文章信息
- Li Rongjin, Zhang Min, Jiang Zeping, Huang Libin, Wang Baosong, Ji Yonghua, Fang Yanming
- 李荣锦, 张敏, 蒋泽平, 黄利斌, 王宝松, 季永华, 方炎明
- Effect of NaCl Stress on Antioxidant Enzyme Activities and Isoenzyme Pattern of Broussonetia papyrifera Plantlets
- NaCl胁迫对构树组培苗抗氧化酶活性及同工酶谱的影响
- Scientia Silvae Sinicae, 2009, 45(2): 40-47.
- 林业科学, 2009, 45(2): 40-47.
-
文章历史
- 收稿日期:2009-07-10
-
作者相关文章
2. 江苏省林业科学研究院 南京 211153
2. Jiangsu Academy of Forestry Nanjing 211153
Salinitization of soil is becoming a critical environmental problem. It was reported that about 20% of the world's cultivated land and nearly half of all irrigated lands are affected by salinity (Rhoades et al., 1990). Salinity severely limits vegetative and reproductive growth of plants by inducing severe physiological dysfunctions and causing widespread direct and indirect harmful effects (Shannon et al., 1994). The stress induced by salinity on plants is the results of two mechanisms: hyperosmotic stress, which causes water deficit and reduces water adsorption, and ion imbalance resulting in accumulation of Na+ and Cl+ (Beritognolo, 2007). As a consequence of these primary effects, secondary stresses such as oxidative damage often occur (Zhu, 2001).
In recent years, considerable attention has been drawn to mechanisms enabling plants to cope with salt stress. Several studies have established the relationship between metabolic changes and salt stress (Adams et al.., 1992; Delauny et al., 1993). Elavumoottil et al. (2003) reported that callus and cell suspension cultures of Brassica olerace. accumulated sucrose and reducing sugars to cope with NaCl treatment. Woody plants may adapt to salinity by variously tolerating or avoiding salt, or both. In some plants, osmotic adjustment results from synthesis in the cytoplasm of compatible organic solutes including proline and other amino acids in addition to sugars (Kozlowski, 1997). Other researchers have aimed to identify components involved in salt-stress tolerance by identifying mRNAs and proteins selectively induced under such condition (Claes et al., 1990; Holland et al., 1993). And a lot of salt stress induced proteins have been found in many plant species (Sadka et al., 1991; Bishnoi et al., 2006; Elavumoottil et al., 2003).
An important cause of damage that high salt concentrations inflict on plants might be reactive oxygen species (ROS) generated by salt stress (Zhu, 2001). These reactive oxygen species are highly deleterious for cell structures and functions (Hideg et al., 1996; Foyer et al., 1997), thus they need to be scavenged to prevent the harmful effects caused by these stresses. A major safeguard mechanism against free radicals is provided by superoxide dismutase (SOD), which catalyzes the conversion of O-2 to H2O2 and then H2O2 is decomposed in the presence of catalase (CAT) and peroxidase (POD) (Guo et al., 2004). A large body of evidence has accumulated from various plant systems showing that drought and salt stress alter the amounts and activities of enzymes involved in scavenging oxygen radicals (Gueta-Dahan et al., 1997; Zhang et al., 1994).
Broussonetia papyrifera widely distributes in China. The roots, leaves and fruits are used in folkloric medicine for treating many diseases. It is also an excellent and financial plant for making paper and firewood, feeding stuff B. papyrifera adapts to most situations and can be used in sprigging operation. A previous study showed that this plant species had a moderate degree of salt tolerance (Wang et al., 2004).
A better understanding of the mechanisms that enable plants to adapt to salt stress could help in selecting salt resistant species. In the present study, the responding mechanism of plants to salt stress was investigated using the plantlets of B. papyrifera. The effect of salinity on the content of total soluble protein and the activities of antioxidant enzyme were analyzed. In addition, the accumulation of malondialdehyde (MDA) and proline were also measured.
1 Materials and methods 1.1 Plant material and NaCl treatmentsCallus culture was induced from scion of B. papyrifera. and then the calli were transferred to bud-inducing medium (MS medium supplemented with 1 mg·L-1 6-BA, 0.1 mg·L-1 IBA, 30 g·L-1 sucrose and 6.5 g·L-1 argrose, pH 5.8). The obtained plantlets were maintained by transferring an inoculum to fresh medium. Salt stress was carried out by transferring the plantlets to the same medium with the addition of 50, 75, 100, 125, 150 mmol·L-1 NaCl. All the cultures were incubated at 25 ℃ and photoperiod of 16 h. Plantlets were harvested on Whatman No. 1 paper filters to soak up the water, immediately frozen in liquid nitrogen and stored at-80 ℃ until use. For the determination of plant dry weight, the plantlets were dried at 70 ℃ until the constant mass was reached.
1.2 Extraction of proteinsFrozen material (1 g fresh weight, FW) was homogenized with an ice-cooled mortar and pestle in 5 mL of potassium phosphate buffer (pH 7.5) containing 1 mmol·L-1 EDTA, 3 mmol·L-1 DTT and 5% (w/v). insoluble PVP (Pereira et al., 2002). The homogenate was then centrifuged at 10 000 g 4 ℃ for 20 min. Total soluble protein was determined by the method of Bradford (1976) using bovine serum albumin as a standard and protein content was expressed as mg protein·g-1 FW.
1.3 Enzyme activity assaysFor enzyme assay, frozen plant materials were homogenized with phosphate buffer (pH 6.8) in a pre-cooled mortar and pestle. The homogenate was filtered through two layers of muslin cloth, and centrifuged at 10 000 g for 20 min at 4 ℃. The supernatants were used for the assays of superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) activity.
SOD activity was determined using the spectrophotometric method according to Cho and Park (2000). The reaction medium comprised 50 mmol·L-1 sodium phosphate buffer (pH 7.8), 0.1 mmol·L-1 EDTA-Na2, 2.2 μmol·L-1 riboflavin, 14.3 mmol·L-1 methionine, and 82.5 μmol·L-1 nitroblue tetrazolium (NBT) and addition of the enzyme extract. One unit of SOD activity was defined as the amount of enzyme required to produce a 50% inhibition of NBT reduction measured at 560 nm.
PODactivity was measured using the procedure of MacAdam et al. (1992) with minor modification. The enzyme activity was measured with guaiacol as the substrate. The oxidation of guaiacol into tetraguaiacol was measured by the increase in absorbance at 470 nm. Enzyme activity was calculated in terms of mmol of guaiacol oxidized min-1 mg-1 protein (Guo et al., 2004).
The activity of CAT was analyzed following Tang et al. (1984) by measuring the consumption of H2O2 at 240 nm using a UV spectrophotometer. Briefly, the reaction mixture contained 1.5 mL 50 mmol·L-1 sodium phosphate buffer (pH 7.0) and 1 mL 0.2% H2O2, and the reaction was initiated by adding 500 μL of the enzyme extract. The results were expressed as U·mg-1 protein.
1.4 PAGE analysis of antioxidant enzymesThe electrophoresis was performed under native condition in 10% polyacrylamide mini-gels for SOD, POD and CAT activity staining. Electrophoresis running was conducted at 4 ℃. Equal amounts of protein were loaded on to each lane.
SOD activity was determined on native PAGE gels according to Pereira et al. (2002). Briefly, the gels were washed with distilled and incubated in the dark for 30 min at room temperature in 50 mmol·L-1 potassium phosphate buffer (pH 7.8) containing 1 mmol·L-1 EDTA, 0.05 mmol·L-1 riboflavin, 0.1 mmol·L-1 nitroblue tetrazolium and 0.3% (v/v) N, N, N′′, N′′-tetramethylethylenediamine (TEMED). At the end, the gels were rinsed with distilled water and placed in distilled-deionized water and exposed to light for 10 min until the bands of SOD activity was visible.
Staining for peroxidase was achieved following the method described by Khedr et al. (2000). Gels were washed with distilled water and then incubated in 1mmol·L-1 3-amino-9-ethyl carbazole in 100 mmol·L-1 acetate buffer (pH 5.0) for 45 min. After incubation, the gels were soaked in 0.3% (v/v) H2O2 and allowed to stand for a few minutes at room temperature until the bands appeared.
CAT isoenzymes were separated on nondenaturating acrylamide gels following the procedure of Jebara et al. (2005). Gels were washed with distilled water for twice, and then soaked in 0.3% H2O2 for 10 min. After a brief rinse, gels were incubated in a solution of 2% potassium ferricyanide and 2% ferric chloride until the bands appeared.
1.5 Measurement of malondialdehyde (MDA)MDA content was measured by the method of Heath et al. (1968).
1.6 Determination of prolineProline concentration was determined as described by Guo et al. (2004). In brief, approximately 0.5 g of frozen plant material was ground in 5 mL 3% sulfosalicylic acid to extract free proline. 2 mL of the extract was mixed with 2 mL water and then reacted with 2 mL glacial acetic acid and 4 mL 2.5% (w/v) acid ninhydrin (dissolved in 3:2 glacial acetic acid and 6 mol·L-1 phosphoric acid, V:V) at 100 ℃ for 1 h. After being cooled, the reaction mixture was extracted with toluene and optical density was measured at 520 nm.
1.7 Statistical analysisStatistical analysis was carried out by one-way ANOVA using SPSS 10.0 software to determine the different significance. When the ANOVA was significant at P < 0.05, the Duncan multiple range test was used for mean comparison. Data presented were mean ± SD of three experiments.
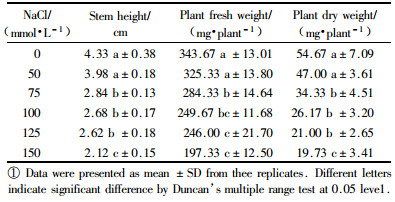
2 Results 2.1 Effect of NaCl on the growth of B. papyriferaThe data in Tab. 1 showed that stem height, plant fresh weight and dry matter accumulation decreased significantly with increasing of salinity. The rate of decline in these growth parameters was greater in higher salinity. These results indicated that the growth of B. papyrifera was inhibited by salt stress.
|
|
The content of total soluble protein in whole plantlet was examined. As shown in Fig. 1, the protein content increased with increasing of NaCl concentrations until 100 mmol·L-1 NaCl. And then the protein content decreased when NaCl concentration was elevated to 125 and 150 mmol·L-1. The maximum increase in protein content was found at 100 mmol·L-1 NaCl (4.92 mg·g-1 FW), and it was increased by 17.88% as compared to control (4.04 mg·g-1 FW).
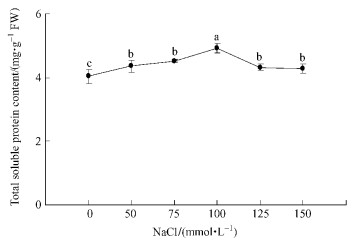
|
Fig.1 Effects of salt stress on protein content of Broussonetia papyrifera Each measurement was conducted with three replicates, and data were mean ±SD. Different letters indicate significant difference by Duncan's multiple range test at 0.05 leve1. FW represents fresh weight. The same below. |
SOD, POD and CAT are important antioxidant enzymes. In our study the activities of these three enzymes under salt stress were investigated. The total SOD activity was slightly decreased at low concentration of NaCl. SOD activity increased when NaCl concentration reached 75 mmol·L-1, and then the activity was declined but it was still higher than the control by 100 mmol·L-1. Thereafter the enzyme activity significantly increased with the increasing of NaCl concentration (Fig. 2A). In Fig. 2B, the isoenzyme pattern of SOD showed six bands in plantlets of control and treatment with 50 mmol·L-1 NaCl. However, band V disappeared when NaCl concentration increased. Moreover, gel staining analysis also demonstrated that SOD activity increased dramatically with increasing of NaCl concentration.
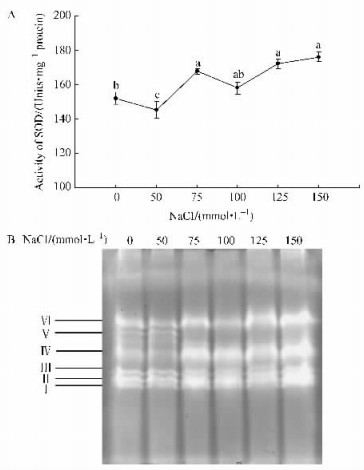
|
Fig.2 Effect of salt stress on superoxide dismutase(SOD) activity and isoenzyme patterns in plantlets of B. papyrifera (A) SOD activity measured by spectrophotometric method; (B) Gel staining analysis. |
Like SOD activity, salt stress resulted in an increase of POD activity especially at 150 mmol·L-1 NaCl, which increased the enzyme activity by more than 162% compared to the control (Fig. 3A). There were three bands appeared on the gel of POD staining, and the three bands were differentially regulated by stress treatments (Fig. 3B).
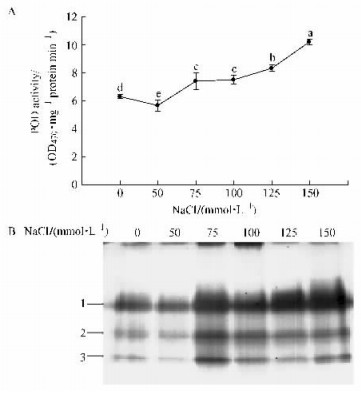
|
Fig.3 Changes in peroxidase (POD) activity and isoenzyme patterns of B. papyrifera plantlets after treatment with NaCl (A) POD activity measured by spectrophotometric method; (B) Gel staining analysis. |
Activity gels showed that there was only one catalase band. The band intensity, as a measure of CAT activity, showed the same trend as the spectrophotometric measurements (Fig. 4 A and B), which may indicate that this band is probably responsible for most of the change in catalase activity.
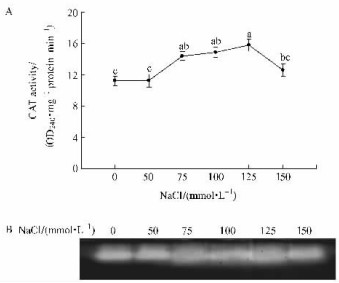
|
Fig.4 Changes in catalase (CAT) activity and isoenzyme patterns in plantlets of B. papyrifera (A) CAT activity measured by spectrophotometric method; (B) Gel staining analysis. |
Membrane lipid peroxidation was examined by measuring MDA content in plantlets with salt stress and control. As shown in Fig. 5, the MDA content increased until 100 mmol·L-1 salinity and then declined. The increase of MDA content was more pronounced at 100 mmol·L-1 NaCl, and it was increased by about 80% compared to control.
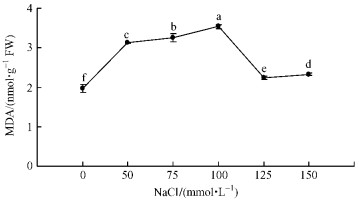
|
Fig.5 Effect of salt stress on MDA content |
The results shown in Fig. 6 demonstrated that the internal proline content increased in response to salt stress. The accumulation of proline in plantlets of B. papyrifera. was in a concentration-dependent manner. The increase of proline content was more evident when treated with higher concentration of NaCl (100~150 mmol·L-1). The accumulation of proline in response to 150 mmol·L-1 NaCl was maximal, and it was increased by 300% compared to control.
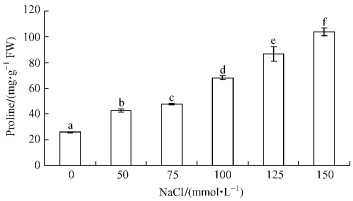
|
Fig.6 Proline contents of B. papyrifera plantlets after NaCl treatment Data represent mean ±SD (n=3). |
In order to learn more about the response of woody plants to salt stress, we examined the physiological and biochemical changes in plantlets of B. papyrifera under NaCl stress. We found that NaCl treatment caused a lot of changes in plant growth, protein content, antioxidant enzyme activities and the content of proline and MDA.
The growth of B. papyrifera plantlets including stem height, fresh weight and dry weight was reduced by salt-stress (Tab. 1). The effect of salt stress on soluble protein content manifested a concentration-dependent manner. At lower levels of NaCl, the protein content increased with elevation of NaCl concentration, but higher concentrations caused it to decline. This suggested that protein synthesis was increased in the initial response to salt stress but it was prevented when the stress became too severe. Accumulation of soluble proteins in plants grown under saline condition has been reported previously. The accumulated proteins may provide a storage form of nitrogen which could be reutilized when stress was over (Singh et al., 1987), and they may play a critical role in osmotic adjustment (Qasim et al., 2003). These proteins may be synthesized de nov in response to salt stress or the expression of formerly present proteins is increased when plants are exposed to salt stress.
The accumulation of reactive oxygen species (ROS) may be an important cause of damage to plants under salt stress (Zhu, 2001). Plant cells are equipped with several free radical detoxifying enzymes to protect them against oxidative damage. These enzymes include superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) etc (Chien et al., 2001). Many studies have shown that environmental stresses, especially drought and salt stress, altered that activities of enzymes involved in scavenging oxygen radicals (Gueta-Dahan et al., 1997; Khedr et al., 2003; Zhang et al., 1994). In this study, SOD activity showed an increase with the increasing of NaCl concentration, and the SOD isoforms were affected by salt treatment. Like superoxide dismutase, peroxidase exhibited a consistent increase in enzyme activity even under severe salt stress. However, the isoforms of POD were slightly changed under salt stress. We found there was only one band in activity gels of CAT, and the band intensity showed the same trend as the spectrophotometric measurements, which may indicate that this band is probably responsible for most of the change in catalase activity.
MDA is an oxidized product of membrane lipids which reflect the extent of oxidative stress. In the present study, we observed that MDA content increased at low levels of salinity and then decreased at 125 mmol·L-1 and 150 mmol·L-1 NaCl concentration. The decrease of MDA content probably results from up-regulation of the antioxidative system in response to salt stress. Similar results correlating lipid peroxidation to antioxidative system activity were also reported by other researchers (Azevedo-Neto et al., 2006; Hernández et al., 2002). With Regard to MDA content reduction, our results were in agreement with those of Azevedo-Neto et al. (2006). These authors suggested the reduction of MDA content was due to increased antioxidative enzyme activities, which reduced H2O2 levels and membrane damage. In our study, the decrease of MDA concentration appeared to be associated with increase in SOD and POD activities, which could lead to reduction of H2O2 concentration and subsequent lipid peroxidation.
Plants under salt stress also need to establish water or osmotic homeostasis. Plants accumulate various compatible osmolytes in the cytosol, thus lowering the osmotic potential to sustain water absorption from saline soil solutions (Zhu et al., 1997). It has been reported that several compounds such as proline and polyamines increased with salt stress (Le Dily et al., 1991; Nanjo et al., 1999). It has been suggested that proline protects plant tissues against osmotic stress because it is an osmosolute and a protectant for enzymes and cellular structures (Stewart et al., 1974; Le-Rudulier et al., 1984). In our study, there was a positive relationship between salt concentration and proline content of tissues. Proline content significantly increased in tissues with increase in salinity. Our result was in consistent with the data reported by Patel et al. (2007). The concomitant increase in proline content of the tissues indicates that proline accumulation may contribute to the alleviation of NaCl stress in the plant. A negative relationship between salt tolerance and proline accumulation was also reported (Soussi et al., 1998; Petrusa et al., 1997). These studies assumed that the accumulation of proline was more a consequence of damage produced by salt stress than of a protective strategy. In the past, most attention has been concerned with the role of proline as a compatible osmolyte (Yancey et al., 1982; Samaras et al., 1995) and osmoprotectant (MacCue et al., 1990; Serrano et al., 1994). However, Nanjo et al. (1999) suggested that in addition to various known roles of proline, it was also involved in the synthesis of key proteins that are necessary for stress responses. In a recent study, Khedr et al. (2003) reported that proline could increase ubiquitin-conjugates and induce the expression of dehydrins in Pancratium maritimum. They came to the conclusion that, proline improved the salt-tolerance of P. maritimu by protecting the protein turnover machinery against stress-damage and up-regulating stress protective proteins. Proline could act as a component of signal transduction pathways that regulate stress responsive genes in addition to its previously described osmoprotective roles, thereby improving the tolerance to salt stress.
In conclusion, our study indicated that salt tolerance occurred in plantlets of B. papyrifera. Salt stress induced oxidative stress and the enhanced activity of anti-oxidative enzymes (SOD, POD and CAT) and accumulation of proline may be responsible for the defenses against this oxidative stress. Our findings may be helpful to understand the physiological and biochemical mechanisms of salt stress adaptation of B. papyrifera.
Adams P, Thomas J C, Vernon D M, et al. 1992. Distinct cellular and organismic responses to salt stress. Plant Cell Physiology, 33: 1215-1223. |
Azevedo-Neto A D, Prisco J T, Eneas J, et al. 2006. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and saltsensitive maize varieties. Environmental and Experimental Botany, 56: 87-94. DOI:10.1016/j.envexpbot.2005.01.008 |
Bates L S, Waldren R P, Teare I D. 1973. Rapid determination of free proline for water stress studies. Plant and Soil, 39: 205-207. DOI:10.1007/BF00018060 |
Beritognolo I, Piazzai M, Benucci S, et al. 2007. Functional characterization of three Italian Populus alba genotypes under salinity stress. Trees, 21: 465-477. DOI:10.1007/s00468-007-0139-x |
Bishnoi S K, Kumar B, Rani C, et al. 2006. Changes in protein profile of pigeonpea genotypes in response to NaCl and boron stress. Biologia Plantarum, 50: 135-137. DOI:10.1007/s10535-005-0088-4 |
Bradford M M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72: 248-254. DOI:10.1016/0003-2697(76)90527-3 |
Chien H F, Wang J W, Lin C C, et al. 2001. Cadmium toxicity of rice leaves is mediated through lipid peroxidation. Plant Growth Regulation, 33: 205-213. DOI:10.1023/A:1017539616793 |
Cho U H, Park J O. 2000. Mercury-induced oxidative stress in tomato plantlets. Plant Science, 156: 1-9. DOI:10.1016/S0168-9452(00)00227-2 |
Claes B, Dekeyser R, Villarroel R, et al. 1990. Characterization of a rice gene showing organ-specific expression in response to salt stress and drought. Plant Cell, 2: 19-27. |
Delauny A J, Verma D P S. 1993. Proline biosynthesis and osmo-regulation in plants. Plant Journal, 4: 215-223. DOI:10.1046/j.1365-313X.1993.04020215.x |
Elavumoottil O C, Martin J P, Moreno M L. 2003. Changes in sugars, sucrose synthase activity and proteins in salinity tolerant callus and cell suspension cultures of Brassica oleracea L. Biologia Plantarum, 46: 7-12. DOI:10.1023/A:1022389428782 |
Foyer C H, Delgado H L, Dat J F, et al. 1997. Hydrogen peroxide and glutathione associated mechanisms of acclamatory stress tolerance and signaling. Physiologia Plantarum, 100: 241-254. DOI:10.1111/ppl.1997.100.issue-2 |
Gueta-Dahan Y, Yaniv Z, Zilinskas B A, et al. 1997. Salt and oxidative stress: similar and specific responses and their relation to salt tolerance in Citrus. Planta, 203: 460-469. DOI:10.1007/s004250050215 |
Guo T R, Zhang G P, Zhou M X, et al. 2004. Effects of aluminum and cadmium toxicity on growth and antioxidant enzyme activities of two barley genotypes with different Al resistance. Plant and Soil, 258: 241-248. DOI:10.1023/B:PLSO.0000016554.87519.d6 |
Heath R L, Packer L. 1968. Photooxidation in chloroplast: kinetics and stoichiometry of fatty acid oxidation. Archives of Biochemistry and Biophysics, 125: 189-198. DOI:10.1016/0003-9861(68)90654-1 |
Hernández J A, Jiménez A, Mullineaux P, et al. 2000. Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defences. Plant Cell Environment, 23: 853-862. DOI:10.1046/j.1365-3040.2000.00602.x |
Hideg E, Vass I. 1996. UV-B induced free radical production in plant leaves and isolated thylakoid membranes. Plant Science, 115: 251-260. DOI:10.1016/0168-9452(96)04364-6 |
Holland D, Ben Hayyim G, Faltin Z, et al. 1993. Molecular characterization of salt-stress-associated protein in citrus: protein and cDNA sequence homology to mammalian glutathione peroxidases. Plant Molecular Biology, 21: 923-927. DOI:10.1007/BF00027124 |
Hurkman W J, Tanaka C K. 1987. The effects of salt on the pattern of protein synthesis in barley roots. Plant Physiology, 83: 517-524. DOI:10.1104/pp.83.3.517 |
Jebara S, Jebara M, Limam F, et al. 2005. Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. Journal of Plant Physiology, 162: 929-936. DOI:10.1016/j.jplph.2004.10.005 |
Khedr A H A, Abbas M A, Wahid A A A, et al. 2003. Proline induces the expression of salt-stress-responsive proteins and may improve the adaptation of Pancratium maritimum L. to salt-stress. Journal of Experimental Botany, 54: 2553-2562. DOI:10.1093/jxb/erg277 |
Kozlowski T T. 1997. Responses of woody plants to flooding and salinity. Tree Physiology Monograph, 1: 1-29. |
Laemmli U K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227: 680-685. DOI:10.1038/227680a0 |
Le Dily F, Billard J P, Boucaud J. 1991. Polyamine levels in relation to growth and NaCl concentration in normal and habituated sugar beet callus cultures. Plant Cell and Environment, 14: 327-332. DOI:10.1111/pce.1991.14.issue-3 |
Le-Rudulier D, Strom A R, Dandekar A M, et al. 1984. Molecular biology of osmoregulation. Science, 224: 1064-1068. DOI:10.1126/science.224.4653.1064 |
MacAdam J W, Nelson C J, Sharp R E. 1992. Peroxidase activity in the leaf elongation zone of tall fescue. Plant Physiology, 99: 872-878. DOI:10.1104/pp.99.3.872 |
MacCue K F, Hanson A D. 1990. Drought and salt tolerance. Trends in Biotechnology, 8: 358-362. DOI:10.1016/0167-7799(90)90225-M |
Nanjo T, Kobayashi M, Yoshiba Y, et al. 1999. Biological functions of proline in morphogenesis and osmotolerance revealed in antisense transgenic Arabidopsis theliana. Plant Journal, 18: 185-193. DOI:10.1046/j.1365-313X.1999.00438.x |
Patel A D, Pandey A N. 2007. Effect of soil salinity on growth, water status and nutrient accumulation in seedlings of Cassia montana (Fabaceae). Journal of Arid Environments, 70: 174-182. DOI:10.1016/j.jaridenv.2006.12.004 |
Pereira G J G, Molina S M G, Lea P J, et al. 2002. Activity of antioxidant enzymes in response to cadmium in Crotalaria juncea. Plant and Soil, 239: 123-132. DOI:10.1023/A:1014951524286 |
Petrusa L M, Winicov L. 1997. Proline status in salt tolerant and salt sensitive alfalfa cell lines and plants in response to NaCl. Plant Physiology and Biochemistry, 35: 303-310. |
Qasim M, Ashraf M, Ashraf M Y, et al. 2003. Salt-induced changes in two canola cultivars differing in salt tolerance. Biologia Plantarum, 46: 629-632. DOI:10.1023/A:1024844402000 |
Rhoades J D, Loveday J. 1990. Salinity in irrigated agriculture//Steward B A, Nielsen D R. American Society of Civil Engineers, Irrigation of Agricultural Crops: Monograph 30. American Society of Agronomists, 1089-1142.
|
Sadka A, Himmelhoch S, Zamir A. 1991. A 150 kilodalton cell surface protein is induced by salt in the halotolerant green alga Dunaliella salina. Plant Physiology, 95: 822-831. DOI:10.1104/pp.95.3.822 |
Samaras Y, Bressan R A, Csonka L N, et al. 1995. Proline accumulation during drought and salinity//Smirnoff N. Environment and plant metabolism: flexibility and accumulation. Oxford, UK: Bios Scientific Publishers, 161-187.
|
Serrano R, Gaxiola R. 1994. Microbial models and stress tolerance in plants. Critical Review of Plant Science, 13: 121-138. DOI:10.1080/07352689409701911 |
Shannon M C, Grieve C M, Francois L E. 1994. Whole-plant response to salinity//Wilkinson R E. Plant-environment interactions. Marcel Dekker, New York, 199-244.
|
Singh N K, Bracken C A, Hasegawa P M, et al. 1987. Characterization of osmotin, a thaumatin-like protein associated with osmotic adjustment in plant cells. Plant Physiology, 85: 529-536. DOI:10.1104/pp.85.2.529 |
Sousa M F, Campos F A P, Prisco J T, et al. 2003/4. Growth and protein pattern in cowpea plantlets subjected to salinity. Biologia Plantarum, 47: 341-346. https://link.springer.com/article/10.1023/B:BIOP.0000023875.63226.67
|
Soussi M, Ocaña A, Lluch C. 1998. Effects of salt stress on growth, photosynthesis and nitrogen fixation in chick-pea (Cicer arietinum L.). Journal of Experimental Botany, 49: 1329-1337. DOI:10.1093/jxb/49.325.1329 |
Sreenivasulu N, Grimm B, Wobus U, et al. 2000. Differential response of antioxidant compounds to salinity stress in salt-tolerant and salt-sensitive plantlets of foxtail millet (Setaria italica). Physiologia Plantarum, 109: 435-442. DOI:10.1034/j.1399-3054.2000.100410.x |
Stewart G R, Lee J A. 1974. The role of proline accumulation in halophytes. Planta, 120: 279-289. DOI:10.1007/BF00390296 |
Tang D, Shi S, Li D, et al. 2007. Physiological and biochemical responses of Scytonema javanicum (cyanobacterium) to salt stress. Journal of Arid Environments, 71: 312-320. DOI:10.1016/j.jaridenv.2007.05.004 |
Wang Y X, Liu J, Qiao L Q, et al. 2004. Evaluation and selection of salt tolerance of 41 introduced species of trees. Journal of Northwest Forestry University, 19: 55-58. |
Yancey P H, Clark M E, Hand S C, et al. 1982. Living with water stress: evolution of osmolyte systems. Science, 217: 1214-1222. DOI:10.1126/science.7112124 |
Zhang J X, Kirkham M B. 1994. Drought-stress-induced changes in activity of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiology, 35: 785-791. DOI:10.1093/oxfordjournals.pcp.a078658 |
Zhu J K, Hasegawa P M, Bressan R A. 1997. Molecular aspects of osmotic stress in plants. Critical Reviews in Plant Sciences, 16: 253-277. DOI:10.1080/07352689709701950 |
Zhu J K. 2001. Plant salt tolerance. Trends Plant Science, 6: 66-71. |
 2009, Vol. 45
2009, Vol. 45