孔雀石绿是一种三苯甲烷染料,作为着色剂被广泛应用于丝绸、棉花、皮革、木材和造纸工业.同时,它还是一种杀菌剂,能防止鱼类体外感染真菌和原生动物,因此也被应用于水产养殖业(Saha et al.,2012;Lee et al.,2012;Moumeni et al.,2012).孔雀石绿由于其低成本、高效率以及缺乏合适的替代品,在许多国家被广泛使用(Srivastava et al.,2004;Shedbalkar et al.,2011).但是研究发现,当孔雀石绿存在于水体当中时,会干扰太阳光在水中的透射,减少水中植物的光合作用,从而影响水生生物的生存环境(Beak et al.,2009).而且孔雀石绿也能够通过影响人体的免疫系统和生殖系统,从而对人体产生危害(Lee et al.,2012;Saha et al.,2012).2002年孔雀石绿被美国食品药品监督管理局列为一种主要的化学致癌物(Srivastava et al.,2004;Shedbalkar et al.,2011).因此,探索有效的方法从水体中移除该物质至关重要.
目前许多物理化学方法已应用于纺织工业废水中孔雀石绿的消除,如芬顿试剂(Chen et al.,2002)、微波辅助催化剂(Ju et al.,2008)和臭氧(Kusvran et al.,2011)等.然而,这些方法自身均具有局限性,如高成本、二次污染和低效率(Patidar et al.,2012).因此,作为一种环境友好、高效和低成本的方法,生物法处理孔雀石绿近几年得到了广泛的关注(Deng et al.,2008).早先研究所报道的能够脱色孔雀石绿的微生物包括细菌中的Cosmarium sp.、Bacillus cereus DC11、Pseudomonas otitidis WL-13、Sphingomonas paucimobilis和Pandoraea pulmonicola YC32等(Daneshvar et al.,2007;Deng et al.,2008;Wu et al.,2009;Ayed et al.,2009; chen et al.,2009),酵母菌中的Saccharomyces cerevisiae MTCC 463(Jadhav et al.,2006)以及丝状真菌中的Trametes sp. SQ01和Penicillium pinophilum(Yang et al.,2009;Jasińska et al.,2012).但是,它们对孔雀石绿的脱色效果较差.因此,本研究着重探究白腐真菌P. eryngii-Co007对孔雀石绿的脱色,并对其降解孔雀石绿的机制进行系统研究,从而为研究三苯甲烷类染料的生物降解途径奠定基础.
2 材料和方法(Materials and methods) 2.1 材料 2.1.1 菌种刺芹侧耳P. eryngii-Co007(陈敏等,2010),由刺芹侧耳GIM 5.280菌株(购自广东省微生物研究所菌种保藏中心)复合诱变选育得到,并由本实验室保藏.
2.1.2 培养基固体种子培养基(g·L-1):麦芽糖10,葡萄糖20,七水合硫酸镁0.5,磷酸二氢钾4,浓缩酵母浸出粉2,胰蛋白胨2,琼脂15-20;液体种子培养基(g·L-1):麦芽糖10,葡萄糖20,七水合硫酸镁0.5,磷酸二氢钾4,浓缩酵母浸出粉2,胰蛋白胨2;液体发酵培养基(g·L-1):麦芽糖10,葡萄糖10,七水合硫酸镁0.5,磷酸氢二钾4,浓缩酵母浸出粉2,胰蛋白胨2,吐温80 0.84.
2.1.3 染料三苯甲烷染料孔雀石绿(C23H25ClN2),分析纯,购自上海佳英化工有限公司,相对分子量364.92,结构式如下:
2.2 方法 2.2.1 脱色实验切取在28℃,固体种子培养基中培养5 d的P. eryngii-Co007菌块,直径10 mm左右,接种到200 mL液体种子培养基中,在28℃、150 r·min-1条件下培养3~5 d.将液体种子培养液按10%的接种量,接入500 mL液体发酵培养基中,28℃、150 r·min-1条件下培养6 d.无菌条件下过滤收集菌丝,按菌丝湿重与乙酸钠-乙酸缓冲液比值为1∶5(W/V)的比例加入缓冲液.接着按实验设定投加染料,150 r·min-1条件下进行染料脱色实验.分别研究染料浓度、脱色pH、脱色温度和脱色时间对脱色率的影响,控制染料浓度为50、100、150、200和250 mg·L-1,脱色pH为3、4、5、6和7,脱色温度为20、25、30、35和40℃,脱色时间为0.5、3、6、9和12 h.
2.2.2 脱色率的测定(李慧蓉等,1999)用分光光度法测定并计算某一培养时间内培养液中剩余染料浓度a1(a为原染料浓度),则染料脱色率η按式(1)计算:
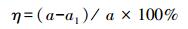
|
(1) |
取200 mg·L-1孔雀石绿溶液、6 h和12 h的降解液上清,用UV-3200 pc Spectrophotometer(购自上海美普达仪器有限公司)在200~800 nm范围内进行紫外-可见扫描.
2.2.4 傅里叶红外光谱分析取200 mg·L-1孔雀石绿溶液和12 h的降解液上清,0.45μm孔径膜过滤.滤液冻干成粉末,经溴化钾压片,用NICOLET-380傅里叶红外光谱仪(购自Thermo公司)进行红外光谱扫描,波数4000~400 cm-1.
2.2.5 GC-MS分析取200 mg·L-1孔雀石绿溶液和12 h的降解液上清,用等体积的三氯甲烷萃取3次,合并萃取液.加入无水硫酸钠充分脱水干燥,旋转蒸发至结晶.然后甲醇溶解结晶物,0.22μm有机膜过滤,用7890A-5975C气相色谱-质谱联用仪(购自Agilent公司)进行GC-MS分析.GC-MS条件:DB-17MS气相色谱柱(30 m×0.25 mm×0.25μm),进样体积1μL,进样口温度280℃,恒流模式,柱流速1 mL·min-1,分流比20:1,柱温35℃保持1 min,后以10℃·min-1升到290℃保持20 min,离子源EI,离子源温度230℃,四级杆温度150℃,EM电压1953 eV,质量数扫描范围33~400 amu.
2.2.6 植物毒性实验取12 h的降解液上清,经三氯甲烷萃取和旋蒸至结晶,加去离子水溶解,使其浓度达到200 mg·L-1以进行植物毒性实验.同时以去离子水和200 mg·L-1孔雀石绿溶液的处理作为对照.采用小麦(Triticum aestivum)为材料,去离子水浸泡8 h,选取90粒膨胀的种子平均置于3个培养皿中的育苗纸上,每天分别用上述3种溶液喷洒种子3~5次,每次1 mL,于室温下萌发.4 d后测定种子的萌发率(%)、胚根长度(cm)、胚芽长度(cm)和种子鲜重(g).
3 结果与讨论(Results and discussion) 3.1 脱色实验 3.1.1 染料浓度对脱色率的影响在pH 5、30℃条件下,考察孔雀石绿的浓度对脱色率的影响.表 1表明,当孔雀石绿浓度为50 mg·L-1和100 mg·L-1时,P. eryngii-Co007脱色24 h,脱色率可接近100%,而当浓度为150~250 mg·L-1时,脱色24 h,脱色率分别为92.12%、85.31%和64.15%,随后随脱色时间增加,脱色率缓慢增大,72 h脱色率分别为96.16%、92.77%和72.49%.染料浓度能提供重要的驱动力,以克服染料水溶液和固相之间所有的质量传递阻力(Lv et al.,2013),然而逐步提高孔雀石绿浓度,脱色率和浓度呈反比关系,这一结果与Kurthia sp.(Sani et al.,1999)、Sphingomonas paucimobilis(Ayed et al.,2009)和Blumea malcolmii(Kagalkar et al.,2011)对孔雀石绿脱色结果一致.主要是因为染料浓度越高,其毒性作用越强,更容易抑制菌丝的生长和产酶,从而影响菌丝对染料的脱色,导致脱色率降低(Hadibarata et al.,2013).考虑到实际应用及脱色率,选择200 mg·L-1孔雀石绿进行后续脱色体系参数的优化.
| 表 1 孔雀石绿浓度对脱色率的影响 Table 1 Effect of malachite green concentration on decolorization efficiency at pH 5 and 30℃ |
染料工业废水的pH具有多样性,因此在染料浓度为200 mg·L-1,脱色温度为30℃以及脱色时间为24 h的条件下,研究P. eryngii-Co007在不同pH下对孔雀石绿的脱色能力(图 1).实验结果表明,pH在5~7范围内P. eryngii-Co007对孔雀石绿具有较好的脱色效果,尤其当pH为6时,脱色效果最佳,24 h脱色率可达98.97%.分析原因是不同的pH对菌丝的生长以及木质素降解酶活性的影响不同,同时pH会改变木质素降解酶所带电荷,从而影响木质素降解酶对染料所带电荷基团的吸附(Hadibarata et al.,2013).Penicillium sp. YW01在pH 6时,对孔雀石绿达到最大脱色(Yang et al.,2011),Pseudomonas sp. DY1对孔雀石绿的最适脱色pH在5.5~8范围内,而达到最大脱色率的pH则为6.6(Du et al.,2011),T. trogii Berk S0301所产漆酶同样在pH 6时,对孔雀石绿的脱色最佳(Yan et al.,2014),本实验结果与以上报道相近.但是,Sphingomonas paucimobili在pH 9时,对孔雀石绿达到最大脱色(Ayed et al.,2009),这主要是因为菌种不同导致孔雀石绿的最佳脱色pH不同.后续研究选取pH 6为脱色体系的pH.
 |
| 图 1 pH对脱色率的影响 Fig. 1 Effect of pH on decolorization efficiency at 30℃and a concentration of 200 mg·L-1 |
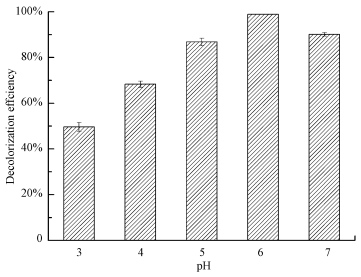
温度对白腐真菌的生长及其分泌的木质素降解酶活性有重要的影响,从而导致温度对染料脱色有影响.一般来说,随着温度的升高脱色率升高,因为在较高温度下酶的溶解度会提高,使得其对染料有较高的降解能力,当温度升高到一定程度,脱色率开始降低,这是因为酶出现失活所致((Hadibarata et al.,2013).因此本实验比较在染料浓度为200 mg·L-1,脱色pH为6以及脱色时间为6 h的条件下,20~40℃范围内菌丝对染料的脱色能力(图 2).当温度为30℃时,P. eryngii-Co007菌丝对孔雀石绿的脱色效果最佳,6 h脱色率可达94.31%,温度低于或者高于30℃,脱色率均有所降低,但是从整体来看,20~40℃范围内脱色率均在80%以上,说明该菌种在较大的温度范围内,对孔雀石绿有较好的脱色效果,在染料工业废水处理中具有广阔的应用前景.Pseudomonas sp. strain DY1对孔雀石绿脱色的最佳温度在25~30℃范围内(Du et al.,2011),本实验结果与该报道相符,而Deinococcus radiodurans(Lv et al.,2013)和Sphingomonas paucimobilis(Ayed et al.,2009)对孔雀石绿的最佳脱色温度为45℃,说明不同的菌种对孔雀石绿的最适脱色温度不同.后续研究选取30℃为脱色体系的温度.
 |
| 图 2 温度对脱色率的影响 Fig. 2 Effect of temperature on decolorization efficiency at pH 6 and a concentration of 200 mg·L-1 |
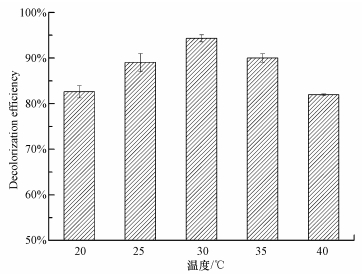
在已优化的脱色参数(pH 6,30℃)下,进行P. eryngii-Co007脱色降解200 mg·L-1孔雀石绿实验,定时取样,结果见图 3.P. eryngii-Co007脱色0.5 h,脱色率可达63.41%,这段时间脱色主要依赖于菌丝的吸附作用.随着时间延长,脱色率增加,9 h脱色率达98.22%,这段时间染料脱色则主要依赖于P. eryngii-Co007分泌的木质素降解酶对染料的降解作用.Bacillus cereus DC11对20 mg·L-1孔雀石绿脱色3 h,脱色率为96%(Deng et al.,2008),Blumea malcolmii对40 mg·L-1孔雀石绿脱色24 h,脱色率为93.4%(Kagalkar et al.,2011),Cosmarium sp.对10 mg·L-1孔雀石绿脱色7 h,脱色率为89.1%(Daneshvar et al.,2007),Penicillium pinophilum对10 mg·L-1孔雀石绿脱色4 d,脱色率为97.2%(Jasińska et al.,2012),Pseudomonas otitidis WL-13对182.5 mg·L-1孔雀石绿脱色12 h,脱色率为95.7%(Wu et al.,2009),Sphingomonas paucimobilis对50 mg·L-1孔雀石绿脱色24 h,脱色率为75%(Ayed et al.,2009),Pandoraea pulmonicola YC32对50 mg·L-1孔雀石绿脱色3.5 h,脱色率为95.6%(chen et al.,2009),Trametes sp. SQ01对100 mg·L-1孔雀石绿脱色6 d,脱色率为70%(Yang et al.,2009),而本实验所用菌种P. eryngii-Co007对200 mg·L-1孔雀石绿脱色,仅脱色9 h,脱色率高达98.22%,说明该菌种相比于所报道菌种,对孔雀石绿有着更高的脱色能力.
 |
| 图 3 时间对脱色率的影响 Fig. 3 Effect of time on decolorization efficiency at pH 6, 30℃and a concentration of 200 mg·L-1 |
取孔雀石绿原溶液(浓度200 mg·L-1)、6 h的降解液(脱色率89.24%)和12 h的降解液(脱色率99.04%)进行紫外-可见扫描,结果见图 4.孔雀石绿脱色6 h,其本身617 nm和315 nm处的特征吸收峰显著下降,420 nm处的特征吸收峰发生蓝移,即吸收峰向短波长方向移动,证明共轭多环芳香化合物孔雀石绿被降解.因为芳香化合物的共轭体系越大,越容易在长波长处产生吸收,若共轭体系遭到破坏,吸收峰则会发生蓝移(Du et al.,2013).脱色12 h,降解液中617 nm和315 nm处的吸收峰消失,在560 nm、360 nm和254 nm处分别产生3个新的吸收峰.有研究表明,如果染料脱色依赖于菌丝吸附作用,吸收峰将会成比例降低,而如果脱色依赖于酶解作用,吸收峰将会消失,同时在其它波长处产生新的吸收峰(Asad et al.,2007),由此可以说明P. eryngii-Co007对孔雀石绿的脱色是以酶解为主,而不是吸附.此外,根据文献报道,4-(二甲氨基)二苯甲酮是孔雀石绿降解的主要产物,其在360 nm处会产生吸收峰(Ju et al.,2009),因此推断360 nm处对应的物质为4-(二甲氨基)二苯甲酮.由图 4及脱色率可知,12 h的降解液中孔雀石绿基本被完全降解,所以用12 h的降解液来进行后续的产物分析研究.
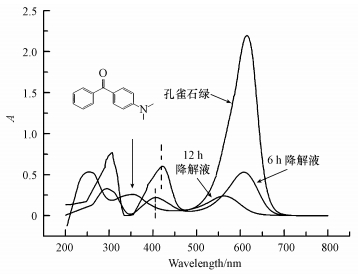 |
| 图 4 降解前后孔雀石绿的紫外-可见吸收光谱 Fig. 4 UV-vis absorption spectra of malachite green before and after degradation |
如图 5所示,孔雀石绿溶液和其12 h降解液的红外光谱图在指纹区(1500~500 cm-1)有明显的不同.对于孔雀石绿本身,1588.26 cm-1处为苯环的CC伸缩振动,1373.67 cm-1处为CH3不对称弯曲振动,1175.09 cm-1处为芳香化合物的C—N伸缩振动.对于孔雀石绿的降解液,1642.70 cm-1处为N—H变形振动,1562.63 cm-1处为与芳环连接的C=O伸缩振动,649.82 cm-1处为N—H面外弯曲振动,由此推断孔雀石绿的降解产物含有官能团C=O,—NH和—NH2.此外,因为900 cm-1以下吸收峰是芳香化合物的特征峰,吸收峰越少,代表芳香化合物因降解发生断裂(Ayed et al.,2009),孔雀石绿降解液的红外光谱图中832.38 cm-1、774.73 cm-1、726.69 cm-1和669.04 cm-1处吸收峰均消失,表明芳环断裂.
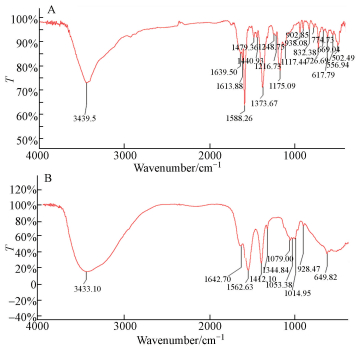 |
| 图 5 降解前后孔雀石绿的红外光谱 Fig. 5 FTIR spectra of malachite green before (A) and after (B) degradation |
如图 6和图 7所示,孔雀石绿的出峰时间为33.731 min,其分子离子峰对应的质荷比为330.2,经过12 h脱色,孔雀石绿本身的色谱峰基本消失,同时产生3个新的色谱峰,出峰时间分别为24.484 min、25.197 min和25.275 min,分子离子峰对应的质荷比分别为196.9、225.1和211.1,由此说明孔雀石绿被P. eryngii-Co007高效降解,并产生新的产物.通过与GS/MS NIST library进行比对,证实新产生的3种产物分别为4-氨基二苯甲酮、4-(二甲氨基)二苯甲酮和4-(甲氨基)二苯甲酮.这一结果与Micrococcus sp. strain BD15(Du et al.,2013)和Pseudomonas sp. strain DY1(Du et al.,2011)降解孔雀石绿的产物基本一致.
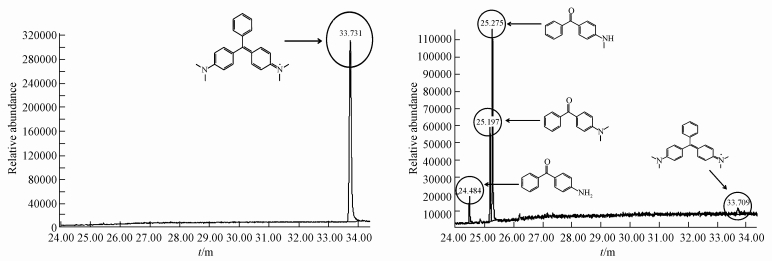 |
| 图 6 降解前后孔雀石绿的GC-MS色谱图 Fig. 6 Chromatography in GC-MS of malachite green before (A) and after (B) degradation |
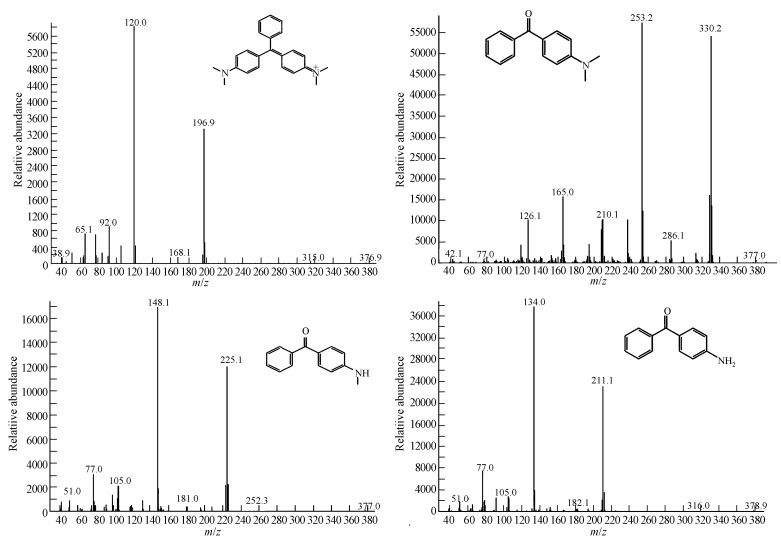 |
| 图 7 孔雀石绿及其降解产物的质谱图(A)孔雀石绿(B)4-(二甲氨基)二苯甲酮(C)4-(甲氨基)二苯甲酮(D)4-氨基二苯甲酮 Fig. 7 Mass spectra of malachite green and its degradation products (A) malachite green (B) 4-(Dimethylamino)benzophenone (C) 4-(methylamino)benzophenone (D) 4-aminobenzophenone |
根据以上产物鉴定并结合文献报道(Chen et al.,2002;Gao et al.,2008;Ju et al.,2009),推测出P. eryngii-Co007降解孔雀石绿的可能降解路径(图 8).首先为孔雀石绿中心碳的羟基化反应,随后中心碳迅速发生碳-碳键断裂,产生4-(二甲氨基)二苯甲酮,4-(二甲氨基)二苯甲酮再经过两个连续的N-去甲基化过程,分别产生4-(甲氨基)二苯甲酮和4-氨基二苯甲酮.Micrococcus sp. strain BD15(Du et al.,2013)对孔雀石绿的降解,中心碳会发生两个位点的碳-碳键断裂,分别产生4-(二甲氨基)二苯甲酮和米蚩酮,从而形成两条路径,而本实验结果与前者一致.
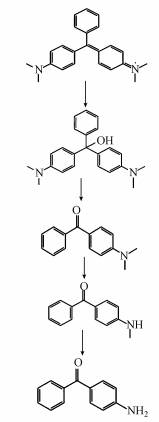 |
| 图 8 刺芹侧耳对孔雀石绿可能的降解路径 Fig. 8 Proposed pathway of malachite green degradation by P. eryngii-Co007 |
染料具有较强的毒性,因此在纺织工业污水释放到水体之前,必须先将染料除去.一般来说,经降解后的染料是无毒或低毒的,但是也存在降解产物比染料本身毒性更强的情况(Kalpana et al.,2011).为了评估P. eryngii-Co007降解孔雀石绿后形成的降解产物毒性,本文进行植物毒性实验(表 2).经3种方式处理的小麦种子,出芽率无差异,均为100%.根长、茎长及鲜重结果表明经孔雀石绿降解液处理的小麦种子与经去离子水处理的小麦种子差异不显著,而经孔雀石绿处理的小麦种子与其它两种相比均差异显著.由此可以说明,200 mg·L-1孔雀石绿溶液对小麦种子有极大的毒害作用,而P.eryngii-Co007对孔雀石绿有较好的脱毒作用.
| 表 2 孔雀石绿及其降解产物对植物的毒性实验结果 Table 2 Results of phytotoxicity tests of malachite green and its degradation products |
P. eryngii-Co007能够高效脱色降解高浓度的孔雀石绿;基于各种产物鉴定方法,推断出一条P. eryngii-Co007降解孔雀石绿的路径;植物毒性实验证明,P. eryngii-Co007对孔雀石绿有较好的脱毒作用.由此说明该菌株在染料工业废水的治理方面具有广阔的应用前景.
| [${referVo.labelOrder}] | Ayed L, Chaieb K, Cheref A, et al. 2009. Biodegradation of triphenylmethane dye malachite green by Sphingomonas paucimobilis[J]. World Journal of Microbiology and Biotechnology , 25 (4) : 705–711. DOI:10.1007/s11274-008-9941-x |
| [${referVo.labelOrder}] | Asad S, Amoozegar M A, Pourbabaee A A. 2007. Decolorization of textile azo dyes by newly isolated halophilic and halotolerant bacteria[J]. Bioresource Technology , 98 (11) : 2082–2088. DOI:10.1016/j.biortech.2006.08.020 |
| [${referVo.labelOrder}] | Beak M H, Ijagbemi C O, Kim D S. 2009. Treatment of malachite green-containing wastewater using poultry feathers as adsorbent[J]. Journal of Environmental Science and Health , 44 (5) : 536–542. DOI:10.1080/10934520902720132 |
| [${referVo.labelOrder}] | Chen F, He J J, Zhao J C, et al. 2002. Photo-Fenton degradation of malachite green catalyzed by aromatic compounds under visible light irradiation[J]. New Journal of Chemistry , 26 (3) : 336–341. DOI:10.1039/b107404k |
| [${referVo.labelOrder}] | Chen C Y, Kuo J T, Cheng C Y, et al. 2009. Biological decolorization of dye solution containing malachite green by Pandoraea pulmonicola YC32 using a batch and continuous system[J]. Journal of Hazardous Materials , 172 (2/3) : 1439–1445. |
| [${referVo.labelOrder}] | 陈敏, 姚善泾.2010. 原生质体复合诱变选育刺芹侧耳木质素降解酶高产菌株[J]. 高校化学工程学报 , 2010, 24 (3) : 462–467. |
| [${referVo.labelOrder}] | Deng D Y, Gao J, Zeng G Q, et al. 2008. Decolorization of anthraquinone, triphenylmethane and azo dyes by a new isolated Bacillus cereus strain DC11[J]. International Biodeterioration and Biodegradation , 62 (3) : 263–269. DOI:10.1016/j.ibiod.2008.01.017 |
| [${referVo.labelOrder}] | Daneshvar N, Ayazloo M, Khataee A R, et al. 2007. Biological decolorization of dye solution containing Malachite Green by microalgae Cosmarium sp.[J]. Bioresource Technology , 98 (6) : 1176–1182. DOI:10.1016/j.biortech.2006.05.025 |
| [${referVo.labelOrder}] | Du L N, Wang S, Li G, et al. 2011. Biodegradation of malachite green by Pseudomonas sp. strain DY1 under aerobic condition:characteristics, degradation products, enzyme analysis and phytotoxicity[J]. Ecotoxicology , 20 (2) : 438–446. DOI:10.1007/s10646-011-0595-3 |
| [${referVo.labelOrder}] | Du L N, Zhao M, Li G, et al. 2013. Biodegradation of malachite green by Micrococcus sp. strain BD15:Biodegradation pathway and enzyme analysis[J]. International Biodeterioration and Biodegradation , 78 (2) : 108–116. |
| [${referVo.labelOrder}] | Gao G D, Zhang A Y, Zhang M. 2008. Photocatalytic degradation mechanism of malachite green under visible light irradiation over novel biominetic photocatalyst HMS-FePcs[J]. Chinese Journal of Catalysis , 29 (5) : 426–430. DOI:10.1016/S1872-2067(08)60043-1 |
| [${referVo.labelOrder}] | Hadibarata T, Adnan L A, Yusoff A R M, et al. 2013. Microbial Decolorization of an Azo Dye Reactive Black 5 Using White-Rot Fungus Pleurotus eryngii F032[J]. Water Air Soil Pollut , 224 (6) : 1595–1603. DOI:10.1007/s11270-013-1595-0 |
| [${referVo.labelOrder}] | Ju Y M, Yang S G, Ding Y C. 2008. Microwave-assisted rapid photocatalytic degradation of malachite green in TiO2 suspensions:mechanism and pathways[J]. Journal of Physical Chemistry A , 112 (44) : 11172–11177. DOI:10.1021/jp804439z |
| [${referVo.labelOrder}] | Jadhav J P, Govindwar S P. 2006. Biotransformation of malachite green by Saccharomyces cerevisiae MTCC 463[J]. Yeast , 23 (4) : 315–323. DOI:10.1002/(ISSN)1097-0061 |
| [${referVo.labelOrder}] | Jasińska A, Róalska S, Bernat P, et al. 2012. Malachite green decolorization by non-basidiomycete filamentous fungi of Penicillium pinophilum and Myrothecium roridum[J]. International Biodeterioration and Biodegradation , 73 (9) : 33–40. |
| [${referVo.labelOrder}] | Ju Y M, Yang S G, Ding Y C. 2009. Microwave-enhanced H2O2-based process for treating aqueous malachite green solution:Intermediates and degradation mechanism[J]. Journal of Hazardous Materials , 171 (1/3) : 123–132. |
| [${referVo.labelOrder}] | Kusvran E, Gulnaz O, Samil A, et al. 2011. Decolorization of malachite green, decolorization kinetics and stoichiometry of ozone-malachite green and removal of antibacterial activity with ozonation processes[J]. Journal of Hazardous Materials , 186 (1) : 134–143. |
| [${referVo.labelOrder}] | Kagalkar A N, Jadhav M U, Bapat V A, et al. 2011. Phytodegradation of the triphenylmethane dye Malachite Green mediated by cell suspension cultures of Blumea malcolmii Hook[J]. Bioresource Technology , 102 (22) : 10312–10318. DOI:10.1016/j.biortech.2011.08.101 |
| [${referVo.labelOrder}] | Kalpana D, Shim J H, Oh B T, et al. 2011. Bioremediation of the heavy metal complex dye Isolan Dark Blue 2SGL-01 by white rot fungus Irpex lacteus[J]. Journal of Hazardous Materials , 198 (2) : 198–205. |
| [${referVo.labelOrder}] | Lee J B, Kim M. 2012. Photo-degradation of malachite green in mudfish tissues-investigation of UV-induced photo-degradation[J]. Food Science and Biotechnology , 21 (2) : 519–524. DOI:10.1007/s10068-012-0066-5 |
| [${referVo.labelOrder}] | 李慧蓉, 李华兵, 李文琼, 等.1999. 黄孢原毛平革菌对6种染料的脱色降解[J]. 环境科学研究 , 1999, 12 (3) : 14–17. |
| [${referVo.labelOrder}] | Lv G Y, Cheng J H, Chen X Y, et al. 2013. Biological decolorization of malachite green by Deinococcus radiodurans R1[J]. Bioresource Technology , 144 (5) : 275–280. |
| [${referVo.labelOrder}] | Moumeni O, Hamdaoui O. 2012. Intensification of sonochemical degradation of malachite green by bromide ions[J]. Ultrasonics Sonochemistry , 19 (3) : 404–409. DOI:10.1016/j.ultsonch.2011.08.008 |
| [${referVo.labelOrder}] | Patidar R, Khana S, Moholkar V S. 2012. Physical features of ultrasound assisted enzymatic degradation of recalcitrant organic pollutants[J]. Ultrasonics Sonochemistry , 19 (1) : 104–118. DOI:10.1016/j.ultsonch.2011.06.005 |
| [${referVo.labelOrder}] | Radha K V, Regupathi I, Arunagiri A, et al. 2005. Decolorization studies of synthetic dyes using Phanerochaete chrysosporium and their kinetics[J]. Process Biochemistry , 40 (10) : 3337–3345. DOI:10.1016/j.procbio.2005.03.033 |
| [${referVo.labelOrder}] | Saha S, Wang J M, Pal A. 2012. Nano silver impregnation on commercial TiO2 and a comparative photocatalytic account to degrade malachite green[J]. Separation and Purification Technology , 89 (2) : 147–159. |
| [${referVo.labelOrder}] | Srivastava S, Sinha R, Roy D. 2004. Toxicological effects of malachite green[J]. Aquatic Toxicology , 66 (3) : 319–329. DOI:10.1016/j.aquatox.2003.09.008 |
| [${referVo.labelOrder}] | Shedbalkar U, Jadhav J P. 2011. Detoxification of malachite green and textile industrial effluent by Penicillium ochrochloron[J]. Biotechnology and Bioprocess Engineering , 16 (1) : 196–204. DOI:10.1007/s12257-010-0069-0 |
| [${referVo.labelOrder}] | Sani R K, Banerjee U C. 1999. Decolorization of triphenylmethane dyes and textile and dye-stuff effluent by Kurthia sp.[J]. Enzyme and Microbial Technology , 24 (7) : 433–437. DOI:10.1016/S0141-0229(98)00159-8 |
| [${referVo.labelOrder}] | Shedbalkar U, Dhanve R, Jadhav J. 2008. Biodegradation of triphenylmethane dye cotton blue by Penicillium ochrochloron MTCC 517[J]. Journal of Hazardous Materials , 15 (2/3) : 472–479. |
| [${referVo.labelOrder}] | Wu J, Jung B G, Kim K S, et al. 2009. Isolation and characterization of Pseudomonas otitidis WL-13 and its capacity to decolorize triphenylmethane dyes[J]. Journal of Environmental Sciences , 21 (7) : 960–964. DOI:10.1016/S1001-0742(08)62368-2 |
| [${referVo.labelOrder}] | Yang X Q, Zhao X X, Liu C Y, et al. 2009. Decolorization of azo, triphenylmethane and anthraquinone dyes by a newly isolated Trametes sp. SQ01 and its laccase[J]. Process Biochemistry , 44 (10) : 1185–1189. DOI:10.1016/j.procbio.2009.06.015 |
| [${referVo.labelOrder}] | Yang Y, Wang G, Wang B, et al. 2011. Decolorization of malachite green by a newly isolated Penicillium sp. YW 01 and optimization of decolorization parameters[J]. Environmental Engineering Science , 28 (8) : 555–562. DOI:10.1089/ees.2010.0172 |
| [${referVo.labelOrder}] | Yan J P, Niu J Z, Chen D D, et al. 2014. Screening of Trametes strains for efficient decolorization of malachite green at high temperatures and ionic concentrations[J]. International Biodeterioration and Biodegradation , 87 (1) : 109–115. |
 2016, Vol. 36
2016, Vol. 36


