文章信息
- Gao Kexiang, Liu Xiaoguang, Dana Friesem, Leonid Chernin, Shi Chengkui
- 高克祥, 刘晓光, Dana Friesem, Leonid Chernin, 时呈奎
- Cell Wall Degrading Enzymes Involved in Mycoparasitism of the Biocontrol Agent Chaetomium spirale ND35
- 生防因子螺旋毛壳ND35的细胞壁降解酶与重寄生作用
- Scientia Silvae Sinicae, 2005, 41(1): 205-210.
- 林业科学, 2005, 41(1): 205-210.
-
文章历史
Received date: 2003-10-09
-
作者相关文章
2. 山东农业大学植物保护学院 泰安 271018;
3. 希伯莱大学农业、食品与环境科学院植病和微生物系及农业生物技术中心 76100 以色列
2. College of Plant Protection, Shandong Agricultural University Taian 271018;
3. Department of plant Pathology and Microbiology, Otto-warburg center for Agricultural Biotechnology, Faculty of Agricultural, Food and Environmental Sciences, The Hebrew University of Jerusalem, PO Box 12, Rehovot 76100, Israel
Some species of the saprophytic ascomycete genera Chaetomium Kunze ex Fr. are potential antagonists of several plant pathogenic fungi, for example Rhizoctonia solani and Pythium ultimum which cause seedling damping-off and Venturia inaequalis which causes the apple scab Chaetomium spirale ND35 is a strain of endophytic fungus from Populus tomentosa and demonstrated antagonistic activity against several common fruit and forest pathogens in vitro, including Valsa sordida, V. mali and R. solani etc.(Liu et al., 1999), the biocontrol trials under greenhouse and field conditions were carried out and showed the biocontrol potential of strain ND35 against Valsa mali, the causal agent of apple canker (Gao et al., 2003). Several antagonistic mechanisms play a role in disease suppression by Chaetomium spp., mainly including three modes of action: mycoparasitism, producing antibiotics and ergosterols that can inhibit plant pathogens and stimulate growth of plants and induce resistance of plants (Cullen et al., 1984; Di Pietro et al., 1992). The ability to produce lytic enzymes has been showed to be a crucial property of the mycoparasitic fungi. Most phytopathogenic fungi have cell wall that contain chitin as a structural backbone arranged in regularly ordered layers and laminarin (β-1, 3-glucan) as a filling material arranged in an amorphic manner. Fungal cell walls contain more than 60% laminarin. The other minor cell-wall components are proteins and lipids (Chernin et al., 2002). The cell wall degrading enzymes (CWDEs) such as chitinases and glucanases, are not only involved in the destruction of the host cell wall; they may also play a role during the initial stages of mycoparasitism, because oligosaccharides generated by CWDEs partially degrading the cell walls of the host may act in turn as elicitors for the general antifungal response of mycoparasitic fungi (Chet et al., 2002).
Although the powerful potential of Chaetomium spp. to control a lot of plant diseases, knowledge concerning the mechanisms accounting for its mycoparasitic activity is rather limited, it is essential both for understanding biocontrol mechanism and for practical use. The present study was performed to determine mycoparasitic activity of C. spirale strain ND35 and the role of cell wall degrading enzymes in its mycoparasitism.
2 Materials and methods 2.1 MicroorganismsThe following strains were used in this work: Chaetomium spirale ND35 isolated from Populus tomentosa in Baoding, Hebei., Saccharomyces cerevisiae, V. sordida , V. mali and R. solani. All these strains are kept in our lab.
2.2 SEM observation of mycoparasitism by Chaetomium spirale ND35A dual-culture technique (Gupta et al., 1999) was modified to study the mode of mycoparasitism. C. spirale ND35 and the pathogenic fungus V. mali were inoculated on the opposite sides of PDA membrane on slides and incubated at high humidity at 25 ℃. Mycelia samples from regions of interaction were taken and fixed in 3% glutaraldehyde in 0.1 mol·L-1 phosphate buffer (pH7.0). After incubation for 6 h at 4 ℃, the samples were washed three times in 0.1 mol·L-1 phosphate buffer and dehydrated in a graded ethanol series(50%, 70%, 80%, 90% and 100%). Dehydrated samples were critical-point dried with liquid carbon dioxide, mounted on stubs and coated with gold in a sputter coater. Electron micrographs were taken in a scanning electron microscope operating at 25 kV.
2.3 Preparation of fungal cell wallsS. cerevisiae was grown in YPD medium (1% yeast extract, 1% peptone, 2% D-glucose), and R. solani and other fungi were grown in potato dextrose broth (PDB) for 7 to 10 days at 25 ℃ with shaking at 140 r·min-1. S. cerevisiae yeast cells and the different fungal mycelia were collected by filtration through filter paper, followed by homogenized with pestle in mortar and frozen in liquid nitrogen for several times. The fungal cell wall preparation (CWP) was washed with 2% MgCl2 and distilled water six times after being sonicated twice for 2 min. CWP was centrifuged at 10 080 g for 15 min before being lyophilized and stored at -20 ℃.
2.4 Effect of carbon source on β-1, 3-glucanase productionStrain ND35 was cultivated in synthetic medium (SM: 3.0 g of NaNO3, 1.0 g of KH2PO4, 0.5 g of KCl, 0.5 g of MgSO4 7H2O, 1.0 g of peptone, 0.01 g of FeSO4, 0.003 g of ZnSO47 H2O, and 0.003 g of CoCl2 6H2O) supplemented with different concentration of glucose, laminarin, colloidal chitin or CWP of different kinds of fungi as the sole carbon source. 40 mL of SM was autoclaved in 150 mL flasks, and two agar disks (ϕ6 mm) from a colony margin of C. spirale ND35 on PDA for five days at 25 ℃ were transferred to each flask and incubated at 28 ℃ for various time periods. Cultural filtrate from each flask was collected by centrifuge. The experiment was arranged in a completely random design and conducted two times with three duplications.
2.5 Induction of β-1, 3-glucanase and chitinase by CWP of R. solaniFor glucanases and chitinases production, the fungus C. spirale ND35 was grown in inductive medium consisting of (g·L-1): CWP of R. solani 5.0 or colloidal chitin 2.0, glucose 0.25, NH4NO3 1.0, MgSO4 0.3, KH2PO4 0.8, KNO3 0.2.Ten mycelium disks (6 mm diameter) of C. spirale ND35 were inoculated in 250 mL-flasks, each containing 50 mL medium, and shaken at 140 r·min-1 for 6 days at 28 ℃.
2.6 Extracellular protein extractionExtracellular protein was prepared as follows: growth media were centrifuged at 4 ℃ and followed by taking the supernatant and discarding the pellets. For protein analysis by gel electrophoresis, the filtered extracellular proteins were concentrated and dialyzed in an ultrafiltration system with a cut-off of 10ku(Vivascience, Linkoln, UK).
2.7 Assays of β-1, 3-glucanase and chitinase activitives in solutionsThe β-1, 3-glucanase activity was determined by measuring the amount of reducing sugar released from laminarin. The standard mixture contained 100μL of culture filtrate and 400μL of laminarin (1 mg·mL-1) dissolved in 50 mmol·L-1 sodium acetate buffer (pH 5.0). Each reaction mixture was incubated at 37 ℃ for 30 min., And the production of reducing sugar was determined using glucose as the standard according to the method of Miller, 1959. One unit of β-1, 3-glucanase activity was defined as the amount of enzyme catalyzing the release of 1mmol of glucose equivalent per min. per mL of enzyme solution. Specific activity was expressed in units per milligram of proteins (specific units [U]). Assays of chitinolytic activity in solutions were performed by the method of Chernin et al., 1995.The following chromogenic oligomers of N-acetyl-β-D-glucosamine (GlcNAc) (Sigma). were used as substrates: ρ-nitrophenyl-N-acetyl-β-D-glucosaminide (pNP-GlcNAc) for just detecting exochitinases, and ρ-nitrophenyl-β-D-N, N′-diacetylchitobiose [pNP-(GlcNAc)2] for all types of chitinases detection. Protein concentration was measured by the Bradford method (Bradford, 1976), with bovine serum albumin as the standard.
2.8 Assay for protease on plateProteolytic activity was assayed on Bacto Litmus milk (Difco) agar plates by monitoring the appearance of haloes of casein lysis around the fungus colony.
2.9 Protein electrophoresis and gel-bases detection of β-1, 3-glucanase and chitinaseExtracted proteins were separated by 10% SDS--PAGE (polyacrylamide gel electrophoresis) or 8% native PAGE according to the method of Laemmli, 1970. Samples were prepared without 2-mercaptoethanol and were not heated prior to loading on the gel. Pre-staining high molecular weight standard proteins were used for molecular weight determination. After electrophoresis, enzymes were reactivated by removing SDS using the casein-EDTA activity buffer and were assayed directly for β-1, 3-glucanase and chitinolytic enzymes activity. β-1, 3-glucanase activity was detected with two assays. The first was adapted from Liang et al., 1995. And used laminarin as a substrate. In this assay, active glucanases appear red bands on the gel by using 2, 3, 5-triphenyltetrazolium chloride (TTC). As a control, the other half of the gel was incubated under the same conditions, except that laminarin was omitted in order to detect proteins that were not β-1, 3-glucanase but might be stained by TTC. In the second assay, exo-β-1, 3-glucanase activity was detected in situ by overlying the gel with dimeric analog 4- methylumbelliferyl-β-D-glucoside (MUG) at a 150μg ml-1 of concentration in 50mM sodium acetate(pH5.0) buffer as a substrate. The active bands were observed on exposure to UV light after incubating 45 min at 30 ℃.Chitinase activity was detected on gels by using fluorescent substrate 4-methylumbelliferyl-β-D- N, N′-diacetylchitobioside.(4-MU-(GlcNAc)2)(all reagents from Sigma).
2.10 Direct detection of β-1, 3-glucanase on isoelectriofocusing (IEF) gelIsoelectric focusing was performed with NOVEX pre-cast IEF gel (pH3 to 7) and a Hoefer system (Amersham Biosciences, USA) according to the manufacturer's instruction. After electrophoresis, β-1, 3-glucanase activity was directly detected on gel using two ways as described above. The gel was run for 2.5 h at low temperature (constant voltage 100 V for 1 h, followed by 200 V for 1 h and 500 V for 30 min.).
3 Results 3.1 Mycoparasitism observed by SEMSEM studies on the hyphal parasitism of Chaetomium on the pathogen of Valsa canker of apple, V. mali indicated that hyphae of C. spirale ND35 frequently grew parallel to the host and attached themselves to host mycelia by forming hooks, followed by coiling around the host and penetrating the host mycelia by partially degrading its cell wall. The holes in the penetration site on the host mycelia can be observed (Fig. 1).

|
Fig.1 Scanning electron micrographs of hyphal interaction between Chaetomium spirale ND35 and Valsa mali a. Coiling of C. spirale ND35 around a hypha of V. mali (×2 500); b. Hook of C. spirale ND35 attached to a hypha of V. mali (×2 500); c. Hypha of ND35 penetrating hyphae of V. mali (×2 500); d. Holes of hyphae of V. mali digested by the antagonist (×2 500). |
The chitinases and β-1, 3-glucanase activities were detected in culture filtrates in SM with CWP, colloidal chitin and laminarin as carbon sources, and implied that CWP of fungi can induce both chitinase and β-1, 3-glucanase. But proteolytic activity was not detected on Bacto Litmus milk (Difco) agar plates.
The β-1, 3-glucanase activity varied considerably when strain ND35 was grown in different carbon sources and in various incubation periods. The β-1, 3-glucanase activity level in SM amended with 1% CWP of R. solani as the sole carbon source increased with time and reached a maximum of 3.95U after six days. So it is the optimum time for extracting proteins under the condition as described above.
Carbon sources play an important role in production of β-1, 3-glucanase. Different kinds of fungal cell wall preparation had a great influence on the induction of β-1, 3-glucanase. Higher enzyme activity was obtained in media with the CWP of Valsa sordida as the sole carbon sources as compared with media added other fungal CWP. After four days of incubation, the activity was 4.38U. This result revealed that the CWP of host fungus is more suitable for induction of β-1, 3-glucanase.The β-1, 3-glucanase activity induced by R. solani was 3.50U, whereas the activity in media with S. cerevisae was 1.33U, the lowest one. Although 1% concentration of laminarin, colloidal chitin and glucose can induce β-1, 3-glucanase, laminarin is able to induce higher level of β-1, 3-glucanase activity as compared with the same concentration of colloidal chitin and glucose. The enzyme activity in SM with 1% laminarin was 7.95U after two days of incubation, about seven times as that one in SM with 1% glucose (1.13U), in addition to 3.64U for 1% colloidal chitin. This result showed that laminarin is a appropriate carbon source for induction of β-1, 3-glucanase.
The glucose has a significant influence on β-1, 3-glucanase production. The enzyme activity was reduced with increasing concentrations of glucose from 0 to 10%. Higher concentration of glucose repressed the β-1, 3-glucanase production. At 2% and 10% concentration of glucose there were both no detection of activity. Although the β-1, 3-glucanase activity could be detected in media with 1% glucose, the specific activity was 1.13U, at a lower level. On the contrary, higher level of activity was detected in SM without any kinds of carbon sources, specific activity was 4.24U. This result maybe implied autolysis occurred under conditions of carbon energy source exhaustion. Therefore starvation could also induce the β-1, 3-glucanase. The result of sample dialysis revealed that the effect of glucose couldn't be because of enzyme inactivation.
3.3 Assay for β-1, 3-glucanase and chitinase on gels from C. spirale ND35The extracted proteins in SM with CWP of R. solani or colloidal chitin were electrophoresed on 8% native PAGE and /or 10%SDS-PAGE gels to separate proteins and detected the activity. Just one band of exo-β-1, 3-glucanase activity with about 110 ku molecular weight and one band of chitinase were detected both on native and SDS-PAGE gels by two ways of assays as described under Materials and Methods (Fig. 2). This result showed that both CWP and colloidal chitin can induce at least one detectable exo-β-1, 3-glucanase and chitinase from C. spirale ND35, and it is a monomer, not polymer because the only one band was detected both in Native and SDS-PAGE gels and MUG is specific for detection of exo-β-1, 3-glucanase.
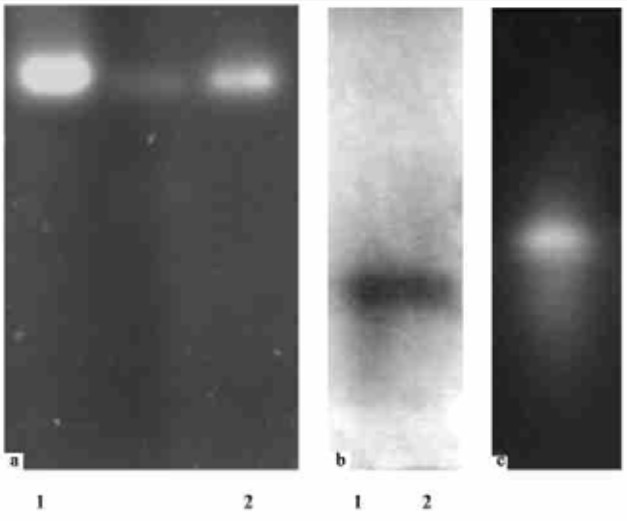
|
Fig.2 Detection of β-1, 3-glucanase and chitins activity on gel a. Detection of an ego-β-1, 3-glucanase by fluorescent substrate 4-MUG after 10% SDS-PAGE; b. Detection of β-1, 3-glucanase by TTC after IEF (pH 3-7) Protein extracts were obtained from SM supplemented with either CWP (lane 1) or colloidal chitin; c. Detection of chitins by fluorescent substrate 4-MU- (Glena)2 after 10% SDS-PAGE. Protein extracts were obtained from SM supplemented with colloidal chitin. All lanes were loaded with 15μg of protein. |
An exo-β-1, 3-glucanase with about 110 ku molecular mass, and a chitinase from C. spirale ND35 were detected both in culture filtrate and on gels. Penetrating the host mycelia by partially degrading its cell wall and a hole in the penetration site on the host mycelia in dual culture with V. mali were observed by SEM, and revealed that these cell wall degrading enzymes involved in mycoparasitic attack on the host, and it is likely related to coordinated action of chitinases and β-1, 3-glucanases, which is a prerequisite to an effective cell wall disruption of the pathogenic fungi.
β-1, 3-glucanase produced by C. spirale ND35 varied considerably when C. spirale ND35 was grown in different carbon source during various incubation times, and might be subjected to both inductions by substrate such as laminarin and CWP etc. and catabolite repression like glucose.
About 110 kDa of exo-β-1, 3-glucanase produced in SM with CWP hydrolyze laminarin by sequentially cleaving glucose residues from the non-reducing end of polymers or oligomers and it is a acidic monomer according to assays of native and SDS-PAGE, as well as IEF. But both endo- and exo-β-1, 3-glucanase are essential for the full digestion of laminarin. Only one exo-β-1, 3-glucanase was detected may be connected to the period taken samples and carbon source, because different polysaccharides or fungal cell walls have been shown to induce different levels of enzyme activity and different enzyme patterns, and regulation of the enzymes' expression is considered a key step inβ-glucan biodegradation and consequently in mycoparasitism.(Chernin et al., 2002). So further studies will be continued on analysis of the β-1, 3-glucanolytic system of the biocontrol agent C. spirale ND35 and synergism between chitinases, glucanases and antibiotics during the antagonism against phytopathogenic fungi.
Acknowledgement
The authors thank all staff in Otto-Warburg Center for Agricultural Biotechnology, Faculty of Agricultural, Food and Environmental Sciences, The Hebrew University of Jerusalem, Israel for kindly help.
Bradford M M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Annual Biotechnology, 72: 248-254. |
Chernin L S, Ismailov Z F, Haran S, et al. 1995. Chitinolytic Enterobacter agglomerans antagonistic to fungal plant pathogen. Appl Environ Microbial, 61: 1720-1726. |
Chernin L, Chet I. 2002. Microbial enzymes in the biocontrol of plant pathogens and pests In: Dick R P and Burns R G. eds. Enzyme in the Environment. New York, Marcel Dekker, 171-225
|
Chet I, Chernin L. 2002. Biocontrol, Microbiol Agents in Soil. In: Bitton G. eds., Encyclopedia of environmental Microbiology. New York, John Willey & Sons Inc, 450-465
|
Cullen D, Berbee F M, Andrews J H. 1984. Chaetomium globosum antagonizes the apple scab pathogen, Venturia inaequalis under field conditions. Canadian Journal of Botany, 62: 1814-1818. DOI:10.1139/b84-245 |
Di Pietro A, Gut-Rella M, Pachlatko J P, et al. 1992. Role of antibiotics produced by Chaetomium globosum in biocontrol of Pythium ultimum, a causal agent of damping-off. Phytopathology, 82: 131-135. DOI:10.1094/Phyto-82-131 |
Gao K X, Liu X G, Li C, et al. 2003. Biocontrol potential of Chaetomium spirale ND35 against canker of apple tree. In: Yang Q eds. Biological Control and Bio-technology Harbin: Heilongjiang Science and Technology Press, 132-140
|
Gupta V P, Tewari S K, Govindaiah. 1999. Ultrastructure of mycoparasitism of Trichoderma, Gliocladium and Laetisaria species on Botryodiplodia theobromae. J Phytopathology, 147: 19-24. DOI:10.1111/j.1439-0434.1999.tb03802.x |
Laemmli U K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227: 680-685. DOI:10.1038/227680a0 |
Liang Z C, Hseu R S, Wang H H. 1995. Partial purification and characterization of a 1, 3-β-D-glucanase from Ganoderma tsugae. Journal Industrial Microbiology, 14: 5-9. DOI:10.1007/BF01570058 |
Liu X G, Gao K X, Gu J C, et al. 1999. Testing on the antagonism of the dominant of endophytic fungi from Populus tomentosa, Chaetomium ND35 in the Laboratory. Scientia Silvae Sinicae, 35: 57-61. |
Miller G L. 1959. Use of dinitrosalicylic reagent for determination of reducing sugar. Annual Biochemistry, 31: 426-428. |
 2005, Vol. 41
2005, Vol. 41

