近年来,采用厌氧消化技术对餐厨垃圾进行处理得到广泛推崇,而如何实现其快速启动及确保稳定高效运行是拟解决的关键问题.对接种污泥进行驯化,使其适应餐厨垃圾的性质及建立稳定的微生物群落结构是有效途径之一.这是因为作为厌氧消化主体的微生物群落若重新达到稳定,则表明系统已适应该底物并处于稳定运行状态.因此,对系统微生物群落特征进行分析是至关重要的.
不同学者对驯化前后微生物群落结构进行了研究,结果表明,驯化后微生物群落发生了变化.例如,Bertin等(2012)以牛粪为接种物进行生活垃圾厌氧消化,并采用DGGE观测驯化过程中微生物群落的变化,结果表明,通过149 d的驯化后,得到了适应于底物性质的微生物菌群;Cho等(2013)对污水厂污泥在干式厌氧消化条件下驯化200 d,并通过454焦磷酸测序技术对驯化前后的古菌群落进行检测,驯化后也观察到显著的微生物转移.此外,还有学者通过接种物对高氨氮、高盐含量底物的驯化,研究驯化对其水解速率的影响,同时通过T-RFLP研究微生物群落的变化(Griffin, 2012).然而,目前的这些研究大都采用DGGE、T-RFLP等分子生物学手段,这些技术能比较直观地观察到微生物群落的变化,但却只能分析有限的优势微生物类群;此外,这些研究大都只停留在微生物群落发生转移及如何转移的层面,鲜有结合底物特性和优势菌的生长特性进行深入分析的.随着分子生物学技术的发展,以454为代表的高通量测序技术以其显著优势受到广泛关注,DGGE等传统的分子指纹图谱获得的微生物DNA序列通常低于100,而新一代高通量技术每次分析获得的基因序列数以百万甚至亿万计.因此,采用该方法不仅能检测到系统中的优势菌,还能检测到某些丰度极低但却起重要作用的功能微生物,同时能灵敏地检测到属和种等较低分类水平上的物种,从而更客观地还原样品本身的菌群结构及丰度比例,得到更准确全面的结果,有助于后续进行深入详细的分析.而前期为数不多的采用高通量测序的研究,又仅仅观测了群落中古菌在驯化前后的演替(Cho et al., 2013).然而,厌氧消化是一个细菌和古菌协调互养的生化过程(Merlin Christy et al., 2014),因此,全面地研究细菌和古菌在驯化前后的演替具有重要作用.
本研究以常温下的消化猪粪为主的农村户用沼气池污泥为接种污泥,在CSTR反应器中进行驯化,以适应餐厨垃圾中温厌氧消化,并对驯化前后系统内微生物群落结构的演替采用454焦磷酸测序技术进行分析,以探索驯化对餐厨垃圾厌氧消化系统微生物群落结构和系统性能的影响.
2 材料与方法(Materials and methods) 2.1 消化物料和接种污泥餐厨垃圾取自重庆大学B区某学生食堂,采用筛网手动滤掉水和油脂后,去除粗颗粒杂质如纸巾、骨头、塑料等,并用粉碎机破碎为5 mm左右,随即分装入4 L的密封袋中,于-18 ℃冰冻保存.临用前1周置于4 ℃冰箱中解冻.接种污泥取自重庆市白市驿镇一个常温下运行的农村户用沼气池.该沼气池以猪粪为主要发酵原料,此外,还添加家禽粪便及一些季节作物(如红薯藤等).接种污泥采样期间沼气池发酵正常.污泥取回后采用2 mm×2 mm筛网过滤后加入反应器中,此时,测得接种污泥浓度为51.5 g·L-1(以VSS计).餐厨垃圾和接种污泥的性质见表 1.
| 表 1 餐厨垃圾及接种污泥的特性 Table 1 Characteristics of food waste and inoculum |
厌氧消化试验在总体积50 L、有效容积30 L的全自动CSTR厌氧消化反应器内进行(BMR-A50U,上海傲中).恒温控制系统保持温度在(36±1) ℃,机械搅拌速率设定为60 r·min-1,运行1 h停2 h.pH、ORP、沼气产量、CH4含量、CO2含量等常规指标可在线监测.
实验初期一次性向反应器内加入30 L接种污泥.预孵化2周后,反应器在3 g·L-1·d-1负荷下启动.约1个月后,日产气量和产气成分基本保持恒定(数据未显示), 此时去除多余的污泥以控制反应器有效体积,并将这一天记为实验第1 d.此后,维持负荷为3 g·L-1·d-1,再持续运行45 d.在此期间,每天出料200 mL用于总VFA、总碱度(TA)等化学指标的测定,此外,每周集中出料1次,以保持有效体积为30 L.
2.3 DNA提取、PCR及焦磷酸测序选取接种污泥及第45 d的样品分别代表驯化前后样品用于微生物群落的测定.样品采集后立即放入-80 ℃冰箱冷冻保存以供后续测定.采用E.Z.N.A Soil DNA kit (OMEGA, USA)进行DNA提取,提取方法按说明书进行.细菌16S rRNA扩增引物采用加标签的27F (5′-AGAGTTTGATCCTGGCTCAG-3′)和533R (5′-TTACCGCGGCTGCTGGCAC-3′)进行扩增,古菌则采用344F (5′-ACGGGGYGCAGCAGGCGCGA-3′)和915R (5′-GTGCTCCCCCGCCAATTCCT-3′)进行扩增.为在测序过程中进行样品倍增,标签连接在553R和915R的5′端.细菌PCR扩增条件为:95 ℃下预变性2 min; 95 ℃下变性30 s,55 ℃退火30 s, 72 ℃下延伸1 min, 25个循环;最终72 ℃下延伸10 min.古菌的PCR扩增条件与细菌类似,只是循环数为27个.扩增后,对PCR产物进行纯化和定量,并合并为相同的浓度.最后,PCR产物送去上海美吉生物医药科技有限公司进行测序.
2.4 焦磷酸测序数据分析焦磷酸测序数据分析同前期文献(Li et al., 2015).最终的核苷酸序列已上传到NCBI数据库,上传凭证号为SRP065754.
2.5 分析方法3 g·L-1·d-1负荷稳定运行期间,pH、CH4、产气量在线实时监测,总VFA、碳酸氢盐碱度(BA)、TA每天测量,乙酸、丙酸、TS、VS每3 d测1次.其中,TS、VS采用标准方法测量(APHA,2005);总VFA、BA和TA的测定方法如Li等(2014)所述.另外,VS去除率(rvs)采用文献中(Koch, 2015)推荐的公式计算.除在线监测外的其余所有化学指标设定3个平行样,取平均值.
3 结果与讨论(Results and discussion) 3.1 反应器性能启动后,系统在3 g·L-1·d-1的负荷下运行45 d,接种污泥及45 d内反应器的性能参数见表 2.由表 2中数据可知,运行期间,各项指标均处于稳定且系统处于高效运行状态,从理化指标的角度说明驯化成功.
| 表 2 接种时及稳定运行期间反应器性能参数 Table 2 The reactor performance parameters at the beginning and stable stage |
为研究驯化对系统微生物群落的影响,接种污泥及第45 d的污泥样品用于DNA提取、扩增及焦磷酸测序以获得16S rRNA基因序列,其结果分析见表 3.由表 3可知,良好的文库覆盖率表明此次测序结果基本能代表样品的真实情况.此外,表 3也展示了更多其他的微生物多样性信息,接种污泥和驯化后样品分别获得5035和6625个细菌优化序列,7419和6221个古菌优化序列.在相似性为97%时,将这些序列归类为OTUs以进行系统发育多样性分析.细菌更多的OTU数,以及ACE、Chao、Shannon和Simpson等多样性指数共同显示出细菌群落结构多样性比古菌更好,这与早期的研究也是一致的(Kim et al., 2014).进一步对比接种污泥和驯化后污泥的这些指标,可以看出,驯化后细菌的多样性降低,古菌的多样性增加.可见,驯化后微生物发生明显转移.
| 表 3 样品信息及焦磷酸测序获得的微生物16S rRNA基因序列具体分析(相似性97%) Table 3 Sample information and statistics analysis of the microbal 16S rRNA gene libraries obtained from the pyrosequencing at 97% similarity |
厌氧消化的水解酸化过程主要是在细菌的作用下完成,对于不同的底物,其成分有所不同,故只有适应该底物的细菌群落建立起来,才表明驯化成功.每个样品在门水平上的分布见图 1.在接种污泥中,优势菌门为拟杆菌门(Bacteroidetes,占27.13%)、绿弯菌门(Chloroflexi,占19.38%)、厚壁菌门(Firmicutes,占18.77%)、变形菌门(Proteobacteria,占11.90%)和螺旋菌门(Spirochaetae,占10.82%).在稳定运行的第45 d,Bacteroidetes和Chloroflexi的相对丰度有小幅度的增加,Firmicutes的相对丰度快速增加,成为仅次于Bacteroidetes的第二优势菌门,Spirochaetae的相对丰度稍有下降,而Proteobacteria的相对丰度则降到0.5%以下.此外,互养菌门(Synergistete)的相对丰度增至6.48%.即在驯化过程中,始终保持优势的菌门为Bacteroidetes、Chloroflexi、Firmicutes、Spirochaetae.
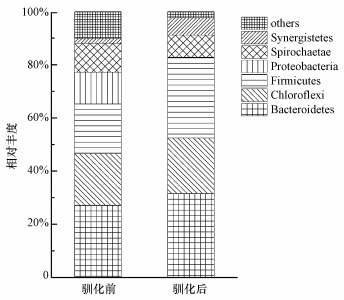 |
| 图 1 驯化前后细菌在门水平上的分布 Fig. 1 Taxonomic compositions of bacterial communities at phyla level in each sample retrieved from pyrosequencing |
据统计,除Spirochaetae外,其他3个菌门也是各种厌氧消化反应器中出现最多的优势菌门(Nelson et al., 2011).近来有学者报道我国云南、青海户用沼气池的微生物群落结构,除Spirochaetae外,前者与本研究的其余4个优势菌的门类及比例极为相似,后者的优势菌门则为Bacteroidetes、Firmicutes和Spirochaetae (李秋敏等, 2015;韩睿等, 2015).究其原因,除地域等其他影响因素外,前者的发酵底物与本研究更为相似,因而具有更相似的群落结构.对于餐厨垃圾中温湿式厌氧消化稳定阶段的细菌优势门类,本研究和其他运行条件相似的研究却有所不同(Yi et al., 2014; Guo et al., 2014),这可能是由于接种污泥的不同所致.由此可见,底物和接种污泥对微生物群落的构建都起着至关重要的作用,这可以从早期不同学者的研究中总结出来(史宏伟等, 2011; Quintero et al., 2012).
为进一步分析驯化过程中微生物对另一种底物的适应情况,即驯化后是否建立适应餐厨垃圾性质的优势功能菌,将驯化前后纲和属水平的菌落情况总结于表 4.由表 4可以看出,接种污泥细菌种类十分丰富,且随着驯化的进行,其微生物群落发生显著变化.随着拟杆菌纲(Bacteroidia)显著增加(由10.71%增至27.58%),该纲中的Petrimonas属也显著增加,并成为能确认的丰度最大的属类.据报道,Petrimonas为发酵细菌,主要发酵易降解的糖类产生乙酸、氢和二氧化碳(Grabowski, 2005),对比沼气池的原料猪粪等和驯化期间原料餐厨垃圾,前者含有更多的纤维素物质,而后者更易水解酸化,因此,Petrimonas显著增长.此外,系统中起发酵作用的菌属还有该纲中的另一丰度较大的属vadinBC27_wastewater-sludge_group、梭菌纲(Clostridia)的Fastidiosipila和互养菌纲(Synergistia)的Thermovirga.Fastidiosipila能利用糖类产生乙酸和丁酸(Falsen, 2005),Thermovirga则和蛋白质的降解相关(Gñker et al., 2012),驯化后它们的丰度也都有所增加.而vadinBC27_wastewater-sludge_group丰度则有所减少,这可能与该属还负责难降解有机物的降解有关(Xie et al., 2014).同样地,降解复杂有机物的vadinHA17(Baldwin et al., 2015)及包含很多纤维素水解物种的梭菌纲(Clostridia)(Nelson et al., 2011)丰度也大幅降低.
| 表 4 驯化前后纲、属水平细菌分类组成(仅列出相对丰度大于1%的类别) Table 4 Taxonomic compositions of bacterial communities at the class and genus level for the sequences retrieved from each sample (only genus with relative abundances higher than 1% at least in one sample were liseted) |
δ-变形菌纲(Deltaproteobacteria)的主要属类密斯氏菌属(Smithella)、互营菌属(Syntrophus),以及螺旋体纲(Spirochaetes)的主要属类Candidatus_Cloacamonas都是互养丙酸氧化菌,当系统中氢分压较低时,与氢营养型产甲烷菌互养氧化丙酸(Siddique et al., 2012; Razaviarani et al., 2015).驯化后反应器中乙酸、丙酸浓度增大且氢营养型产甲烷菌大量增长,理论上应该有更丰富的互养菌,然而其丰度却都下降甚至消失了,这可能预示着系统内存在其他具有相似功能却未被鉴定的微生物,取代了它们的生态位.
此外,厚壁菌门下的Erysipelotrichia显著增加.Erysipelotrichia包含的种是肠道菌的组成部分(Ogawa et al., 2011; Bang et al., 2015),并有研究发现当老鼠转向高脂肪饮食时,其在老鼠肠道微生物中的比例提高(Greiner et al., 2011),可见该菌可能与脂肪降解有关,驯化后其增长可能是由于餐厨垃圾具有高油脂含量导致的.
3.4 驯化对古菌群落的影响驯化前后古菌群落在属水平的序列分布情况见图 2.从图 2中可知,在接种污泥中,甲烷鬃菌属(Methanosaeta)占据绝对主导地位,其丰度占古菌总量的85.01%.该菌和甲烷八叠球菌属(Methanosarcina)是已知仅有的乙酸营养型产甲烷菌.一般认为Methanosaeta在乙酸浓度不超过100~150 mg·L-1时占主导,而后者在乙酸浓度高于250~500 mg·L-1时具有竞争优势(De Vrieze et al., 2012).接种污泥中的乙酸浓度为(154.0±0.3) mg·L-1,因此,Methanosaeta的主导地位是可预见的.这也与上文中提到的与本研究细菌群落极为相似的我国云南户用沼气池的古菌优势菌属一致(李秋敏等, 2015).驯化后,甲烷螺菌属(Methanospirillum)显著增长(由2.25%增至35.35%),成为仅次于Methanosaeta(46.97%)的第二属类;此外,甲烷囊菌属(Methanoculleus)的相对丰度也从0.80%增至9.89%.这两类菌都是严格氢营养型产甲烷菌,因此,驯化后的古菌群落转变为乙酸营养型与氢营养型产甲烷菌共同主导.餐厨垃圾较沼气池底物含有更多的易水解物质,具有更高的水解发酵速率,在用餐厨垃圾进行驯化时,系统中VFA浓度升高,而丙酸、丁酸等VFA在降解为乙酸的过程中都会产生H2(Merlin Christy et al., 2014),系统内H2浓度增加,会诱导氢营养型产甲烷菌产生(Kampmann et al., 2012).这也与前文中驯化后细菌群落中发酵菌快速增长一致.由此可见,驯化后的这种古菌群落更有利于餐厨垃圾的厌氧发酵.值得注意的是,尽管驯化后系统中的乙酸浓度高达1725 mg·L-1,Methanosaeta依然是最主导的乙酸营养型产甲烷菌,其相对丰度高达46.97%,Methanosarcina的相对丰度稍有上升,却仍不超过5%.这似乎和早期的研究不一致(De Vrieze et al., 2012),但Tale等(2011)的研究表明,接种污泥来源对特定甲烷菌的富集具有强烈影响.本研究接种污泥中的Methanosaeta相对丰度为85.01%,而Methanosarcina只有0.39%,这就奠定了Methanosaeta在系统中的主导地位,这也进一步说明了接种污泥对微生物群落构建的重要作用.
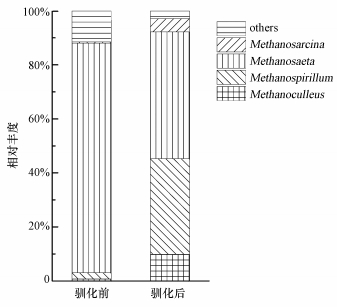 |
| 图 2 驯化前后古菌在属水平上的分布 Fig. 2 Taxonomic compositions of methanogens at the genus level in each sample retrieved from pyrosequencing |
1) 以猪粪为主要发酵原料的农村户用沼气池污泥在经过预孵化、启动后,在3 g·L-1·d-1负荷下连续运行45 d且保持性能稳定,说明接种污泥已适应餐厨垃圾这种底物,驯化成功.
2) 通过对驯化前后的微生物群落焦磷酸测序结果分析可知,驯化后,细菌的多样性降低,古菌的多样性增加.
3) 驯化后,细菌群落发生明显变化,与复杂有机物降解相关的菌类显著下降(如Clostridia和vadinHA17),同时与易降解碳水化合物发酵相关的菌类(如Petrimonas)及参与脂肪降解的菌类(Erysipelotrichia)显著增加,说明系统中已建立适应餐厨垃圾性质的稳定的细菌群落.
4) 古菌群落结构的塑造受到底物和原始接种污泥的双重影响.接种污泥中Methanosaeta占据绝对优势,使得驯化后即使在非最佳竞争条件下,该菌依然保持主导地位;同时,底物的变化也导致了氢营养型产甲烷菌Methanospirillum和Methanoculleus显著增长.驯化后乙酸型产甲烷菌和氢营养型产甲烷菌丰度类似,有利于保证平衡、稳定的厌氧消化过程.
| [${referVo.labelOrder}] | APHA.2005.Standard Methods for the Examination of Water and Wastewater (21st ed)[M].New York: American Public Health Association, American Water Works Association, Water Environment Federation, Washington, USA |
| [${referVo.labelOrder}] | Baldwin S A, Khoshnoodi M, Rezadehbashi M, et al. 2015. The microbial community of a passive biochemical reactor treating arsenic, zinc, and sulfate-rich seepage[J]. Frontiers in Bioengineering and Biotechnology, 3 : 1–13. DOI:10.12970/2311-1755.2015.03.01.1 |
| [${referVo.labelOrder}] | Bang B, Rhee M, Chang D, et al. 2015. Erysipelothrix larvae sp[J]. nov., isolated from the larval gut of the rhinoceros beetle, Trypoxylus dichotomus (Coleoptera: Scarabaeidae)[J].Antonie van Leeuwenhoek, 107(2) : 443–451. |
| [${referVo.labelOrder}] | Bertin L, Bettini C, Zanaroli G, et al. 2012. Acclimation of an anaerobic consortium capable of effective biomethanization of mechanically-sorted organic fraction of municipal solid waste through a semi-continuous enrichment procedure[J]. Journal of Chemical Technology & Biotechnology, 87(9) : 1312–1319. |
| [${referVo.labelOrder}] | Cho S, Im W, Kim D, et al. 2013. Dry anaerobic digestion of food waste under mesophilic conditions: Performance and methanogenic community analysis[J]. Bioresource Technology, 131 : 210–217. DOI:10.1016/j.biortech.2012.12.100 |
| [${referVo.labelOrder}] | De Vrieze J, Hennebel T, Boon N, et al. 2012. Methanosarcina: The rediscovered methanogen for heavy duty biomethanation[J]. Bioresource Technology, 112 : 1–9. DOI:10.1016/j.biortech.2012.02.079 |
| [${referVo.labelOrder}] | Falsen E. 2005. Fastidiosipila sanguinis gen[J]. nov., sp.nov., a new Gram-positive, coccus-shaped organism from human blood[J].International Joural of Systematic and Evolutionary Microbiology, 55(2) : 853–858. |
| [${referVo.labelOrder}] | Göker M, Saunders E, Lapidus A, et al. 2012. Genome sequence of the moderately thermophilic, amino-acid-degrading and sulfur-reducing bacterium Thermovirga lienii type strain (Cas60314T)[J]. Standards in Genomic Sciences, 6(2) : 230–239. DOI:10.4056/sigs.2726028 |
| [${referVo.labelOrder}] | Grabowski A. 2005. Petrimonas sulfuriphila gen[J]. nov., sp.nov., a mesophilic fermentative bacterium isolated from a biodegraded oil reservoir[J].International Joural of Systematic and Evolutionary Microbiology, 55(3) : 1113–1121. |
| [${referVo.labelOrder}] | Greiner T, Bäckhed F. 2011. Effects of the gut microbiota on obesity and glucose homeostasis[J]. Trends in Endocrinology & Metabolism, 22(4) : 117–123. |
| [${referVo.labelOrder}] | Griffin L P.2012.Anaerobic digestion of organic wastes: The impact of operating conditions on hydrolysis efficiency and microbial community composition[D].Fort Collins:Colorado State University |
| [${referVo.labelOrder}] | Guo X, Wang C, Sun F, et al. 2014. A comparison of microbial characteristics between the thermophilic and mesophilic anaerobic digesters exposed to elevated food waste loadings[J]. Bioresource Technology, 152 : 420–428. DOI:10.1016/j.biortech.2013.11.012 |
| [${referVo.labelOrder}] | 韩睿, 陈来生, 李莉, 等. 2015. PCR-DGGE研究青海农村户用沼气池微生物群落结构[J]. 中国环境科学, 2015, 35(6) : 1794–1804. |
| [${referVo.labelOrder}] | Kampmann K, Ratering S, Baumann R, et al. 2012. Hydrogenotrophic methanogens dominate in biogas reactors fed with defined substrates[J]. Systematic and Applied Microbiology, 35(6) : 404–413. DOI:10.1016/j.syapm.2012.07.002 |
| [${referVo.labelOrder}] | Kim S, Bae J, Choi O, et al. 2014. A pilot scale two-stage anaerobic digester treating food waste leachate (FWL): Performance and microbial structure analysis using pyrosequencing[J]. Process Biochemistry, 49(2) : 301–308. DOI:10.1016/j.procbio.2013.10.022 |
| [${referVo.labelOrder}] | Koch K. 2015. Calculating the degree of degradation of the volatile solids in continuously operated bioreactors[J]. Biomass and Bioenergy, 74 : 79–83. DOI:10.1016/j.biombioe.2015.01.009 |
| [${referVo.labelOrder}] | 李秋敏, 吴燕, 董明华, 等. 2015. 云南热带户用沼气池的原核生物群落结构研究[J]. 微生物学通报, 2015, 42(1) : 54–63. |
| [${referVo.labelOrder}] | Li L, He Q, Ma Y, et al. 2015. Dynamics of microbial community in a mesophilic anaerobic digester treating food waste: Relationship between community structure and process stability[J]. Bioresource Technology, 189 : 113–120. DOI:10.1016/j.biortech.2015.04.015 |
| [${referVo.labelOrder}] | Li L, He Q, Wei Y, et al. 2014. Early warning indicators for monitoring the process failure of anaerobic digestion system of food waste[J]. Bioresource Technology, 171 : 491–494. DOI:10.1016/j.biortech.2014.08.089 |
| [${referVo.labelOrder}] | Merlin Christy P, Gopinath L R, Divya D. 2014. A review on anaerobic decomposition and enhancement of biogas production through enzymes and microorganisms[J]. Renewable and Sustainable Energy Reviews, 34 : 167–173. DOI:10.1016/j.rser.2014.03.010 |
| [${referVo.labelOrder}] | Nelson M C, Morrison M, Yu Z. 2011. A meta-analysis of the microbial diversity observed in anaerobic digesters[J]. Bioresource Technology, 102(4) : 3730–3739. DOI:10.1016/j.biortech.2010.11.119 |
| [${referVo.labelOrder}] | Ogawa Y, Ooka T, Shi F, et al. 2011. The genome of Erysipelothrix rhusiopathiae, the causative agent of swine erysipelas, reveals new insights into the evolution of firmicutes and the organism's intracellular adaptations[J]. Journal of Bacteriology, 193(12) : 2959–2971. DOI:10.1128/JB.01500-10 |
| [${referVo.labelOrder}] | Quintero M, Castro L, Ortiz C, et al. 2012. Enhancement of starting up anaerobic digestion of lignocellulosic substrate: fique's bagasse as an example[J]. Bioresource Technology, 108 : 8–13. DOI:10.1016/j.biortech.2011.12.052 |
| [${referVo.labelOrder}] | Razaviarani V, Buchanan I D. 2015. Anaerobic co-digestion of biodiesel waste glycerin with municipal wastewater sludge: Microbial community structure dynamics and reactor performance[J]. Bioresource Technology, 182 : 8–17. DOI:10.1016/j.biortech.2015.01.095 |
| [${referVo.labelOrder}] | Siddique T, Penner T, Klassen J, et al. 2012. Microbial communities involved in methane production from hydrocarbons in oil sands tailings[J]. Environmental Science & Technology, 46(17) : 9802–9810. |
| [${referVo.labelOrder}] | 史宏伟, 邹德勋, 左剑恶, 等. 2011. 原料差异对厌氧消化微生物群落的影响[J]. 农业环境科学学报, 2011, 30(8) : 1675–1682. |
| [${referVo.labelOrder}] | Tale V P, Maki J S, Struble C A, et al. 2011. Methanogen community structure-activity relationship and bioaugmentation of overloaded anaerobic digesters[J]. Water Research, 45(16) : 5249–5256. DOI:10.1016/j.watres.2011.07.035 |
| [${referVo.labelOrder}] | Xie Z, Wang Z, Wang Q, et al. 2014. An anaerobic dynamic membrane bioreactor (AnDMBR) for landfill leachate treatment: Performance and microbial community identification[J]. Bioresource Technology, 161 : 29–39. DOI:10.1016/j.biortech.2014.03.014 |
| [${referVo.labelOrder}] | Yi J, Dong B, Jin J, et al. 2014. Effect of increasing total solids contents on anaerobic digestion of food waste under mesophilic conditions: performance and microbial characteristics analysis[J]. PLos One, 9(7) : e102548. DOI:10.1371/journal.pone.0102548 |
 2016, Vol. 36
2016, Vol. 36


