饮用水通常采用氯气、氯胺和臭氧等强氧化方法进行消毒(袁孟阳等,2013).由于源水中往往存在不同分子量的有机质和卤素,从而导致卤代消毒副产物的产生(Liu et al., 2015;梅红等,2013).有关研究表明,由于源水存在碘化物,在强氧化消毒过程中还会导致碘代消毒副产物的生成(Krasner et al., 2006),其中,碘代乙酸是常被检出的代表性碘代消毒副产物之一(Fabbricino et al., 2009).据报道,2002年首先在美国饮用水厂出水中检出碘代乙酸(赵玉丽等,2011).Richardson等(2008)对美国多个饮用水厂调研发现,碘代乙酸平均浓度约为1.7 μg·L-1.沿海地区以溴碘离子浓度相对较高的地表水作为饮用水源,氯化消毒过程易于形成碘代消毒副产物(Richardson,2005).由于碘代消毒副产物普遍具有细胞毒性和遗传毒性,因而其健康风险日益被人们所关注(Cemeli et al., 2006).
碘代消毒副产物中的碘离子易被细胞内GSH或巯基代谢活化形成活性强亲电子剂(Plewa et al., 2008).碘乙酸对CHO细胞的毒性比溴乙酸和氯乙酸分别强3和287倍,而致突变毒性分别是溴乙酸和氯乙酸的4和54倍(Plewa et al., 2004).碘代消毒副产物的细胞毒性主要表现为引起细胞膜通透性改变,导致细胞发生凋亡,例如,碘代乙酰胺能够使细胞中蛋白硫醇烷基化,从而发生细胞凋亡(van de Water et al., 1999).细胞凋亡是细胞程序性死亡的一种方式,可由多种外源环境污染物诱导发生(曹谨玲等,2013).氯乙酸等多种消毒副产物都能诱导不同类型动物细胞发生凋亡,引起细胞毒效应(Richardson et al., 2007).Lu等(2015)报道,氯乙酸能够通过诱导活性氧产生从而导致神经细胞发生凋亡;氯乙腈能诱导小鼠肝细胞发生细胞凋亡(Abdel-Naim et al., 2009).碘代乙酸是较新发现的消毒副产物(Woo et al., 2002),对其细胞水平毒作用机理的认知有限,尚待深入研究.
细胞凋亡是生物进化中保存下来的细胞程序性死亡的调节机制,受不同因素调控,细胞凋亡的途径也不完全相同(Mei et al., 2015).导致细胞凋亡发生的途径主要有外源性和内源性两条通路(Joo et al., 2015).其中,外源性凋亡主要由细胞膜上的死亡受体TNF-α、Fas和FasL等介导,Bid基因及Caspase-8等酶参与调控(Park et al., 2015);内源性凋亡过程主要受到线粒体凋亡通路的调控,Bcl-2家族基因和Caspase-3和Caspase-9等家族酶参与该调控过程(Qin et al., 2015).目前,有关碘代乙酸诱导细胞凋亡的机理还未见有报道.
饮用水消毒的碘代副产物普遍具有较强的水溶性,进入水生生态系统后,鱼类是极易遭受此胁迫的重要类群(Hua et al., 2006).因此,本文以模式水生动物斑马鱼为试验生物,采用体外细胞暴露试验,研究环境浓度碘代乙酸诱导斑马鱼淋巴细胞凋亡的内源性通路机制,以期为碘代消毒副产物的水生态风险评价研究提供科学资料.
2 材料与方法(Materials and methods) 2.1 试剂、材料和设备碘代乙酸(C2H3IO2)购自百灵威公司;总RNA提取试剂盒购自杭州浩基生物科技有限公司;细胞色素C凋亡检测试剂盒(美国BioVision)购自艾美捷科技有限公司;Caspase-3和Caspase-9活性检测试剂盒均购自南京建成生物工程研究所;细胞凋亡率、蛋白含量检测及线粒体膜电位检测试剂盒购自上海碧云天生物技术有限公司;Gibco® RPMI 1640培养基、淋巴细胞分离液、SYBR Green I染料、磷酸盐缓冲液(PBS)、细胞滤网和细胞培养96孔板均购自生工生物工程(上海)股份有限公司;细胞毒理研究实验用水为灭菌超纯水;实验用成年斑马鱼(Danio rerio)体重约0.4 g,购自浙江杭州市花鸟市场;试验用水为曝气除氯自来水.试验用鱼在实验室驯养3周后进行试验,水温25 ℃.
主要设备:流式细胞仪(GuavaeasyCyte 8HT,美国Millipore公司)、紫外分光光度计(Ultrospec 2100 pro,美国GE公司)、基因扩增仪(T100,美国Bio-Rad公司)、实时荧光定量PCR仪(iQTM4,美国Bio-Rad公司)、超纯水仪(RiOsTM5,美国Millipore公司).
2.2 淋巴细胞的提取和体外暴露试验随机取健康斑马鱼,参照Zhang等(2014)方法,将斑马鱼冰晕后,取出肾脏.眼科剪剪碎成小块后,转移至100目细胞筛,一边用玻棒研磨,一边用4 ℃预冷的PBS缓冲液冲洗筛网,用灭菌后的小烧杯收集.将含细胞的PBS缓冲液转至10 mL离心管中,小心加入等体积的淋巴细胞分离液,以4000 r·min-1的速度离心10 min,用吸管吸取离心管中间的白色云雾层,转移至灭菌后的1.5 mL离心管中.收集到的细胞用PBS缓冲液吹打,3000 r·min-1的速度离心5 min后,弃上清液,将收集的细胞转移至RPMI1640培养基中27 ℃培养5 h以去除杂细胞.将未贴壁的淋巴细胞小心收集后,重新置于RPMI1640培养基中培养.应用显微镜观察淋巴细胞的纯度,确保收集到的细胞为淋巴细胞.
淋巴细胞置于96孔板中培养,每孔1.0×106个细胞.体外暴露试验共分成6组,包括对照组、12、24、36、48和96 h诱导组.根据文献报道,水环境中碘代乙酸的检出浓度通常为μg·L-1量级(Hua et al., 2006),本研究中碘代乙酸暴露浓度为1 μg·L-1/106个细胞.
2.3 细胞凋亡率测定参考Zhang等(2014)方法检测细胞凋亡率.取诱导后的淋巴细胞1.0×106个,前述方法离心收集,用70%的乙醇于4 ℃下固定24 h.离心收集细胞后,用预冷的PBS缓冲液洗2次,然后用100 mg·L-1的RNase和50 mg·L-1的碘化丙锭在室温下共同孵育30 min.使用细胞滤网过滤细胞,然后使用流式细胞仪检测凋亡率,激发波长为488 nm,发射波长为630 nm.
2.4 细胞色素C含量测定参考Stoyanovsky等(2008)方法检测细胞色素C含量.取诱导后的细胞,前述方法离心收集,用预冷的PBS洗2次.应用细胞色素C凋亡检测试剂盒将淋巴细胞线粒体组分分离,以试剂盒提供的细胞色素C抗体做Western杂交,来检测释放出来的细胞色素C.根据试剂盒说明进行化学发光显影,然后用Quantity One软件对条带进行光度分析,最终结果以不同实验组细胞色素C光度相对于对照组光度的倍数变化表示.
2.5 线粒体膜电位的测定参考Del Bino等(1991)方法检测线粒体膜电位.取诱导后的淋巴细胞1.0×106个,前述方法离心收集,重悬到1 mL预冷的PBS缓冲液中,然后在室温下用终浓度为5 mg·L-1的罗丹明123(Rhodamine123)染色30 min.然后,淋巴细胞用预冷的PBS缓冲液冲洗,离心收集后用荧光光度计检测,激发波长为507 nm,发射波长为529 nm.应用蛋白含量检测试剂盒检测细胞的蛋白含量,线粒体膜电位采用罗丹明123荧光值与蛋白含量的比值表示,单位为 U·mg-1.
2.6 凋亡相关基因在mRNA水平相对表达量的测定参考Pan等(2015)的方法检测凋亡相关基因在mRNA水平相对表达量.诱导后的斑马鱼淋巴细胞采用前述离心法收集,用总RNA提取试剂盒提取总RNA,采用RNA纯化试剂盒进一步纯化RNA,并使用紫外分光光度计根据260 nm与280 nm处吸光度的比值确定RNA浓度.采用基因扩增仪进行逆转录实验.RT-PCR实验需要的引物根据Xiang等(2008)的研究结果设计,具体如表 1所示.反应体系包括SYBR Green I染料、各种引物、去离子水和cDNA,共20 μL,反应条件为:95 ℃预变性10 min,95 ℃变性30 s,55 ℃退火40 s,72 ℃延伸30 s,45次循环.基因表达水平使用实时荧光定量PCR仪进行相对荧光定量检测.所有测量重复3次,基因相对表达水平以2(Ct内参基因-Ct目的基因)(Ct代表基因扩增的循环次数)进行分析,结果以实验组基因相对于对照组基因表达量的倍数表示.
| 表 1 斑马鱼β-Actin、Bax和Bcl-2基因表达检测引物设计序列 Table 1 Sequences of primers of β-Actin, Bax and Bcl-2 for Zebrafish (Danio rerio) |
参考Zhang等(2010)的方法检测Caspase-3和Caspase-9酶活性的变化情况.诱导后的淋巴细胞采用离心法收集,转速为2000 r·min-1,离心5 min.然后根据Caspase酶活性检测试剂盒说明书操作,利用Caspase酶剪切底物偶联的发光基团(pNA,p-nitroaniline)的原理,检测Caspase-3和Caspase-9的酶活性,检测激发波长为405 nm,酶活性结果以相对于对照组的变化倍数来表示.
2.8 数据的统计分析每个试验组设置3个平行样,采用SPSS软件(12.0版)进行分析,应用单侧ANOVA法进行差异性检验,p<0.05表示差异显著,p<0.01表示差异极显著.数据用均数±标准差(mean±S.D.)表示.
3 结果(Results) 3.1 细胞凋亡率的变化采用溴化乙锭染色法结合流式细胞仪检测碘代乙酸体外暴露条件下诱导斑马鱼淋巴细胞凋亡随时间的变化规律,结果如图 1所示.1 μg·L-1的碘代乙酸暴露12、24、36、48和96 h后,相对于对照组,细胞凋亡率从3.52%分别增加到6.12%、15.89%、22.47%、40.76%和52.13%,其中,暴露12 h组与对照组显著差异(p<0.05),随着暴露时间增加,暴露24 h以后,所有实验组与对照组均呈现极显著差异(p<0.01),表明该细胞凋亡过程具有时间-效应特征.
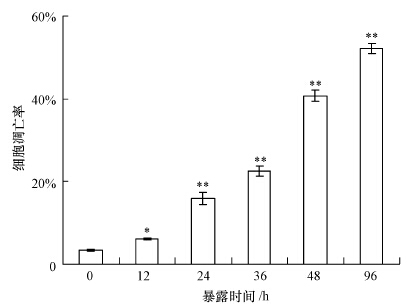 |
| 图 1 斑马鱼淋巴细胞体外暴露于碘代乙酸诱导过程中细胞凋亡率(*p<0.05,**p<0.01) Fig. 1 Apoptosis of zebrafish lymphocytes exposed to iodine acetic acid in vitro |
线粒体膜通透性的变化通常被认为是早期细胞凋亡的典型特征(Zhang et al., 2015),采用罗丹明123染色技术可以灵敏地检测到线粒体膜电位的变化(Shirakawa et al., 2015).图 2的结果表明,1 μg·L-1的碘代乙酸暴露12 h和24 h后,相对于对照组,线粒体膜电位分别下降了16.1%和32.9%;当暴露时间达到36 h后,线粒体膜电位下降达到50.1%;当暴露时间增加到48 h和96 h后,线粒体膜电位崩溃,分别下降了68.6%和81.5%.统计分析表明,所有实验诱导组淋巴细胞线粒体膜电位的下降都与对照组呈现极显著差异(p<0.01).
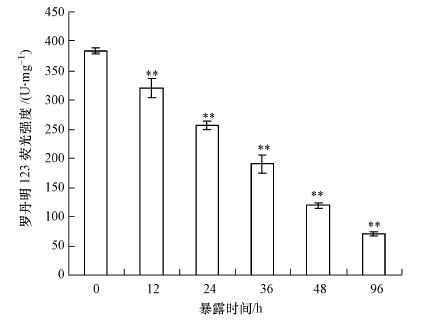 |
| 图 2 斑马鱼淋巴细胞体外暴露于碘代乙酸诱导过程中线粒体膜电位变化(**p<0.01) Fig. 2 Mitochondrial membrane potential disruptions of zebrafish lymphocytes exposed to iodine acetic acid in vitro |
细胞色素C是生物氧化过程中的电子传递体(Babbitt et al., 2015),有关细胞凋亡的研究表明,细胞色素C与细胞凋亡有关,从线粒体中泄露出的细胞色素C有诱导细胞凋亡的作用(Mohan et al., 2015).图 3的结果表明,1 μg·L-1的碘代乙酸暴露12、24、36、48和96 h后,相对于对照组,细胞色素C分别增加了0.31、0.85、1.37、1.86和2.66倍,呈现出明显的时间-效应特征.这些数据表明,在碘代乙酸体外诱导斑马鱼淋巴细胞凋亡过程中,细胞色素C持续从线粒体中释放出来,这与细胞凋亡下游通路关系密切.
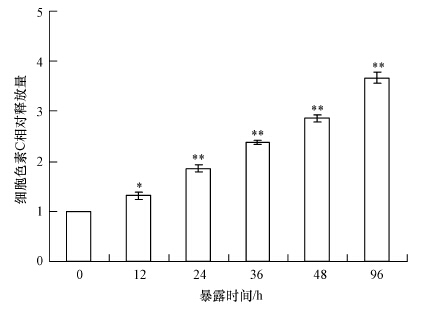 |
| 图 3 斑马鱼淋巴细胞体外暴露于碘代乙酸诱导过程中细胞色素C含量变化(*p<0.05,**p<0.01) Fig. 3 Cytochrome C levels of zebrafish lymphocytes exposed to iodine acetic acid in vitro |
Caspase-3和Caspase-9被认为是细胞凋亡通路的关键调控酶(Sobenin et al., 2015),Caspase-9能够调控Caspase-3的活性(Jiang et al., 2015),而Caspase-3能够最终激活细胞凋亡的发生(Chen et al., 2015).图 4的结果表明,随着碘代乙酸体外暴露时间的增加,两种Caspase酶活性都呈现出随暴露时间增加而增强的时间-效应特征.体外暴露24 h后,Caspase-9酶活性显著增强(p<0.05);暴露36、48和96 h后,两种酶活性与对照组相比,差异均极显著(p<0.01),Caspase-3酶活性分别增加了0.49、0.86和1.43倍;Caspase-9酶活性分别增加了0.73、1.41和1.88倍.
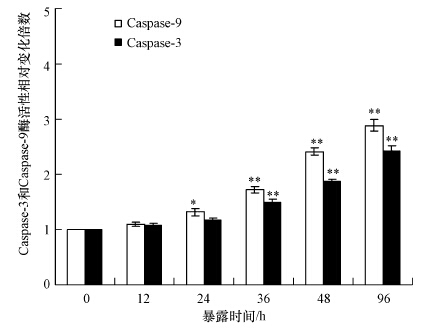 |
| 图 4 斑马鱼淋巴细胞体外暴露于碘代乙酸诱导过程中Caspase酶活性变化(*p<0.05,**p<0.01) Fig. 4 Caspase-3 enzyme and caspase-9 enzyme activities of zebrafish lymphocytes exposed to iodine acetic acid in vitro |
Bcl-2家族基因在调控细胞凋亡过程中发挥了重要的作用,其中,Bcl-2是典型的抑制凋亡基因,而Bax是经典的促进凋亡基因(Burlacu,2003).图 5的结果清晰地表明,随着碘代乙酸体外暴露时间的增加,Bcl-2基因表达量明显呈现下降趋势,相对于对照组,暴露24 h后,Bcl-2基因表达量呈现显著差异(p<0.05),当暴露时间增加到36、48和96 h后,Bcl-2基因的相对表达量分别下降27.0%、35.3%和52.3%(p<0.01).对于Bax基因来说,变化趋势完全相反,相对于对照组,暴露12 h后Bax基因的相对表达量显著上升(p<0.05),当暴露时间增加到24、36、48和96 h后,Bax基因的相对表达量分别增加0.67、1.1、2.3和3.2倍(p<0.01),表现出明显的促进凋亡效应.
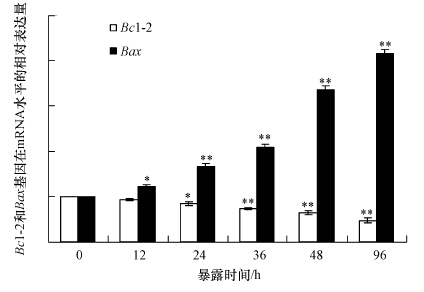 |
| 图 5 斑马鱼淋巴细胞体外暴露于碘代乙酸诱导过程中Bcl-2和Bax基因在mRNA水平相对表达量的时间-效应变化 Fig. 5 Time-dependent relative quantities of Bcl-2 and Bax genes of zebrafish lymphocytes exposed to iodine acetic acid in vitro |
研究发现,多种消毒副产物都具有细胞毒性.采用经典的MTT实验证实,新型消毒副产物夹二氮蒽能够对T24和HegG2细胞产生明显毒性,半致死浓度分别为0.50和2.04 mmol·L-1(Zhou et al., 2012);Liviac等(2011)研究发现,溴代硝基甲烷、三溴乙醛、二溴代丁烯醛酸、二氯甲酰丙烯酸、三氯硝基甲烷和水合三氯乙醛6种消毒副产物都能够对小鼠淋巴细胞产生毒性;Ali等(2014)研究发现,碘乙酸、溴乙酸和氯乙酸能够体外诱导人体外周淋巴细胞DNA损伤.本研究发现,碘代乙酸能够体外诱导斑马鱼淋巴细胞凋亡,细胞凋亡率呈现明显时间-效应特征,证明低剂量体外暴露的碘代乙酸对鱼体免疫细胞具有毒效应,表现为诱导细胞发生凋亡.
细胞凋亡的发生受多种因素调控和影响(Paschall et al., 2015).本研究采用罗丹明123染色实验发现,碘代乙酸诱导斑马鱼淋巴细胞凋亡过程中,与对照组相比,诱导实验组淋巴细胞线粒体膜电位不断下降,同样呈现明显的时间-效应,在诱导12 h后,即可以检测到线粒体膜电位下降16.1%,差异极显著(p<0.01).这表明线粒体膜电位下降是碘代乙酸诱导斑马鱼淋巴细胞凋亡过程的早期事件,这与其它污染物导致细胞凋亡过程线粒体膜电位变化趋势相一致(井长勤等,2013).线粒体膜电位的改变说明线粒体膜通透性增强,这会导致线粒体中细胞因子及细胞凋亡信号分子的释放.采用Western杂交实验发现,线粒体中的细胞色素C释放量明显增加,碘代乙酸暴露12 h即导致细胞质细胞色素C含量增加0.3倍,暴露96 h,细胞色素C含量增加2.66倍,而细胞色素C能够显著增强细胞凋亡信号通路关键酶Caspase-9的活性(Kirkland et al., 2015),因此,细胞色素C应当是调控该过程细胞凋亡下游通路的关键因子.
Caspase-3和Caspase-9等Caspase家族酶是调控细胞凋亡内源性通路的重要信使(Shakor et al., 2015).本研究发现,碘代乙酸诱导斑马鱼淋巴细胞凋亡过程中,两种Caspase酶活性均随着碘代乙酸暴露时间增加而活性显著增强,这表明Caspase-3和Caspase-9都参与到该细胞凋亡的调控过程.然而值得注意的是,在相对较短的12 h暴露时间内,两种酶活性的变化并不明显,当暴露时间增加到24 h后,Caspase-3的酶活性与对照组仍无显著差异,而Caspase-9的酶活性与对照组呈显著差异(p<0.05);当暴露时间达到36 h以后,Caspase-3和Caspase-9的酶活性都与对照组呈极显著差异(p<0.01).上述结果表明,在该细胞凋亡过程中Caspase-9明显比Caspase-3优先启动,有可能Caspase-9的启动对Caspase-3起到了诱导活化作用.Sobenin等(2015)的研究中同样发现Caspase-9对Caspase-3的活化作用.
细胞凋亡内源性通路受Bcl-2家族基因调控(Weaver et al., 2008),其中,Bcl-2和Bax基因是最为经典的调控基因,Bcl-2能够抑制细胞凋亡的发生,而Bax基因则能够促进细胞凋亡,它们是细胞凋亡过程的重要标志性基因(Vandaele et al., 2008).正常情况下,Bcl-2和Bax表达相对平衡,而外界诱因可以强化Bax基因从线粒体膜释放到细胞质中,从而刺激细胞凋亡的发生.本研究结果证实,碘代乙酸诱导斑马鱼淋巴细胞凋亡过程中Bax基因表达明显上升,而Bcl-2基因的表达明显下调,充分说明该细胞凋亡过程与这两种基因的参与有关.
综合上述分析,碘代乙酸诱导斑马鱼淋巴细胞凋亡过程可能的内源性通路如图 6所示.该通路主要表现为线粒体膜电位的降低,导致线粒体内细胞色素C释放,激活Caspase-9,进而活化Caspase-3,并且Bcl-2和Bax基因参与了该调控过程.此外,碘代乙酸体外暴露诱导斑马鱼淋巴细胞发生凋亡过程呈现明显的时间-效应特征.
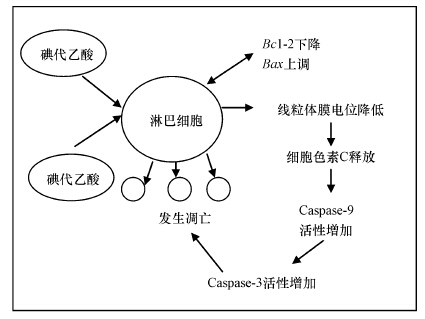 |
| 图 6 碘代乙酸体外诱导斑马鱼淋巴细胞内源性凋亡机理的假想模型 Fig. 6 Hypothetical model for iodine acetic acid -induced intrinsic apoptosis pathway of Zebrafish (Danio rerio) lymphocytes in vitro |
| [1] | Abdel-Naim A B, Nagy A A, Mohamadin A M, et al. 2009.Chloroacetonitrile induces oxidative stress and apoptosis in mouse fetal liver[J]. Toxicology Letters, 190 (2): 123–127. |
| [2] | Ali A, Kurzawa-zegota M, Najafzadeh M, et al. 2014.Effect of drinking water disinfection by-products in human peripheral blood lymphocytes and sperm[J]. Mutation Research-Fundamental and Molecular Mechanisms of Mutagenesis, 770 : 136–143. |
| [3] | Babbitt S E, Sutherland M C, Francisco B S, et al. 2015.Mitochondrial cytochrome c biogenesis:no longer an enigma[J]. Trends in Biochemical Sciences, 40 (8): 446–455. |
| [4] | Burlacu A. 2003.Regulation of apoptosis by Bcl-2 family proteins[J]. Journal of Cellular and Molecular Medicine, 7 (3): 249–257. |
| [5] | 曹谨玲, 陈剑杰, 王俊东, 等.2013.氟对鲤鱼脑抗氧化系统及细胞凋亡的影响[J].环境科学学报, 33 (3):861–866. |
| [6] | Cemeli E, Wagner E D, Anderson D, et al. 2006.Modulation of the cytotoxicity and genotoxicity of the drinking water disinfection byproduct iodoacetic acid by suppressors of oxidative stress[J]. Environmental Science & Technology, 40 (6): 1878–1883. |
| [7] | Chen H X, Zhang J J, Gao Y M. 2015.Sensitive cell apoptosis assay based on caspase-3 activity detection with graphene oxide-assisted electrochemical signal amplification[J]. Biosensors & Bioelectronics, 68 : 777–782. |
| [8] | Del Bino G, Lassota P, Darzynkiewicz Z. 1991.The S-phase cytotoxicity of camptothecin[J]. Experimental Cell Research, 193 (1): 27–35. |
| [9] | Fabbricino M, Korshin G V. 2009.Modelling disinfection by-products formation in bromide-containing waters[J]. Journal of Hazardous Materials, 168 (2/3): 782–786. |
| [10] | Hua G H, Reckhow D A, Kim J S. 2006.Effect of bromide and iodide ions on the formation and speciation of disinfection byproducts during chlorination[J]. Environmental Science & Technology, 40 (9): 3050–3056. |
| [11] | Jiang W D, Wen H L, Liu Y. 2015.The tight junction protein transcript abundance changes and oxidative damage by tryptophan deficiency or excess are related to the modulation of the signalling molecules,NF-κB p65,TOR,caspase-(3,8,9) and Nrf2 mRNA levels,in the gill of young grass carp(Ctenopharyngodon idellus)[J]. Fish & Shellfish Immunology, 46 (2): 168–180. |
| [12] | 井长勤, 陈红丽, 李效宇.2013.离子液体氯化1-辛基-3-甲基咪唑对 EMT6 细胞的毒性及其机理研究[J].环境科学学报, 33 (6):1809–1814. |
| [13] | Joo J H, Ueda E, Bortner C D, et al. 2015.Farnesol activates the intrinsic pathway of apoptosis and the ATF4-ATF3-CHOP cascade of ER stress in human T lymphoblastic leukemia Molt4 cells[J]. Biochemical Pharmacology, 97 (3): 256–268. |
| [14] | Kirkland R A, Franklin J L. 2015.Bax and caspases regulate increased production of mitochondria-derived reactive species in neuronal apoptosis:LACK of A role for depletion of cytochrome c from the mitochondrial electron transport chain[J]. Biochemistry and Biophysics Reports, 4 : 158–168. |
| [15] | Krasner S W, Weinberg H S, Richardson S D, et al. 2006.Occurrence of a new generation of disinfection byproducts[J]. Environmental Science & Technology, 40 (23): 7175–7185. |
| [16] | Liu K, Lu J H, Ji Y F. 2015.Formation of brominated disinfection by-products and bromate in cobalt catalyzed peroxymonosulfate oxidation of phenol[J]. Water Research, 84 : 1–7. |
| [17] | Liviac D, Creus A, Marcos R. 2011.Mutagenic analysis of six disinfection by-proucts in the Tk gene of mouse lymphoma cells[J]. Journal of Hazardous Materials, 190 (1/3): 1045–1052. |
| [18] | Lu T H, Su C C, Tang F C, et al. 2015.Chloroacetic acid triggers apoptosis in neuronal cells via a reactive oxygen species-induced endoplasmic reticulum stress signaling pathway[J]. Chemico-Biological Interactions, 225 : 1–12. |
| [19] | 梅红, 丁国际, 黄鑫, 等.2011.含溴黄浦江水消毒过程中溴代三卤甲烷和卤乙酸的生成特性[J].环境科学学报, 31 (10):2162–2168. |
| [20] | Mei M, Tang F T, Lu M L, et al. 2015.Astragaloside IV attenuates apoptosis of hypertrophic cardiomyocyte through inhibiting oxidative stress and calpain-1 activation[J]. Environmental Toxicology and Pharmacology, 40 (3): 764–773. |
| [21] | Mohan R, Atreja S K. 2015.Tyrosine phosphorylation of cytochrome c as a signaling event in frozen thawed buffalo spermatozoa at the cross-roads of capacitation and apoptosis[J]. Cryobiology, 70 (3): 253–261. |
| [22] | Pan Y Y, Cui Y, Baloch A R, et al. 2015.Insulinlike growth factor I improves yak(Bos grunniens) spermatozoa motility and the oocyte cleavage rate by modulating the expression of Bax and Bcl-2[J]. Theriogenology, 84 (5): 756–762. |
| [23] | Park M R, Kim S G, Cho I A, et al. 2015.Licochalcone-A induces intrinsic and extrinsic apoptosis via ERK1/2 and p38 phosphorylation-mediated TRAIL expression in head and neck squamous carcinoma FaDu cells[J]. Food and Chemical Toxicology, 77 : 34–43. |
| [24] | Paschall A V, Liu K. 2015.Epigenetic regulation of apoptosis and cell cycle regulatory genes in human colon carcinoma cells[J]. Genomics Data, 5 : 189–191. |
| [25] | Plewa M J, Muellner M G, Richardson S D, et al. 2008.Occurrence,synthesis,and mammalian cell cytotoxicity and genotoxicity of haloacetamides:an emerging class of nitrogenous drinking water disinfection byproducts[J]. Environmental Science & Technology, 42 (3): 955–961. |
| [26] | Plewa M J, Wagner E D, Richardson S D, et al. 2004.Chemical and biological characterization of newly discovered iodoacid drinking water disinfection byproducts[J]. Environmental Science & Technology, 38 (18): 4713–4722. |
| [27] | Qin G Q, Wu L P, Liu H Y, et al. 2015.Artesunate induces apoptosis via a ROS-independent and Bax-mediated intrinsic pathway in HepG2 cells[J]. Experimental Cell Research, 336 (2): 308–317. |
| [28] | Richardson S D, Fasano F, Ellington J J, et al. 2008.Occurrence and mammalian cell toxicity of iodinated disinfection byproducts in drinking water[J]. Environmental Science & Technology, 42 (22): 8330–8338. |
| [29] | Richardson S D, Plewa M J, Wagner E D, et al. 2007.Occurrence,genotoxicity,and carcinogenicity of regulated and emerging disinfection by-products in drinking water:A review and roadmap for research[J]. Mutation Research/Reviews in Mutation Research, 636 (1/3): 178–242. |
| [30] | Richardson S D. 2005.New disinfection by-product issues:emerging DBPs and alternative routes of exposure[J]. Global NEST Journal, 7 (1): 43–60. |
| [31] | Shakor A B A, Atia A, Alshehri A S, et al. 2015.Ceramide generation during curcumin-induced apoptosis is controlled by crosstalk among Bcl-2,Bcl-xL,caspases and glutathione[J]. Cellular Signalling, 27 (11): 2220–2230. |
| [32] | Shirakawa M, Sekine S, Tanaka A, et al. 2015.Metabolic activation of hepatotoxic drug(benzbromarone) induced mitochondrial membrane permeability transition[J]. Toxicology and Applied Pharmacology, 288 (1): 12–18. |
| [33] | Sobenin I A, Bobryshev Y V, Korobov G A, et al. 2015.Quantitative analysis of the expression of caspase 3 and caspase 9 in different types of atherosclerotic lesions in the human aorta[J]. Experimental and Molecular Pathology, 99 (1): 1–6. |
| [34] | Stoyanovsky D A, Vlasova L L, Belikova N A, et al. 2008.Activation of NO donors in mitochondria:Peroxidase metabolism of(2-hydroxyamino-vinyl)-triphenyl-phosphonium by cytochrome c releases NO and protects cells against apoptosis[J]. FEBS Letters, 582 (5): 725–728. |
| [35] | van De Water B, Wang Y, Asmellash S, et al. 1999.Distinct endoplasmic reticulum signaling pathways regulate apoptotic and necrotic cell death following iodoacetamide treatment[J]. Chemical Research in Toxicology, 12 (10): 943–951. |
| [36] | Vandaele L, Goossens K, Peelman L, et al. 2008.mRNA expression of Bcl-2,Bax,caspase-3 and-7 cannot be used as a marker for apoptosis in bovine blastocysts[J]. Animal Reproduction Science, 106 (1/2): 168–173. |
| [37] | Weaver C V, Liu S P. 2008.Differentially expressed pro-and anti-apoptogenic genes in response to benzene exposure:Immunohistochemical localization of p53,Bag,Bad,Bax,Bcl-2,and Bcl-w in lung epithelia[J]. Experimental and Toxicologic Pathology, 59 (5): 265–272. |
| [38] | Woo Y T, Lai D, McLain J L, et al. 2002.Use of mechanism-based structure-activity relationships analysis in carcinogenic potential ranking for drinking water disinfection by-products[J]. Environmental Health Prospect, 110 (1): 75–87. |
| [39] | Xiang L X, Peng B, Dong W R, et al. 2008.Lipopolysaccharide induces apoptosis in Carassius auratus Iymphocytes,a possible role in pathogenesis of bacterial infection in fish[J]. Developmental and Comparative Immunology, 32 (8): 992–1001. |
| [40] | 袁孟阳, 林冲, 欧桦瑟, 等.2013.焦化废水尾水O3氧化消除消毒副产物生成潜能的影响分析[J].环境科学学报, 33 (8):2166–2173. |
| [41] | Zhang B, Bian W L, Pal A, et al. 2015.Macrophage apoptosis induced by aqueous C-60 aggregates changing the mitochondrial membrane potential[J]. Environmental Toxicology and Pharmacology, 39 (1): 237–246. |
| [42] | Zhang H J, Fang W D, Wang D D, et al. 2014.The role of interleukin family in perfluorooctanoic acid(PFOA)-induced immunotoxicity[J]. Journal of Hazardous Materials, 280 : 552–560. |
| [43] | Zhang H J, Wu Y Z, Fang W D, et al. 2014.Regulatory effect of quercetin on hazardous microcystin-LR-induced apoptosis of Carassius auratus lymphocytes in vitro[J]. Fish & Shellifsh Immunology, 37 (2): 278–285. |
| [44] | Zhang Z F, Lu J, Zheng Y L, et al. 2010.Purple sweet potato color protects mouse liver against D-galactose-induced apoptosis via inhibiting caspase-3 activation and enhancing PI3K/Akt pathway[J]. Food and Chemical Toxicology, 48 (8/9): 2500–2507. |
| [45] | Zhou W J, Lou L J, Zhu L F, et al. 2012.Formation and cytotoxicity of a new disinfection by-product(DBP) phenazine by chloramination of water containing diphenylamine[J]. Journal of Environmental Sciences-China, 24 (7): 1217–1224. |
| [46] | 赵玉丽, 李杏放.2011.饮用水消毒副产物:化学特征与毒性[J].环境化学, 30 (1):20–33. |
 2016, Vol. 36
2016, Vol. 36


