P. alba × P. glandulosa. From about 1 000 leaf discs used for transformation, 123 independent Km-resistant shoots were recovered. The shoots, 1~1.5 cm, were further transferred to a rooting medium with 50 mg·L-1 Km, and 102 transformed plants were obtained(Fig. 2).

|
Fig.2 Km-resistant shoots (left) and plantlets (right) of P. alba × P. glandulosa |
To confirm the transgenic nature of these Km-resistant plants, PCR amplication was performed initially using specific primers for the chimeric gene. Of 102 Km-resistant lines, 99 were PCR positive. The size of the PCR products was as expected (1 270 bp) and no bands were detected in the untransformed plant (data not shown).
The integration of transgene was examined further by Southern dot hybridization of undigested genomic DNA using the SacB gene as a probe. The SacB gene was confirmed to be in 97 genomes of the 99 PCR-positive lines, and no signal was detected using DNA from the untransformed control line (Fig. 3).
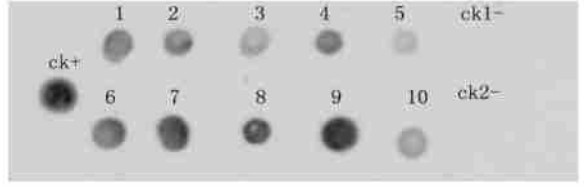
|
Fig.3 Southern dot blot analysis of transformed plants ck+: pKP-positive control; ck1- and ck2-: non-transformed control; 1~10: transgenic lines T10, T13, T23, T39, T45, T21, T34, T55, T58, T69. |
To analyze the expression of the transferred SacB gene, we prepared total RNA from leaf tissue of 62 Southern blot-positive transgenic poplar lines and subjected it to RT-PCR analysis. The expected amplification product corresponding to a 477 bp internal SacB gene fragment was reproducibly detected in 50 SacB-transgenic plants (Fig. 4).
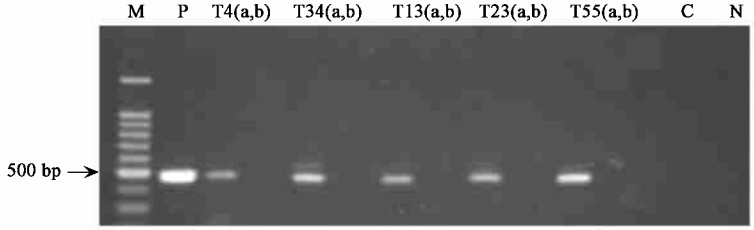
|
Fig.4 RT-PCR analysis of SacB-transgenic plants M: 100 bp DNA Ladder marker; P: pKP-positive control; T4, T34, T13, T23, T55: SacB-transgenic lines; C: non-transformed control; N: negative control with no template. Reactions using SacB primers were carried out with reverse transcriptase(a) and without reverse transcriptase (b). |
The 50 RT-PCR positive SacB-transgenic poplar(P. alba×p. glandulosa) plants exhibited no persistent morphological difference from the corresponding isogenic control plants over 5 months under normal watering in the greenhouse. In primary observation, the growth of some transgenic lines was less than that of the controls, but most lines appeared to grow at least as well as or even better than the control by primary observation.
3 DiscussionIn this study, we describe the introduction of a bacterial levansucrase gene to poplar (P. alba ×P. glandulosa) and the preliminary evaluation of some selected transgenic lines under normal watering in a greenhouse. A total of 102 Km-resistant plantlets were produced. Southern dot blot analysis confirmed the presence of the transgene in 97 of 99 PCR positive poplar lines, using the SacB gene coding sequence as a probe. It has been reported that the SacB transcripts could not be detected by Northern hybridization in SacB-transgenic tobacco (Ebskamp et al., 1994) and Lolium multiflorum(Ye et al., 2001). Furthermore, the levels of levansucrase in SacB-transgenic maize plant have been reported to be below the limit of immunodetection (Caimi et al., 1996). Therefore, we used RT-PCR to detect the expression of chimeric SacB gene in our transgenic poplar plants. Fifty of 62 transgenic lines reproducibly expressed the target gene.
Fructan accumulation in SacB-transgenic plants has been reported to cause leaf damage and necrotic lesions in some cases (van der Meer et al., 1994; Röber et al., 1996; Caimi et al., 1997; Turk et al., 1997). In the current study, no leaf damage or significant morphological differences were observed in SacB-transgenic poplar lines during 5 months of growth in a greenhouse. Growth inhibition has been reported in several transgenic plants expressing the chimeric SacB gene: potato (Pilon-Smits et al., 1996; Caimi et al., 1997), tobacco (Turk et al., 1997), L. mutiflorum(Ye et al., 2001) and white clover (Colin et al., 2002). It has also been reported that under well-watered (or unstressed) conditions no significant growth differences were observed between transgenic and wild-type plants in SacB-transformed tobacco (Pilon-Smits et al., 1995) and sugar beet (Pilon-Smits et al., 1999). In our case, growth under normal watering was apparently inhibited in some of the transgenic lines, while most transgenic lines appeared to grow at least as well as the control by primary observation. The reasons for the varying effects on growth of SacB transformation in different kind of plants are not clear. Pilon-Smits et al. (1996) suggested that the growth inhibition in SacB transgenic potato was most likely due to the perturbation, by high MW fructans, of the source-to-sink transport.
The accumulation of fructan is considered to be an important mechanism in plants for overcoming water stress (Hendry et al., 1993). Increased fructan concentrations increase the osmotic potential, or prevent lipid condensation and phase transitions taking place when plants are under water stress (Demel et al., 1998). Transfer of SacB to tobacco (Pilon-Smits et al., 1995) and sugar beet (Pilon-Smits et al., 1999) has resulted in increased drought tolerance. In our experiments, a widely-grown commercial poplar clone was used for the transformation, and the expression of SacB gene was confirmed in 50 transgenic lines. Most lines grew at least as well as the untransformed controls. Although further field drought-stress tests are needed to assess the effect of SacB gene on drought tolerance in our transgenic lines, the successful expression of SacB gene in this poplar, and the initial growth of some transformed plants, indicate that this material may be the basis for obtaining a more drought-resistant poplar.
Acknowledgement
Prof. Yin-Mei Zhang, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, are gratefully acknowledged for providing the pKP plasmid. We would like to thank Mr. Alan Brown and Dr. Simon Southerton Forestry and Forest Products, CSIRO, Australia, for critical reading of the manuscript. This work was funded by Ministry of Science and Technology of the People's Republic of China (J2002-B-004).
Caimi P G, McCole L M, Klein T M, et al. 1996. Fructan accumulation and sucrose metabolism in transgenic maize endosperm expressing a Bacillus amyloliquefaciens SacB gene. Plant Physiology, 110: 355-363. DOI:10.1104/pp.110.2.355 |
Caimi P G, McCole L M, Klein T M, et al. 1997. Cytosolic expression of the Bacillus amyloliquefaciens SacB protein inhibits tissue development in transgenic tobacco and potato. The New Phytologist, 136: 19-28. DOI:10.1111/j.1469-8137.1997.tb04727.x |
Colin L D J, Annette J S, Richard J S, et al. 2002. Fructan formation in transgenic white clover expressing a fructosyltransferase from Streptococcus salivarius. Functional Plant Biololgy, 29: 1287-1298. DOI:10.1071/FP02029 |
Confalonieri M, Allegro G, Balestrazzi A, et al. 1998. Regeneration of Populus nigra transgenic plants expressing a Kunitz proteinase inhibitor(KTi3)gene. Molecular Breeding, 4: 137-145. DOI:10.1023/A:1009640204314 |
Confalonieri M, Belenghi B, Balestrazzi A, et al. 2000. Transformation of elite white poplar (Populus alba L.) cv. 'Billafranca' and evaluation of herbicide resistance. Plant Cell Reports, 19: 978-982. DOI:10.1007/s002990000230 |
Csaikl U M, Bastian H, Brettschneider R, et al. 1998. Comparative analysis of different DNA extraction protocols. A fast, universal maxi-preparation of high quality plant DNA for genetic evaluation and phylogenetic studies. Plant Molecular Biology Reporter, 61: 69-86. |
Delledonne M, Allegro G, Belenghi B, et al. 2001. Transformation of white poplar(Populus alba L.) with a novel Arabidopsis thaliana cysteine proteinase inhibitor gene and analysis of insect pest resistance. Molecular Breeding, 7: 35-42. DOI:10.1023/A:1009605001253 |
Demel R A, Dorrepaal E, Ebskamp M J M, et al. 1998. Fructans interact strongly with model membranes. Biochimica et Biophysica Acta, 1375: 36-42. DOI:10.1016/S0005-2736(98)00138-2 |
Ebskamp M J M, van der Meer I M, Spronk B A, et al. 1994. Accumulation of fructose polymers in transgenic tobacco. Biotechnology, 12: 272-275. DOI:10.1038/nbt0394-272 |
Franke R, McMichael C M, Meyer K, et al. 2000. Modified lignin in tobacco and poplar plants overexpressing the Arobidopsis gene encoding ferulate 5-hydroxylase. Plant Journal, 22: 223-234. DOI:10.1046/j.1365-313x.2000.00727.x |
Hendry G A F. 1993. Evolutionary origins and natural functions of fructans—a climatological, biogeographic and mechanistic appraisal. New Phytologist, 123: 3-14. |
Hu J J, Tian Y C, Han Y F, et al. 2001. Field evaluation of insect-resistant transgenic Populus nigra trees. Euphytica, 121: 123-127. DOI:10.1023/A:1012015709363 |
Liang H, Maynard C A, Allen R D, et al. 2001. Increased Septoria musiva resistance in transgenic poplar leaves expressing a wheat oxalate oxidase gene. Plant Molecular Biology, 45: 619-629. DOI:10.1023/A:1010631318831 |
Olsen J E, Junttila O, Nilsen J, et al. 1997. Ectopic expression of oat phytochrome A in hybrid aspen changes critical daylength for growth and prevents cold acclimatization. Plant Journal, 12: 1339-1350. DOI:10.1046/j.1365-313x.1997.12061339.x |
Pilon-Smits E A H, Ebstamp M J M, Paul M J, et al. 1995. Improved performance of transgenic fructan-accumulating tobacco under drought stress. Plant physiology, 107: 125-130. DOI:10.1104/pp.107.1.125 |
Pilon-Smits E A H, Ebstamp M J M, Jeuken M J W, et al. 1996. Microbial fructan production in transgenic potato plants and tubers. Industrial Crops and Products, 5: 35-46. DOI:10.1016/0926-6690(95)00051-8 |
Pilon-Smits E A H, Terry N, Sear S, et al. 1999. Enhanced drought resistance in fructan-producing sugar beet. Plant Physiology and Biochemistry, 37: 313-317. DOI:10.1016/S0981-9428(99)80030-8 |
Rottmann W H, Meilan R, Sheppard L A, et al. 2000. Diverse effects of overexpression of LEAFY and PTLE, a poplar(Populus) homolog of LEAFY/FLORICAULA, in transgenic poplar and Arabidopsis. Plant Journal, 22: 235-245. DOI:10.1046/j.1365-313x.2000.00734.x |
Röber M, Geider K, Müller-Röber B, et al. 1996. Synthesis of fructans intubers of transgenic starch-deficient potato plants does not result in an increased allocation of carbohydrates. Planta, 199: 528-536. |
Sambrook H, Fritsch E F, Maniatis T. 1989. Molecular Cloning. In: A Laboratory Manual. Vol. 2. Cold Springs Harbor Laboratory Press, Cold Spring Harbor, N Y
|
Sawahel W. 2003. Improved performance of transgenic glycinebetaine-accumulating rice plants under drought stress. Biologia Plantarum, 47: 39-44. |
Steinmetz M, Le Coq D, Aymerich S, et al. 1985. The DNA sequence of the gene for the secreted Bacillus subtilis enzyme levansucrase and its genetic control sites. Molecular & General Genetics, 200: 220-228. |
Strohm M, Eiblmeier M, Langebartels C, et al. 1999. Responses of transgenic poplar(Populus tremula×P. alba) over-expressing glutathione synthetase or glutathione reductase to acute ozone stress: visible injury and leaf gas exchange. Journal of Experimental Botany, 50: 365-374. DOI:10.1093/jxb/50.332.365 |
Tatyana K, Daniela P, Atanas A, et al. 2002. Freezing tolerant tobacco, transformed to accumulate osmoprotectants. Plant Science, 163: 157-164. DOI:10.1016/S0168-9452(02)00090-0 |
Turk S C H J, De Roos K, Scotti P A, et al. 1997. The vacuolar sorting domain of sporamin transports GUS, but not levansucrase, to the plant vacuole. The New Phytologist, 136: 29-38. DOI:10.1111/j.1469-8137.1997.tb04728.x |
van der Meer I M, Ebskamp M J M, Visser R G F, et al. 1994. Fructan as a new carbohydrate sink in transgenic potato plants. The Plant Cell, 6: 561-570. DOI:10.2307/3869935 |
Wang W, Levin N, Tzfira T, et al. 1998. Plant tolerance to water and salt stress, the expression pattern of a water stress responsive protein(BspA) in transgenic aspen plants. In: Abstracts 9th International Congress on Plant Tissue and Cell Culture. Jerusalem, Israel, 184
|
Ye X D, Wu X L, Zhao H, et al. 2001. Altered fructan accumulation in transgenic Lolium multiflorum plants expressing a Bacillus subtilis sacB gene. Plant Cell Reports, 20: 205-212. DOI:10.1007/s002990000304 |
Yeo E T, Kwon H B, Han S E, et al. 2000. Genetic engineering of drought resistant potato plants by introduction of the trehalose-6-phosphate synthase (TPS1) gene from Saccharomyces cerevisiae. Molecules and Cells, 10: 263-268. |
Zhang C S, Lu Q, Verma D P S. 1997. Characterization of Delta(1)-pyrroline-5-carboxylate synthetase gene promoter in transgenic Arabidopsis thaliana subjected to water stress. Plant Science, 129: 81-89. DOI:10.1016/S0168-9452(97)00174-X |
 2005, Vol. 41
2005, Vol. 41


