2. 内蒙古民族大学生命科学学院;
3. Bio-Synthesis, Inc.-DNA lab Department, Lewisville, TX, USA(美国德克萨斯州路易斯维尔百奥生生物科技公司
多溴联苯醚(polybrominated diphenyl ethers,PBDEs)是一种非常优秀的阻燃剂,有良好热稳定性与高效阻燃性,被广泛地应用于电子电器与建材等诸多行业,在家具、室内装潢中也广泛使用[1-2]。近年来,环境研究者发现PBDEs在鱼体内以及淤泥中大量蓄积[3-5],并且发现PBDEs在人体内同样蓄积,引起胎儿的畸形以及甲状腺功能的失调[6]。我国学者黄玉妹等[7]报道婴幼儿通过尘土摄入PBDEs量远大于成年人,其对婴幼儿的暴露水平也明显偏高。这种“母性影响”通过哺乳动物试验得到验证,母性染毒可引起子代神经毒性,以及相应的行为学变化[8]。这种神经毒性机理极有可能是由于母性甲状腺激素紊乱而影响了子代[9],或是PBDEs代谢产物直接转移到胎儿而影响了子代甲状腺素系统的形成于发育[10]。与胎儿或者婴幼儿相比,PBDEs对成人几乎没有影响,但希腊研究者Kalantzi等[11]监测到长期使用电脑的雇员体内血清中的PBDEs含量显著高于非使用者;生产PBDEs时,会在燃烧及高温状态下产生剧毒致癌物质多溴二苯并二英(polybrominated dibenzo-p-dioxin, PBDDs)和多溴二苯并呋喃(polybrominated dibenzofuran, PBDFs)[12]。鉴于PBDEs毒性的重要性,2009年5月,《斯德哥尔摩公约》第四次缔约方大会讨论并通过了将四溴联苯醚、五溴联苯醚、六溴联苯醚和七溴联苯醚列为新增的受控环境中持久性有机污染物(POPs)[13]。
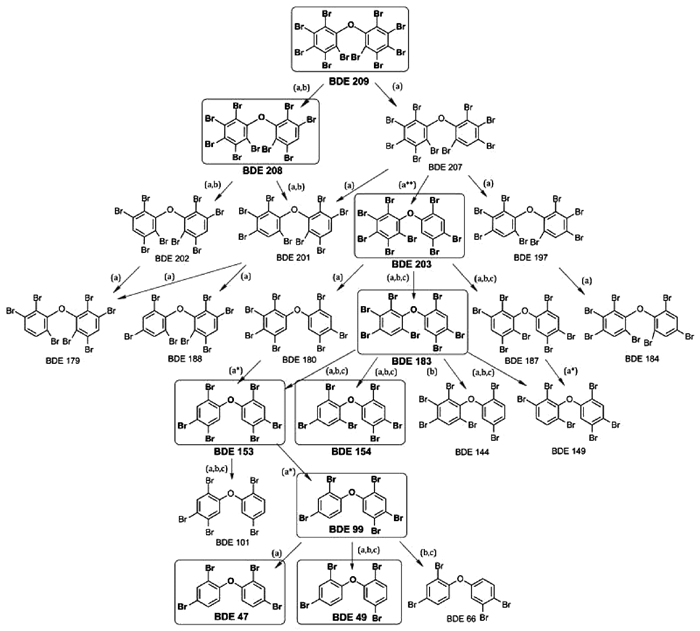
1 PBDEs的结构及在鱼体内的代谢PBDEs的结构是一种以溴为中心命名的化合物,按照所含有溴原子数目分为209种以上同类物质,PBDEs进入有机体内后会被代谢降解。PBDE 209在鱼体内代谢较为复杂(图 1)。其中十溴二苯谜(deca-BDEs)、八溴二苯谜(octa-BDEs)、六溴二苯谜(hexa-BDEs)、五溴二苯谜(pentr-BDEs)和四溴二苯谜(tetra-BDEs)等分别含有10,8,6,5和4个溴原子。其中最为引起研究者重视并且取得一定研究成果的是BDE 47、BDE 99以及BDE 209等多溴二苯谜[14]。
有关BDE 99,Pamela等[16]报道的更为详细,BDE 99在鱼体内降解为BDE 66 (占有比率为14%~23%)、BDE 47 (67%~80%)和BDE 49 (5%~6%),而BDE 66继续降解为BDE 33和BDE 28,BDE 47只是降解为BDE 28。
2 PBDEs的胚胎毒性作用近年来,很多学者使用斑马鱼作为模型动物来评价PBDEs毒性,观察PBDEs对胚胎初期的影响。毒性作用表现为免疫毒性以及神经毒性。PBDEs中6-OH-PBDE 47的毒性较强,影响主要表现为延迟胚胎发育、死亡、心囊水肿、尾部细胞死亡以及色素沉积减弱等毒性症状[17]。有关毒性机制不是很明了, Walpita等[18]使用基因敲出技术敲出DI2,引起色素沉着明显降低,但追加T3 50 nmol/L后,减弱的色素沉积被恢复。Stencel等[19]最新研究结果表明6-OH-BDE47能够与THR结合。此结果说明6-OH-BDE 47在动物体内与THR结合,使T3不能够与THR结合造成甲状腺素调节系统失调,也就是有机体不能得到应有的T3(T3需求不足),即产生正反馈,增高DI1和/或DI2的基因表达。我国研究人员He等[20]使用BDE 47给成神经细胞瘤(SH-SY5Y)染毒,发现4 μg/mL BDE 47增加细胞死亡率,引起细胞凋亡等细胞毒性和基因毒性。夏涛等[21]使用10 mg/kg·bw BDE 47给10 d大鼠染毒,发现大鼠神经系统损伤,BDE 47引起海马区钙信号诱导的凋亡相关基因mRNA表达水平,而PCB53可增强PBDE 47的神经毒性作用。Ma等[22]研究者调查神经管缺陷的西部农村,发现发病胎儿胎盘的PCBs(平均浓度为0.91 ng/g脂肪)和PBDEs(0.55 ng/g脂肪)浓度高于正常组胎儿胎盘。
DE 71是PBDE的混合物,含有BDE 47、BDE 100、BDE 99、BDE 153、BDE 154、BDE 28和BDE 183等物质,其中BDE 47的含量最高。使用DE 71给5个月龄成年斑马鱼(F0) 染毒,可引起子代(F1) T3和T4水平显著增高、孵化率降低以及生长发育抑制。继续给子代F1染毒,幼鱼畸形率显著高于未染毒组,Yu等[10]认为母体PBDEs以及甲状腺激素转移到了子代。
3 去碘酶的作用与基因表达去碘酶是调节甲状腺素含量的关键酶,PBDEs对去碘酶产生毒性作用。其中BDE 99及其代谢产物OH-BDEs能抑制人肝脏细胞去碘酶活性[23],人类甲状腺素中3, 5, 3’-triiodothyronine (T3) 会产生很大的影响。人类T3在细胞分化以及生长发育中起重要作用,如对早期胚胎的生长发育有重要影响[24-26],尤其是保障中枢神经系统发育[25-27]。但是T3的含量很少,T3来源是由thyroxine (T4) 经去碘酶作用去掉一个碘而生成。去碘酶在甲状腺素调节中非常关键。多数哺乳动物以及鱼类的T3也同样由T4经过一型去碘酶(DI1) 和二型去碘酶(DI2) 外环脱碘(ORD)或者内环脱碘(IRD)而成,T3能够与甲状腺素受体结合(thyroid hormone nuclear receptors, THR)[28]。与DI1和DI2相比,三型去碘酶DI3只是把T4转变成没有生物活性的反向T3 (reverse triiodothyronine, rT3)[29]。有关去碘酶的基因表达,DI1主要分布在肾脏、肝脏、以及甲状腺[30-33];DI2主要分布在大脑、垂体、胎盘、甲状腺、肌肉以及心脏等器官[34-40];DI3主要表达在大脑、子宫以及胎盘[30, 41-43]。与哺乳动物相比,硬骨鱼类DI1表达在肝脏、肾脏以及大脑[24, 44];DI2在大脑、肝脏、肾脏以及性腺,而没有发现在心脏和肌肉表达;DI3主要在腮弓表达[45]。
研究人员发现,不同的PBDEs染毒水平非常显著影响去碘酶的转录水平以及基因表达[46-48]。如使用0.625 ppm 2, 2, 4, 4’-tetrabromodiphenyl ether (BDE 47) 给24 hpf到28 hpf的斑马鱼染毒,DI1和DI2 mRNA显著被诱导上调[17];使用商业deca-BDE flame retardant (BDE 209) 给斑马鱼[49]或者中华鲟[50]染毒,同样上调DI1和/或DI2 mRNA表达。Stapleton等[23]使用BDE 99和BDE 209给人红细胞染毒也得到类似结果。与PBDE相比,PBDE的代谢产物羟化PBDE(hydroxylated PBDEs, OH-BDEs),具有比母体更强的毒性作用,给人肝脏培养细胞染毒,发现OH-PBDEs显著抑制碘酶活性[23]。Dong等[51]报道DI1和DI2在早期斑马鱼胚胎(22 hpf)头部脑室周边区域表达,而DI3在前肾管表达,并且发现6-OH-BDE显著上调DI1和DI2的基因表达。根据以上研究结果,有研究者使用去碘酶来作为生物学标记来评价环境污染物质。
PBDEs的分布检测逐渐受到研究人员的关注,但有关检测方法、详细分布状况还有待进一步加强研究。有关PBDEs及其羟化产物OH-BDEs的毒性以及对动物有机体的毒性机制,虽然与甲状腺系统以及神经系统干扰有一定关联,但作用机理还有待进一步研究。
| [1] | Schreiber T, Gassmann K, Gotz C, et al. Polybrominated diphenyl ethers induce developmental neurotoxicity in a human in vitro model:evidence for endocrine disruption[J]. Environ Health Perspect, 2010, 118(4): 572–578. |
| [2] | 刘汉霞, 张庆华, 江桂斌, 等. 多溴联苯醚及其环境问题[J]. 化学进展, 2005, 17(03): 554–562. doi: 10.3321/j.issn:1005-281X.2005.03.022 |
| [3] | Andersson ÖaB G. Polybrominated aromatic pollutants found in fish in Sweden[J]. Chemosphere, 1981: 1051–1060. |
| [4] | Watanabe IaT, R. Formation of brominated dibenzofurans from the photolysis of ame retardant decabromobiphenyl ether in hexane solution by UV and sunlight[J]. EnvironCont Toxicol, 1987, 39(6): 953–959. |
| [5] | de Wit CA. An overview of brominated flame retardants in the environment[J]. Chemosphere, 2002, 46(5): 583–624. doi: 10.1016/S0045-6535(01)00225-9 |
| [6] | Szabo DT, Richardson VM, Ross DG, et al. Effects of perinatal PBDE exposure on hepatic phase Ⅰ, phase Ⅱ, phase Ⅲ, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups[J]. Toxicol Sci, 2009, 107(1): 27–39. doi: 10.1093/toxsci/kfn230 |
| [7] | 黄玉妹, 陈来国, 许振成, 等. 家庭尘土中多溴联苯醚的含量及人体暴露水平研究[M]. 第五届全国环境化学大会摘要集2009, 大连. |
| [8] | Costa LG, Giordano G, Tagliaferri S, et al. Polybrominated diphenyl ether (PBDE) flame retardants:environmental contamination, human body burden and potential adverse health effects[J]. Acta bio-medica:Atenei Parmensis, 2008, 79(3): 172–183. |
| [9] | Harley KG, Marks AR, Chevrier J, et al. PBDE concentrations in women's serum and fecundability[J]. Environ Health Perspect, 2010, 118(5): 699–704. doi: 10.1289/ehp.0901450 |
| [10] | Yu L, Lam JC, Guo Y, et al. Parental transfer of polybrominated diphenyl ethers (PBDEs) and thyroid endocrine disruption in zebrafish[J]. Environ Sci Technol, 2011, 45(24): 10652–10659. doi: 10.1021/es2026592 |
| [11] | Kalantzi OI GT, Covaci A, Siskos PA. Distribution of polybrominated diphenyl ethers (PBDEs) and other persistent organic pollutants in human serum from Greece. Environ Int[J]. 2011, 37(2):349-353. http://www.sciencedirect.com/science/article/pii/S0160412010002047 |
| [12] | Thoma H HG, Knorr E. Polybrominated dibenzofurans (PBDF) and dibenzodioxins (PBDD) from the pyrolysis of neat brominated diphenylethers, biphenyls and plastic mixtures of these compounds[J]. Chemosphere, 1987, 16(1): 277–285. doi: 10.1016/0045-6535(87)90132-9 |
| [13] | 关于持久性有机污染物的斯德哥尔摩公约[M]. 缔约方大会第五次会, 2011, 25-29. |
| [14] | 魏爱雪, 王学彤, 徐晓白. 环境中多溴联苯醚类(PBDEs)化合物污染研究[J]. 化学进展, 2006, 18(9): 1227–1233. |
| [15] | Roberts SC, Noyes PD, Gallagher EP, et al. Species-specific differences and structure-activity relationships in the debromination of PBDE congeners in three fish species[J]. Environ Sci Technol, 2011, 45(5): 1999–2005. doi: 10.1021/es103934x |
| [16] | Imm P, Knobeloch L, Buelow C, et al. Household exposures to polybrominated diphenyl ethers (PBDEs) in a Wisconsin Cohort[J]. Environ Health Perspect, 2009, 117(12): 1890–1895. doi: 10.1289/ehp.0900839 |
| [17] | Usenko CY, Hopkins DC, Trumble SJ, et al. Hydroxylated PBDEs induce developmental arrest in zebrafish[J]. Toxicol Appl Pharmacol, 2012, 262(1): 43–51. doi: 10.1016/j.taap.2012.04.017 |
| [18] | Walpita CN, Van der Geyten S, Rurangwa E, et al. The effect of 3, 5, 3'-triiodothyronine supplementation on zebrafish (Danio rerio) embryonic development and expression of iodothyronine deiodinases and thyroid hormone receptors[J]. Gen Comp Endocrinol, 2007, 152(2-3): 206–214. doi: 10.1016/j.ygcen.2007.02.020 |
| [19] | Stencel K, Dong W, Akshay S, et al. Development of a fluorescence polarization, competitive receptor-ligand binding assay of thyroid hormone disruption by PBDEs and PCBs in zebrafish[M]. SETAC North America 33rd Annual Meeting, Long Beach, California 2012, Nov. 11-15:TP186. |
| [20] | He W, He P, Wang A, et al. Effects of PBDE-47 on cytotoxicity and genotoxicity in human neuroblastoma cells in vitro[J]. Mutat Res, 2008, 649(1-2): 62–70. doi: 10.1016/j.mrgentox.2007.08.001 |
| [21] | 夏涛, 何平, 王爱国, 等. PBDE-47和PCB153染毒致大鼠神经毒性作用[J]. 中国公共卫生, 2009, 25(5): 564–566. doi: 10.11847/zgggws2009-25-05-30 |
| [22] | Ma J, Qiu X, Ren A, et al. Using placenta to evaluate the polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) exposure of fetus in a region with high prevalence of neural tube defects[J]. Ecotoxicol Environ Saf, 2012, 86: 141–146. doi: 10.1016/j.ecoenv.2012.09.005 |
| [23] | Stapleton HM, Kelly SM, Pei R, et al. Metabolism of poly brominated diphenyl ethers(PBDEs) by human hepatocytes in vitro[J]. Environ Health Perspect, 2009, 117(2): 197–202. doi: 10.1289/ehp.11807 |
| [24] | Butt CM, Wang D, Stapleton HM. Halogenated phenolic contaminants inhibit the in vitro activity of the thyroid-regulating deiodinases in human liver[J]. Toxicol Sci, 2011, 124(2): 339–347. doi: 10.1093/toxsci/kfr117 |
| [25] | Chan S, Kachilele S, Hobbs E, et al. Placental iodothyronine deiodinase expression in normal and growth-restricted human pregnancies[J]. The Journal of Clinical Endocrinology and Metabolism, 2003, 88(9): 4488–4495. doi: 10.1210/jc.2003-030228 |
| [26] | Johnson KM, Lema SC. Tissue-specific thyroid hormone regulation of gene transcripts encoding iodothyronine deiodinases and thyroid hormone receptors in striped parrotfish (Scarus iseri)[J]. Gen Comp Endocrinol, 2011, 172(3): 505–517. doi: 10.1016/j.ygcen.2011.04.022 |
| [27] | Calvo RM, Jauniaux E, Gulbis B, et al. Fetal tissues are exposed to biologically relevant free thyroxine concentrations during early phases of development[J]. The Journal of Clinical Endocrinology and Metabolism, 2002, 87(4): 1768–1777. doi: 10.1210/jcem.87.4.8434 |
| [28] | Kilby MD, Gittoes N, McCabe C, et al. Expression of thyroid receptor isoforms in the human fetal central nervous system and the effects of intrauterine growth restriction[J]. Clin Endocrinol (Oxf), 2000, 53(4): 469–477. doi: 10.1046/j.1365-2265.2000.01074.x |
| [29] | Fisher DA. Thyroid function in very low birthweight infants[J]. Clin Endocrinol (Oxf), 1997, 47(4): 419–421. doi: 10.1046/j.1365-2265.1997.3021106.x |
| [30] | Kohrle J. The deiodinase family:selenoenzymes regulating thyroid hormone availability and action[J]. Cell Mol Life Sci, 2000, 57(13-14): 1853–1863. |
| [31] | Darras VM, Van Herck SL. Iodothyronine deiodinase structure and function:from ascidians to humans[J]. The Journal of endocrinology, 2012, 215(2): 189–206. doi: 10.1530/JOE-12-0204 |
| [32] | Richard K, Hume R, Kaptein E, et al. Ontogeny of iodothyronine deiodinases in human liver[J]. The Journal of clinical endocrinology and metabolism, 1998, 83(8): 2868–2874. |
| [33] | Visser TJ, Kaptein E, Terpstra OT, et al. Deiodination of thyroid hormone by human liver[J]. J Clin Endocrinol Metab, 1988, 67(1): 17–24. doi: 10.1210/jcem-67-1-17 |
| [34] | Brtko J, Bobalova J, Podoba J, et al. Thyroid hormone receptors and type Ⅰ iodothyronine 5'-deiodinase activity of human thyroid toxic adenomas and benign cold nodules[J]. Exp Clin Endocrinol Diabetes, 2002, 110(4): 166–170. doi: 10.1055/s-2002-32147 |
| [35] | Schoenmakers CH, Pigmans IG, Visser TJ. Species differences in liver type Ⅰ iodothyronine deiodinase[J]. Biochim Biophys Acta, 1992, 1121(1-2): 160–166. doi: 10.1016/0167-4838(92)90349-I |
| [36] | Bianco AC, Salvatore D, Gereben B, et al. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases[J]. Endocr Rev, 2002, 23(1): 38–89. doi: 10.1210/edrv.23.1.0455 |
| [37] | Bernal J. Action of thyroid hormone in brain[J]. J Endocrinol Invest, 2002, 25(3): 268–288. doi: 10.1007/BF03344003 |
| [38] | Croteau W, Davey JC, Galton VA, et al. Cloning of the mammalian type Ⅱ iodothyronine deiodinase. A selenoprotein differentially expressed and regulated in human and rat brain and other tissues[J]. J Clin Invest, 1996, 98(2): 405–417. doi: 10.1172/JCI118806 |
| [39] | Salvatore D, Tu H, Harney JW, et al. Type 2 iodothyronine deiodinase is highly expressed in human thyroid[J]. J Clin Invest, 1996, 98(4): 962–968. doi: 10.1172/JCI118880 |
| [40] | Hosoi Y, Murakami M, Mizuma H, et al. Expression and regulation of type Ⅱ iodothyronine deiodinase in cultured human skeletal muscle cells[J]. J Clin Endocrinol Metab, 1999, 84(9): 3293–3300. |
| [41] | Salvatore D, Bartha T, Harney JW, et al. Molecular biological and biochemical characterization of the human type 2 selenodeiodinase[J]. Endocrinology, 1996, 137(8): 3308–3315. doi: 10.1210/endo.137.8.8754756 |
| [42] | Murakami M, Araki O, Hosoi Y, et al. Expression and regulation of type Ⅱ iodothyronine deiodinase in human thyroid gland[J]. Endocrinology, 2001, 142(7): 2961–2967. doi: 10.1210/endo.142.7.8280 |
| [43] | Darras VM, Hume R, Visser TJ. Regulation of thyroid hormone metabolism during fetal development[J]. Mol Cell Endocrinol, 1999, 151(1-2): 37–47. doi: 10.1016/S0303-7207(99)00088-X |
| [44] | Galton VA, Martinez E, Hernandez A, et al. Pregnant rat uterus expresses high levels of the type 3 iodothyronine deiodinase[J]. J Clin Invest, 1999, 103(7): 979–987. doi: 10.1172/JCI6073 |
| [45] | Salvatore D, Low SC, Berry M, et al. Type 3 lodothyronine deiodinase:cloning, in vitro expression, and functional analysis of the placental selenoenzyme[J]. J Clin Invest, 1995, 96(5): 2421–2430. doi: 10.1172/JCI118299 |
| [46] | Klaren PH, Geven EJ, Nagelkerke A, et al. Kinetics and thiol requirements of iodothyronine 5'-deiodination are tissue-specific in common carp (Cyprinus carpio L.)[J]. Comp Biochem Physiol B Biochem Mol Biol, 2012, 161(3): 275–282. doi: 10.1016/j.cbpb.2011.12.005 |
| [47] | Sambroni E, Gutieres S, Cauty C, et al. Type Ⅱ iodothyronine deiodinase is preferentially expressed in rainbow trout (Oncorhynchus mykiss) liver and gonads[J]. Mol Reprod Dev, 2001, 60(3): 338–350. doi: 10.1002/(ISSN)1098-2795 |
| [48] | Lema SC, Dickey JT, Schultz IR, et al. Dietary exposure to 2, 2', 4, 4'-tetrabromodiphenyl ether (PBDE-47) alters thyroid status and thyroid hormone-regulated gene transcription in the pituitary and brain[J]. Environ Health Perspect, 2008, 116(12): 1694–1699. doi: 10.1289/ehp.11570 |
| [49] | Egloff C, Crump D, Chiu S, et al. In vitro and in ovo effects of four brominated flame retardants on toxicity and hepatic mRNA expression in chicken embryos[J]. Toxicol Lett, 2011, 207(1): 25–33. doi: 10.1016/j.toxlet.2011.08.015 |
| [50] | Li W, Zhu L, Zha J, Wang Z. Effects of decabromodiphenyl ether (BDE-209) on mRNA transcription of thyroid hormone pathway and spermatogenesis associated genes in Chinese rare minnow (Gobiocypris rarus)[J]. Environ Toxicol, in press. |
| [51] | Dong.W, M L, K K, et al. Development of in situ hybridization-based analysis for examining mRNA expression of thyroid regulation deiodinase in Zebrafish (Danio rerio)[M]. SETAC North America 33rd Annual Meeting, Long Beach, California, 2012, Nov. 11-15:MP177. |