文章信息
- 杨玉盛, 郭剑芬, 陈银秀, 陈光水, 郑燕明.
- Yang Yusheng, Guo Jianfen, Chen Yinxiu, Chen Guangshui, Zheng Yanming.
- 福建柏和杉木人工林凋落物分解及养分动态的比较
- Comparatively Study on Litter Decomposition and Nutrient Dynamics Between Plantations of Fokienia hodginsii and Cunninghamia lanceolata
- 林业科学, 2004, 40(3): 19-25.
- Scientia Silvae Sinicae, 2004, 40(3): 19-25.
-
文章历史
- 收稿日期:2002-07-25
-
作者相关文章
2. 福建农林大学林学院 南平 353001;
3. 福建农林大学莘口教学林场 三明 365002
2. Forestry College of Fujian Agriculture and Forestry University Nanping 353001;
3. Xinkou Experimental Forest Farm of Fujian Agriculture and Forestry University Sanming 365002
森林凋落物(forest litter)是土壤动物、微生物重要物质和能量的来源,凋落物分解是森林生态系统生物地球化学循环的重要组成部分,决定了枯枝落叶层现存量及其性质,其分解速率则对森林生态系统生产力有重要影响,已愈来愈引起林学家、生态学家、微生物学家、土壤学家以及森林经营工作者的重视(田大伦等, 1989; 赵其国等, 1991; Berg, 2000; Waring et al., 1985)。早在1876年,德国的E.Ebermayer就开始研究凋落物在养分循环中的作用,而后,国外许多学者大量报道世界范围内森林凋落物的分解及养分释放(Adams et al., 1996; Berg et al., 1993; Edmonds et al., 1995; Singh et al., 1999)。而我国直到20世纪80年代后才有类似研究报道,涉及的森林类型有山地雨林、半落叶季雨林、红树林、阔叶林(常绿或落叶)、针叶林及针阔混交林等(贾黎明等, 1998; 林鹏等, 1990; 卢俊培等, 1989; 莫江明等, 1996; 沈海龙等, 1996; 田大伦等, 1989; 屠梦照等, 1993; 杨玉盛等, 2002; 赵其国等, 1991)。本文仅从凋落物分解及养分释放角度,比较福建柏(Fokienia hodginsii)与杉木(Cunninghamia lanceolata)林生态学差异。
1 试验地概况试验地位于三明莘口教学林场小湖工区(北纬26°11′30″,东经117°26′00″),福建柏和杉木人工林前身均为格氏栲(Castanopsis kawakamii)、米槠(Castanopsis carlesii)等为主的天然林,1966年经皆伐劈草炼山,穴状整地,1967年初用福建柏和杉木实生苗营造人工纯林。试验地气候条件、林分抚育措施、标准地概况、土壤理化性质等详见文献(杨玉盛等,2004)。
2 研究方法 2.1 凋落物分解于1999年4月分别在福建柏和杉木林内收集新凋落的叶片及小枝(杉木小枝与叶片连在一起脱落),风干后分别称取干重约为50 g的福建柏与杉木落叶和落枝,装入尼龙网袋(大小20 cm×20 cm,孔径0.5 mm,每种样品80袋),同时取样测定含水率及化学分析。网袋于19 99年5月分别置于福建柏与杉木林内枯枝落叶层表面,放置30、60、90、150、210、270、330、390、510、630、750 d后,分别随机回收每种分解样品各6袋,清除样品中的附着杂物后105℃烘干恒重后称重求干物质重,再把相同样品6袋混合、磨细,过60目筛,连同分解前样品(0 d)贮存于广口瓶中备用。
2.2 凋落物N、P、K、C元素测定采用硫酸-高氯酸消煮法,在上海嘉定纤检仪器厂生产的KDN-消化炉上制备待测液,KDN-C型定氮仪测定全N,钼锑抗比色法测定全P,火焰光度计法测定全K;重铬酸钾氧化-外加热法测定有机C( 中华人民共和国林业行业标准, 1999)。
2.3 统计分析与计算平均相对分解速率(mean relative decomposition rate, RDR)用如下公式计算:
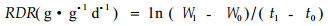
|
(1) |
其中W0和W1分别为凋落物分解t0和t1时间后的数量,t1-t0为取样的时间间隔(d)。
凋落物瞬时分解系数(k)采用Olson (1963)指数方程进行计算:
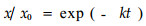
|
(2) |
其中为x为分解t时间后干重或养分残留量,x0为分解初始干重或养分含量,并采用F-检验法进行差异显著性检验。
凋落物养分年释放量(M)用如下公式计算:
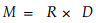
|
(3) |
其中R和D分别为养分年归还量和养分年分解率。
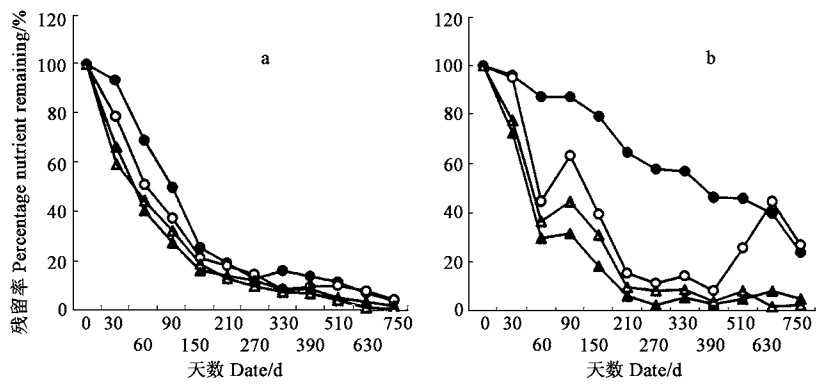
3 结果 3.1 凋落物分解速率福建柏落叶在150天前分解快,失重率达72.26%,比杉木落叶高出43.61%,之后分解速率明显减慢;分解1年后,福建柏落叶残留率为16.53%,而杉木落叶则为39.22%(图 1)。福建柏和杉木落枝分解过程中的失重率变化比落叶的平稳,福建柏落枝在150天前分解速率略高于杉木落枝,之后则低于杉木落枝;分解1年后福建柏落枝残留率为80.57%,比杉木的高出5.59%(图 1)。
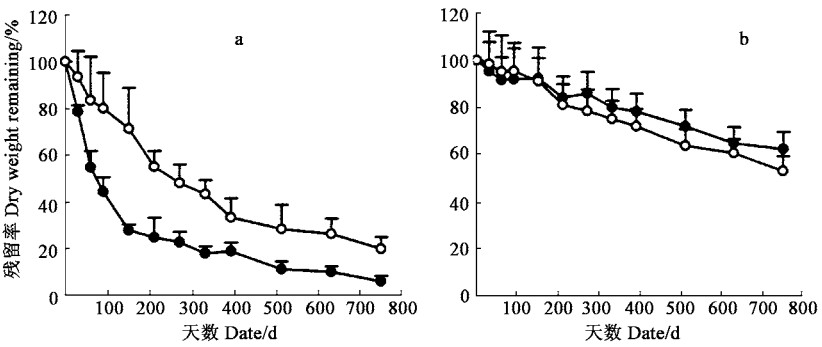
|
图 1 落叶(a)、落枝(b)分解过程中干重残留率变化 Fig. 1 Changes of dry matter during decomposition of needle litter (a) and branch litter(b) ●福建柏F. hodginsii; ○杉木C. lanceolata. |
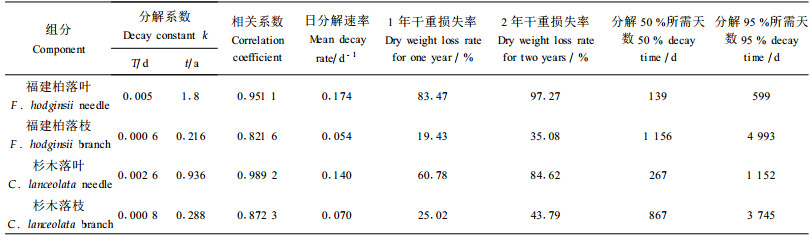
应用Olsen (1963)指数方程模拟凋落物分解过程,拟合程度较好(R2>0.8, p<0.0 5),福建柏落叶、杉木落叶、福建柏落枝和杉木落枝分解1年后干重损失率分别为83.47%、60.78%、19.43%和25.02%,分解50%所需时间分别为139、267、1 156和867 d(表 1)。
|
|
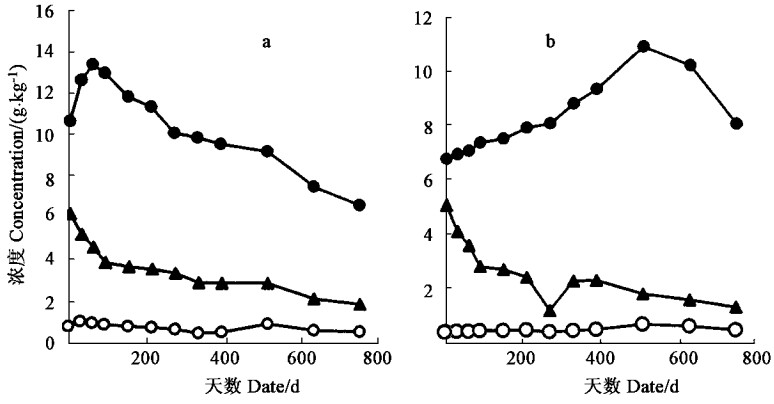
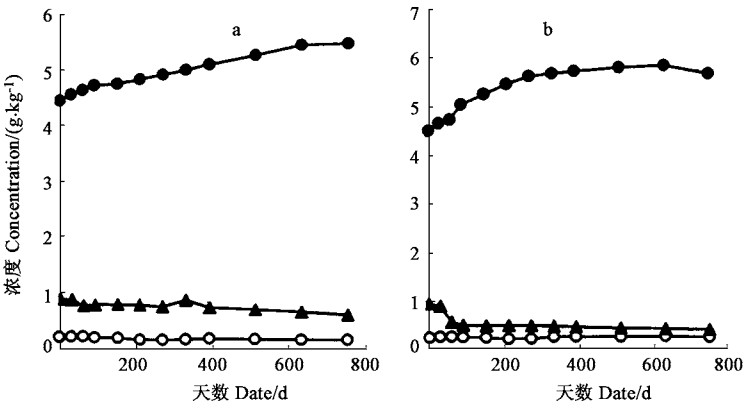
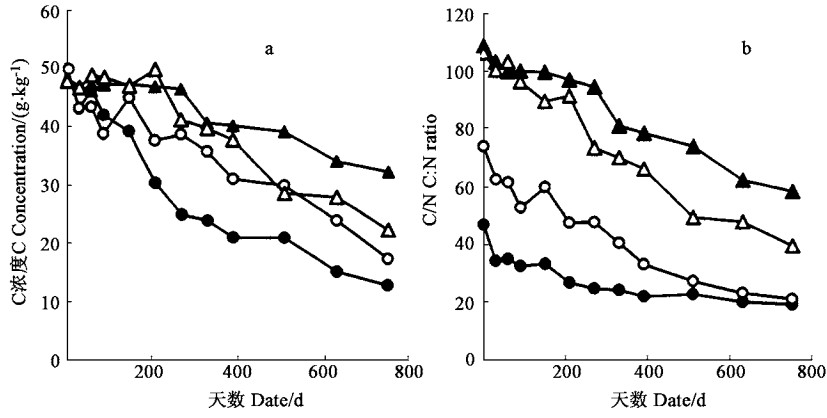
福建柏和杉木落叶分解过程中P浓度呈上升趋势,而K浓度则有明显下降(图 2)。两种落叶分解过程中N浓度变化差异较大,福建柏落叶分解的最初阶段,N浓度有较大幅度的上升,60天后,N浓度逐渐下降;而杉木落叶N浓度在分解过程中基本呈单调上升趋势(图 2)。落枝分解过程中,福建柏和杉木各元素浓度均为N单调上升,K单调下降,P先升后降(图 3)。福建柏、杉木落叶和落枝分解过程中C元素浓度均呈下降的趋势,福建柏落叶C浓度降低速率高于杉木,但福建柏落枝的C浓度变化却比杉木平缓(图 4)。分解过程中各组分C/N值均不断降低,降低的速率以杉木落叶最快,福建柏落枝最慢;福建柏落枝C/N值分解初期(150 d)低于杉木的,150天后则高于杉木的,福建柏落叶C/N值在分解过程中始终小于杉木(图 4)。
|
图 3 福建柏(a)、杉木(b)落枝分解过程中养分浓度变化 Fig. 3 Changes of nutrient concentrations during decomposition of branch of F. hodginsii(a) and C.lanceolata(b) |
|
图 4 落叶和落枝分解过程中C浓度(a)及C/N比(b)变化 Fig. 4 Changes of C concentration(a) and ratio of C:N(b) in Litter of needle and branch during decomposition ●福建柏落叶Needle litter of F. hodginsii; ○杉木落叶Needle litter of C. lanceolata; ▲福建柏落枝Branch litter of F. hodginsii; △杉木落枝Branch litter of C. lanceolata. |
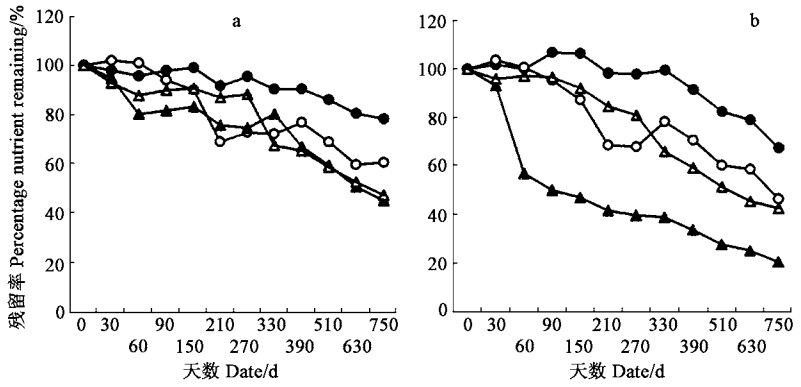
福建柏和杉木落叶分解过程中N、P、K养分残留率与其干重残留率变化趋势相似,分解前150 d落叶N、P、K养分残留率下降较快,随后养分残留率下降比较平缓(图 5)。不同元素的分解速率以K最快,其次为C和P,而N最小;福建柏落叶中N和P养分年残留率分别比杉木的低了35.9%和3.98%,但福建柏落叶中K年残留率却比杉木的高4.32%(图 5)。
|
图 6 福建柏(a)、杉木(b)落枝分解过程中N、P、K、C残留率变化 Fig. 6 Percentages of N, P, K and C remaining in branch litter of F. hodginsii(a) and C. lanceolata(b) |
虽然落枝中N、P、K、C分解速率比落叶的慢得多,且分解过程阶段性亦不如落叶的明显,但各元素分解速率大小模式却与落叶的相似(图 6)。福建柏落枝C和N元素年分解残留率比杉木的小,而P和K元素年分解残留率却比杉木的大(图 6)。
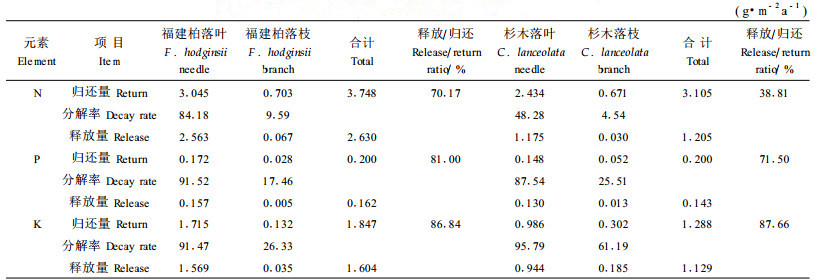
3.4 凋落物分解过程中养分释放量福建柏和杉木落叶和枝养分释放速率最快为K,最慢为N;福建柏凋落物落叶和落枝的N、P、K养分年释放量分别为2.630、0.162和1.604 g·m-2,分别是杉木(1.205、0.143和1.129 g·m-2)的2.18倍、1.13倍和1.42倍;其中福建柏落叶N、P、K年释放量分别是杉木的2.18倍、1.21倍和1.66倍;福建柏落枝除了N的年释放量是杉木2.23倍外,P和K均比杉木的小(表 2)。从表 2可见各养分释放/归还比值大小为K>P>N;福建柏落叶和落枝的N和P释放/归还的比值分别比杉木的高出31.36%和9.50%,而K释放/归还的比值则较接近。
|
|
通过森林凋落物和林木枯死细根分解作用,向土壤归还大量养分与有机物质,这是森林生态系统自肥的重要机制之一(Waring et al., 1985)。森林凋落物的分解既有物理过程,又有生物化学过程,一般由淋溶作用(凋落物中可溶性物质通过降水而被淋溶)、自然粉碎作用(主要由腐食动物的啃食完成,但非生物因素如土壤干湿交替、冻融作用亦可使枯叶破碎)、代谢作用(由腐生微生物的活动把复杂的有机化合物转化成简单无机化合物)等共同完成。气候(常用AET作为指标,actual evapotranspiration)、凋落物质量、微生物和土壤动物等是影响凋落物分解的主要因素(胡肄慧等, 1987; 许新健等, 1995; Aerts, 1997; Berg et al., 1993)。本研究的福建柏和杉木凋落物年分解速率,均在已报道的亚热带凋落物年分解速率40%~70%范围内(田大伦等, 1989;屠梦照等, 1993; 许新健等, 1995),高于温带森林凋落物的平均分解速率20%~30% (胡肄慧等, 1987; 沈海龙等, 1996; Brofas et al., 2001; Caldentey et al., 2001; Gholz et al., 1985; Pedersen et al., 1999),但低于热带雨林或季雨林凋落物的大于70%年均分解速率(卢俊培等, 1989; Bubb et al., 1998; Lisanework et al., 1994; Louzada et al., 1997),体现了凋落物分解速率的气候地带性(屠梦照等, 1993; Aerts, 1997; Berg et al., 1993)。
福建柏和杉木落叶分解速率与时间均呈指数关系,这与凋落物分解过程中先后出现分解速率较快和较慢两个阶段的结果相吻合(胡肄慧等, 1987; 许新健, 1995; Edmonds et al., 1995; Jamaa et al., 1996; Kumar et al., 1992)。初期出现较快分解速率,与水溶性物质和易分解的碳水化合物的快速淋失和降解有关,N、P、S等元素的浓度对此阶段分解速率有主要影响。随着分解继续,木质素等难分解物质不断累积(达到45%~51%),凋落物的进一步分解受抑制,分解速率明显减慢(Aerts, 1997; Berg, 1986; Jamaludheen et al., 1999; Taylor et al., 1989)。
凋落物质量直接影响其分解速率(胡肄慧等, 1987; Aerts, 1997; Berg, 2000; Couteaux et al., 1995; Gallardo et al., 1999; Taylor et al., 1989)。由于福建柏落叶的易分解物质(水溶性物、半纤维素和粗蛋白)含量高于杉木,而杉木落叶纤维素、木质素含量及C/N、C/P及木质素与N和P的比值明显高于福建柏(杨玉盛等,2004),这是本研究中福建柏落叶分解比杉木快的主要原因之一;此外,还与福建柏林地土壤微生物数量比杉木林地高有关。福建柏林年凋落物量比杉木林的高(杨玉盛等,2004),但林内枯枝落叶层现存量(2.652 t·hm-2)却比杉木林(3.155 t·hm-2)的低,亦从另一角度验证了福建柏凋落物分解比杉木的快。
4.2 凋落物分解过程中养分动态森林凋落物的分解过程中元素迁移有:(1)淋溶一富集—释放;(2)富集一释放;(3)直接释放等模式;针叶和木质凋落物的淋溶阶段可能不明显甚至不存在(Berg, 2000; Blair, 1998)。福建柏和杉木落叶分解过程中,N浓度先升后降,与马尾松(Pinus massoniana)、杨树(Populus spp.)、刺槐(Robinia pseudoacacia)、柳桉木(Shorea robusta)、非洲圆柏(Juniperus procera)等落叶分解结果相似(贾黎明等, 1998; 莫江明等, 1996; Lisanework et al., 1994; Singh et al., 1999),而与樟子松(Pinus sylvestris var.mongolica)落叶分解过程N浓度始终保持下降相异(沈海龙,1996)。本研究中落叶和落枝K浓度随分解过程不断下降,与国内外大量研究结果相似(田大伦等, 1989; 杨玉盛等,2002; Bubb et al., 1998; Jamaludheen et al., 1999)。而落叶P分解过程中一直处于富集状态,这与混交林中毛竹(Phyllostachys pubescens)落叶分解结果相一致(许新健,1995),但与温带的刚松、欧洲黑松、欧洲水青冈等落叶分解略有不同(Blair, 1998; Kavvadias et al., 2001)。
福建柏和杉木落叶养分元素残留率与其干重残留率变化相似,这与红松(Pinus koraiensis)落叶研究结果一致(张放等, 1990),但不同于米槠(Castanopsis carlesii)落叶(许新健等,1995)。K元素残留率最低则与他人的研究结果一致,表明K元素周转快,利用率高(Lisanework et al., 1994; Adams et al., 1996; 贾黎明等, 1998; 莫江明等, 1996)。本研究中杉枝的N残留率在分解初期上升,这与杉枝初始C/N值高(106)有关,也可能与枝条富集降雨输入的N有关(莫江明等, 1996; 杨玉盛等, 2002; Bubb et al., 1998)。
Waring和Schlesinger (1985)认为,凋落物分解过程中每年释放的营养元素可满足林木生长所需量的69%~87%。与其他亚热带森林类型凋落物分解过程中养分释放量相比,本研究中凋落物P释放量在多数已报道的0.06~0.19 g·m-2a-1范围内,而N和K释放量则较高,这与凋落物中营养元素的初始含量及分解速率有关(Gholz et al., 1985; Louzada et al., 1997; Singh et al., 1999; 田大伦等, 1989; 杨玉盛等, 2002)。
胡肄慧, 陈灵芝, 陈清朗, 等. 1987. 几种树木枯叶分解速率的试验研究. 植物生态学与地植物学学报, 11(2): 124-132. |
贾黎明, 方陆明, 胡延杰. 1998. 杨树刺槐混交林及纯林枯落叶分解. 应用生态学报, 9(5): 463-467. |
林鹏, 卢昌义, 王恭礼, 等. 1990. 海南岛河港海莲红树林凋落物动态的研究. 植物生态学与地植物学学报, 14(1): 69-73. |
卢俊培, 刘其汉. 1989. 海南岛尖峰岭热带林凋落叶分解过程的研究. 林业科学研究, 2(1): 25-32. |
莫江明, 布朗, 孔国辉, 等. 1996. 鼎湖山生物圈保护区马尾松林凋落物的分解及其营养动态研究. 植物生态学报, 20(6): 534-542. |
沈海龙, 丁宝永, 沈国舫, 等. 1996. 樟子松人工林下针阔叶凋落物分解动态. 林业科学, 32(5): 393-402. |
田大伦, 朱小年, 蔡宝玉, 等. 1989. 杉木人工林生态系统凋落物的研究Ⅱ.凋落物的养分含量及分解速率. 中南林学院学报, 9(增): 45-55. |
屠梦照, 姚文华, 翁轰, 等. 1993. 鼎湖山南亚热带常绿阔叶林凋落物的特征. 土壤学报, 30(1): 35-41. |
文启孝, 杜丽娟, 张晓华等编著.土壤有机质研究法.北京:农业出版社, 1984: 256-271
|
许新健, 陈金耀, 俞新妥. 1995. 武夷山六种杉木伴生树种落叶养分归还的研究. 福建林学院学报, 15(3): 213-217. |
杨玉盛, 陈光水, 郭剑芬, 等. 2002. 杉木观光木混交林凋落物分解及养分释放的研究(英文). 植物生态学报, 26(3): 275-282. DOI:10.3321/j.issn:1005-264X.2002.03.004 |
杨玉盛, 陈银秀, 何宗明, 等. 2004. 福建柏和杉木人工林凋落物性质的比较. 林业科学, 40(1): 2-10. DOI:10.3321/j.issn:1001-7488.2004.01.001 |
张放, 谭学仁, 史凤友. 1990. 红松人工林枝叶分解速度及养分动态的研究. 生态学杂志, 9(5): 14-18. |
赵其国, 王明珠, 何园球. 1991. 我国热带亚热带森林凋落物及其对土壤的影响. 土壤, 23(1): 8-15. DOI:10.3321/j.issn:1009-2242.1991.01.005 |
中华人民共和国林业行业标准. 森林土壤分析方法. 北京: 中国标准出版社, 1999
|
Aerts R. 1997. Climate, leaf chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos, 79: 439-449. DOI:10.2307/3546886 |
Adams M B, Angradi T R. 1996. Decomposition and nutrient dynamics of hardwood leaf litter in the Fernow whole-watershed acidification experiment. For Ecol Manage, 83: 61-69. DOI:10.1016/0378-1127(95)03695-4 |
Berg B. 1986. Nutrient release from litter anlhumus in coniferous forest soils—a mini review. Scand J For Res, 1: 359-369. DOI:10.1080/02827588609382428 |
Berg B. 2000. Litter decomposition and organic matter turnover in northern forest soils. For Ecol Manage, 133: 13-22. DOI:10.1016/S0378-1127(99)00294-7 |
Berg B, Berg M P, Bottner P. 1993. Litter mass loss rates in pine forests of Europe and Eastern United States: some relationships with climate and litter quality. Biogeochemistry, 20: 127-153. DOI:10.1007/BF00000785 |
Blair J M. 1998. Nitrogen, sulfur and phosphorus dynamics in decomposing deciduous leaf litter in the southern Appalachians. Soil Biology and Biochemistry, 20: 693-701. |
Brofas G, Stamatelos G. 2001. Litterfall, litter accumulation and litter decomposition rates in four forest ecosystems in northern Greece. For Ecol Manage, 144: 113-127. DOI:10.1016/S0378-1127(00)00365-0 |
Bubb K A, Xu Z H, Simpson J A. 1998. Some nutrient dynamics associated with litterfall and litter decomposition in hoop pine plantations of southern Queensland, Australia. For Ecol Manage, 110: 343-352. DOI:10.1016/S0378-1127(98)00295-3 |
Caldentey J, Ibrarra M, Hernandez J. 2001. Litter fluxes and decomposition in Nothofagus pumilio stands in the region of Magallanes, Chile. For Ecol Manage, 148: 145-157. DOI:10.1016/S0378-1127(00)00532-6 |
Couteaux M M, Bottner P, Berg B. 1995. Litter decomposition, climate and litter quality. Trend Ecol Evol, 10: 63-66. DOI:10.1016/S0169-5347(00)88978-8 |
Edmonds R L, Thomas T B. 1995. Decomposition and nutrient release from green needles of western hemlock and Pacific silver fir in an old-growth temperate rain forests. Olympic National Park Washington. Can J For Res, 25: 1049-1057. DOI:10.1139/x95-115 |
Gallardo A, Merino J. 1999. Control of leaf litter decomposition in a Mediterranean shrubland as indicated by N, P and lignin concentrations. Pedobiologia, 43: 64-72. |
Gholz H L, Perry C S, Cropper W P. 1985. Litterfall, decomposition, and nitrogen and phosphorus dynamics in a chronosequence of Slash Pine (Pinus elliottii) plantations. For Sci, 31(2): 463-478. |
Jamaa B A, Nair P K R. 1996. Decomposition and nitrogen mineralization patterns of Leucaena leucocephala and Cassia siamea mulch under tropical semiarid conditions in Kenya. Plant and Soil, 179: 275-285. DOI:10.1007/BF00009338 |
Jamaludheen V, Kumar B M. 1999. Litter of multipurpose trees in Kerala, India: variations in the amount, quality, decay rates and release of nutrients. For Ecol Manage, 115: 1-11. DOI:10.1016/S0378-1127(98)00439-3 |
Kavvadias V A, Alifragis D, Tsiontsis A, et al. 2001. Litterfall, litter accumulation and litter decomposition rates in four forest ecosystems in northern Greece. For Ecol Manage, 144: 113-127. DOI:10.1016/S0378-1127(00)00365-0 |
Kumar B M, Deepu J K. 1992. Litter production and decomposition dynamics in moist deciduous forests of the Western Ghats in Peninsular-India. For Ecol Manage, 50: 181-201. DOI:10.1016/0378-1127(92)90335-7 |
Lisanework N, Michelsen A. 1994. Litterfall and nutrient release by decomposition in three plantations compared with a natural forest in the Ethiopian highland. For Ecol Manage, 65: 149-164. DOI:10.1016/0378-1127(94)90166-X |
Louzada J N C, Schoereder J H, Marco P D. 1997. Litter decomposition in semideciduous forest and Eucalyptus spp. Crop in Brazil: a comparison. For Ecol Manage, 94: 31-36. DOI:10.1016/S0378-1127(96)03986-2 |
Olson J S. 1963. Energy storage and the balance of producers and decomposers in ecological systems. Ecology, 44: 323-331. |
Pedersen L B, Hansen J B. 1999. A comparison of litterfall and element fluxes in even aged Norway spruce, sitka spruce and beech stands in Denmark. For Ecol Manage, 114: 55-70. DOI:10.1016/S0378-1127(98)00381-8 |
Singh K P, Singh P K, Tripathi S K. 1999. Litterfall, litter decomposition and nutrient release patterns in four native tree species raised on coal mine spoil at Singrauli, India. Biol Fertil Soils, 29: 371-378. DOI:10.1007/s003740050567 |
Taylor B R, Parkinson D, Parsons W F J. 1989. Nitrogen and lignin content as predictor of litter decay rates: a microcosm test. Ecology, 70: 97-104. DOI:10.2307/1938416 |
Waring R H, Schlesinger W H. 1985. Forest ecosystem concepts and management. Orlando: Academic Press, INC, 181-210.
|
 2004, Vol. 40
2004, Vol. 40