随着科技的迅速发展,工业化和城市化程度的不断提高,水体富营养化的问题日益严重,使得水资源更加紧张.而氮是引起水体富营养化的主要因素.所以越来越多的国家和地区制定了氮排放标准.因此,研究开发经济、高效的脱氮技术已成为水污染控制工程领域的研究重点.
生物处理法作为19 世纪末废水处理新型技术,与物化处理法相比具有处理费用低,不会对环境造成二次污染等优点.因此,生物处理法至今已成为世界各国污水二、三级处理的主要手段.众所周知氮元素可在相应微生物的作用下转化成各种氧化态和化学形式(目前已知的生物氮循环途径如 图 1所示),因此在污水生物脱氮处理中衍生了大量组合工艺.而厌氧氨氧化过程是目前最捷径的生物脱氮过程,因此被誉为最具前景的污水脱氮工艺.为了更好的将厌氧氨氧化工艺应用到实际规模中,本文着重对厌氧氨氧化菌的发现及其与污水处理中常见细菌的协同与竞争关系进行了详细的综述.旨在为厌氧氨氧化工艺在污水生物处理中的应用提供理论依据,并为今后厌氧氨氧化工艺的研究方向提出一些意见.
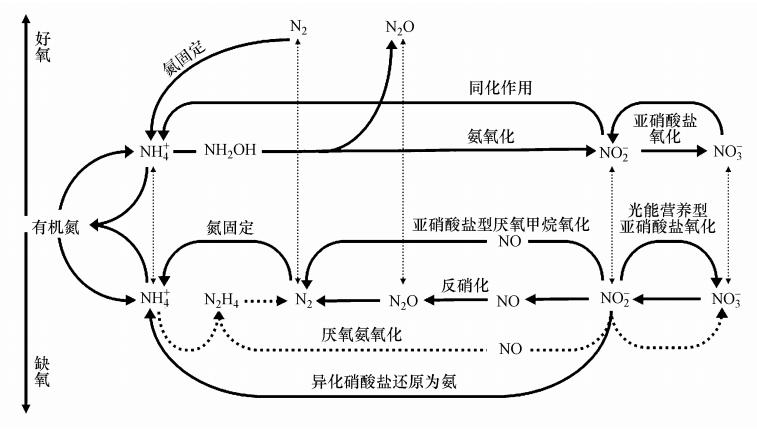 |
| 图 1 氮循环示意图(Thamdrup,2012) Fig. 1 Nitrogen cycle |
早在1976年,Broda(Broda,1977)预言在自然界中存在一种以NO-2或NO-3作为电子受体把NH+4氧化成N2的化能自养型细菌.直到1995年,Mulder等(Mulder et al.,1995)在处理酵母废水的反硝化流化床反应器内发现了NH+4消失的现象,从而证实了厌氧氨氧化反应的存在.
厌氧氨氧化(Anaerobic ammonium oxidation,Anammox)是在缺氧条件下以亚硝酸盐(NO-2)为电子受体将氨(NH+4)转化成氮气(N2)(Vand egraaf et al.,1995),同时伴随着以亚硝酸盐为电子供体固定CO2并产生硝酸盐(NO-3)的生物过程(Strous et al.,1999a).执行该过程的微生物称之为厌氧氨氧化菌(Anaerobic ammonium oxidation bacteria,AAOB),其化学计量学方程式(Strous et al.,1998)如下:
1NH+4+1.32NO-2+0.066HCO-3+0.13H+→ 1.02N2+0.26NO-3+0.066CH2O0.5N0.15+2.03H2O
由于氨氧化细菌(Ammonium oxidation bacteria,AOB)可将氨氧化成亚硝酸盐,为AAOB提供基质,所以目前对厌氧氨氧化工艺的应用通常与短程硝化(亚硝化)联系在一起. 图 2为亚硝化-厌氧氨氧化工艺与传统的硝化-反硝化工艺的对比,通过对比可知亚硝化-厌氧氨氧化工艺具有如下优点(Kartal et al.,2010):
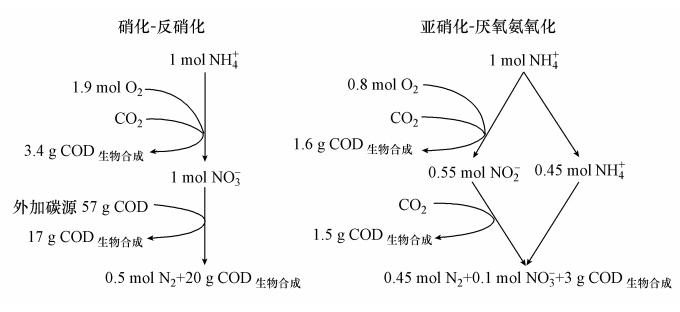 |
| 图 2 硝化-反硝化工艺与亚硝化-厌氧氨氧化工艺的比较(Siegrist et al., 2008) Fig. 2 Comparison between nitrification-denitrification(left) and the nitritation-anammox process(right) |
①厌氧氨氧化在缺氧条件下进行,无需氧气的供应,可节省62.5%的能源消耗.
②厌氧氨氧化以无机碳(CO2或HCO-3)为碳源,无需投加有机碳,大大节省了碳源.
③亚硝化-厌氧氨氧化所产生的CO2与普通的硝化-反硝化系统相比减少90%.
④AAOB生长缓慢、产率低,因此工艺剩余污泥量少,污泥处置费用低.
⑤厌氧氨氧化氮去除率及氮去除负荷较高,从而能够减少工艺占地面积,降低工艺基建成本.
AAOB是一群分支很深的浮霉状菌(Strous et al.,1999a).AAOB生长缓慢,在30~40℃条件下,其倍增时间为10~14 d(Strous et al.,2006),细胞产率为0.11 g(VSS)/g(NH+4)(Strous et al.,1999a),如果对培养条件进行优化,其倍增时间可缩短至4.8 d(Tang et al.,2011b),甚至1.8 d(Isaka et al.,2006).如表 1所示为目前已发现的5属17种Anammox菌.相关研究表明,AAOB为地球氮循环做出了巨大贡献,目前已在自然环境和人工环境中发现了大量AAOB的存在.其中厌氧的自然生态系统包括:海洋沉积物和海洋水体(Galán et al.,2009; Rich et al.,2008)、淡水沉积物和淡水水体(Moore et al.,2011;Yoshinaga et al.,2011)、红树林地区(Meyer et al.,2005)以及陆地生态系统(Zhu et al.,2011b)等.而在人工生态系统中包括:污水处理厂(van der Star et al.,2007)、海洋循环水产养殖系统(Tal et al.,2006)、垃圾渗滤液处理系统(Egli et al.,2001).此外,人们以各种环境中发现的AAOB作为基础,将其引入污水处理系统,循序渐进地对AAOB进行驯化培养以处理各类废水,包括实验室规模、中试规模及实际规模的污泥消化液(Joss et al.,2009)、垃圾渗滤液(Liang et al.,2009)、焦化废水(Toh and Ashbolt,2002)、味精废水(Shen et al.,2012)、养猪废水(Yamamoto et al.,2008)以及制药废水(Tang et al.,2011a)等.
| 表1 目前已发现的5属17种AAOB Table.1 The five genera and seventeen species of AAOB discovered to date |
厌氧氨氧化工艺是一种高效脱氮工艺,其最大氮负荷可达9.5 kg · m-3 · d-1,且处理污水成本只需0.75C =· kg-1,因此厌氧氨氧化是一种极具前景的生物处理工艺(van der Star et al.,2007).但由于厌氧氨氧化至今未能获得纯培养,为了得到高纯度的AAOB,目前只能用密度梯度离心的方法获得纯度为99.8%的AAOB(Strous et al.,1999a),因此对AAOB的研究仍受到很大的阻碍,这意味着对厌氧氨氧化工艺的应用及推广也会带来诸多难题.然而“在单个细胞水平上对基因组进行测序”的单细胞测序技术能够解决上述难题(de Souza,2012).因此对AAOB的进一步了解有赖于单细胞测序技术的成熟应用.
3 AAOB与其他微生物之间的关系(The relationship between the AAOB and other microorganisms)在自然生态系统和人工生态系统中,AAOB不仅与环境有着密切的关系,而且与其他微生物也有着密切的关系.微生物之间的关系有种内和种间关系.其中AAOB的种内关系主要体现在群体感应系统,而AAOB的种间关系主要是与氨氧化菌、亚硝酸盐氧化菌、反硝化菌、厌氧甲烷氧化菌的合作与竞争关系.
3.1 AAOB的种内关系(群体感应系统)群体感应作为一种通讯机制在微生物细胞之间普遍存在,它能够根据菌群密度和周围环境的变化来调节基因表达,从而控制菌群行为.由于AAOB 至今未能得到纯培物,所以对AAOB 群体感应的深入研究无从下手. 但根据目前对AAOB的研究来看,Ding等(Ding et al.,2013)认为有3个现象可以推测厌氧氨氧化菌之间存在群体感应系统.
(1)厌氧氨氧化活性存在密度依赖性.根据Strous等(Strous et al.,1999a)的研究发现,只有当AAOB细胞浓度大于1010~1011 cell · mL-1时,AAOB才具有厌氧氨氧化活性.
(2)目前认为AAOB属于革兰氏阴性细菌,而对于革兰氏阴性细菌的群体感应系统而言其自诱导物为N-乙酰化高丝氨酸内酯(N-acyl-homoserine lactones,AHLs)类化合物,主要用作种内“语言”,且对于革兰氏阴性菌中AHLs的合成来说,S-腺苷甲硫氨酸(S- adenosyl methionine,SAM)和乙酰化载体蛋白(acyl-acyl carrier protein,acyl-ACP)是必要的生物分子.Strous等(Strous et al.,2006)利用宏基因组学技术对C and idatus Kuenenia stuttgartiensis 的脂肪酸代谢基因组学信息进行了研究,发现其具有编码SAM和acyl-ACP的合成酶的相关基因,证明其具有自诱导物合成潜力.
(3)De Clippeleir 等(De Clippeleir et al.,2011)在研究长链高丝氨酸内酯对OLAND生物膜中厌氧氨氧化速率的影响时发现,在OLAND生物膜系统 中,添加N-十二酰基高丝氨酸内酯(N-dodecanoyl homoserine lactone,C12-HSL)可以增强AAOB活性,而对AOB没有影响.C12-HSL属于AHLs中碳链较长的一种,其对革兰氏阴性细菌的自诱导起到关键的作用.
3.2 AAOB的种间关系 3.2.1 AAOB的种间竞争关系目前已发现的AAOB有5属17种,根据目前对AAOB的研究来说,除了Schmid等(2003)用AAOB处理垃圾渗滤液的研究中发现C and idatus Scalindua brodae和C and idatus Scalindua wagneri有等量共存的现象,绝大多数情况下很少能发现两种AAOB在相同单一生境中等量共存的现象(Kartal et al.,2007),这意味着AAOB的确存在种间的竞争关系.不论在自然生态系统还是人工生态系统C and idatus Brocadia和C and idatus Kuenenia都最为常见,现以该两种菌属为例讨论AAOB的种间竞争关系.
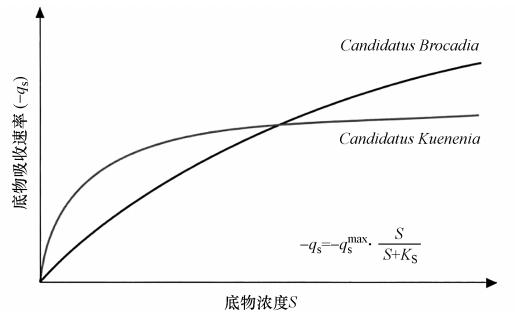 |
| 图 3 C and idatus Kuenenia与C and idatus Brocadia的竞争曲线 Fig. 3 The competition curve of C and idatus Kuenenia and C and idatus Brocadia |
van der Star(van der Star et al.,2008)等,用膜生物反应器富集AAOB时发现,在富集过程中存在种群结构逐渐由原来的C and idatus Brocadia转变为C and idatus Kuenenia的现象.他们认为C and idatus Brocadia的繁殖策略属于r-策略,而C and idatus Kuenenia则属于K-策略. 图 3为C and idatus Kuenenia与C and idatus Brocadia的竞争曲线,其中C and idatus Kuenenia的亚硝酸盐半饱和常数KS=0.2~0.3 μmol · L-1,而C and idatus Brocadia的亚硝酸盐半饱和常数KS≈5 μmol · L-1(Strous et al.,1999b).因此C and idatus Kuenenia较C and idatus Brocadia生长缓慢,所以在底物浓度较低的情况下,C and idatus Kuenenia占主导作用,相反在底物浓度较高的情况下,C and idatus Brocadia占主导作用.因此,对于污水处理系统的高效运行来说,C and idatus Brocadia可能是很好的选择.而对于处理低浓度废水C and idatus Kuenenia则更为合适.
3.2.2 AAOB与硝化菌的种间关系(1)AAOB与硝化菌基于底物协同作用
AOB将氨氧化成亚硝酸盐,为AAOB提供基质,与此同时AAOB也为AOB去除有毒物质(亚硝酸盐).在自然生态系统中,AOB和AAOB经常共同生长在氧最小区域(oxygen minimum zones,OMZs).目前已在多处海洋的OMZs处发现AOB和AAOB的存在,如智利北部OMZs发现AAOB(Galán et al.,2009),且在同一地平线处的秘鲁OMZs中发现AOB(Ward et al.,1989).如 图 4所示,为海洋生态系统中AAOB与其他微生物的竞争协同关系.据报道,在海洋50 m深处,AAOB的生物量(高达3000 cell · mL-1)和多样性最高,而当深度达到300 m时,AAOB的生物量降低到450 cell · mL-1.这是因为深达300 m处的海洋极度缺氧,导致AOB不能生存,因此不能为AAOB提供充足的亚硝酸盐,所以在300 m深处AAOB活性丧失(Galán et al.,2009).同时在黑海的OMZs中也存在着AOB-AAOB互利共存的现象(Lam et al.,2007).
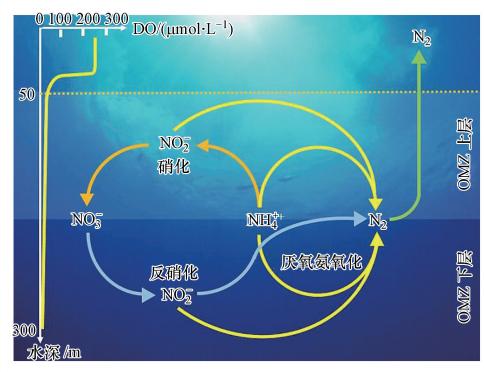 |
| 图 4 海洋生态系统中AAOB与其他微生物的竞争协同作用示意图 Fig. 4 The competition and synergism between AAOB and other microorganisms in marine ecosystem |
在人工生态系统中,CANON(completely autotrophic nitrogen removal over nitrite)工艺(Sliekers et al.,2002)和OLAND(oxygen limited autotrophic nitrification and denitrification)工艺(Philips et al.,2002)结合AOB和AAOB进行脱氮.其原理为AOB首先将氨转化成亚硝酸盐,之后由AAOB将剩余的氨和AOB产生的亚硝酸盐转化成氮气.Sliekers等(Sliekers et al.,2004)研究表明AAOB不具备水解尿素的能力,因此AAOB不能以尿素作为氮源,但是AOB具有将尿素转化为氨氮的能力,因此在处理含尿素废水时,AOB不但能为AAOB提供亚硝酸盐,而且还能提供氨氮(Liu et al.,2008a).AAOB和AOB的协同作用主要依赖氨和亚硝酸盐,其中亚硝酸盐占主导地位.
(2)AAOB与硝化菌空间协同作用
空间协同作用主要存在于颗粒污泥和生物膜中,AAOB、AOB、NOB在颗粒污泥或生物膜中的位置主要由DO决定.因为AAOB对氧气极为敏感,耐氧能力极低(Strous et al.,1997),所以AOB通常长在颗粒污泥或生物膜的表层,而AAOB则长在内部(Nielsen et al.,2005).这是因为AOB可以消耗氧气为AAOB提供缺氧环境(Tsushima et al.,2007),同时还可以提供亚硝酸盐基质.对NOB进行检测发现其在颗粒污泥中的含量远低于AOB,究其原因主要是在低氧环境下AOB对氧的亲和力比NOB要强(Blackburne et al.,2008). 图 5所示为AAOB与AOB的空间协同作用关系.
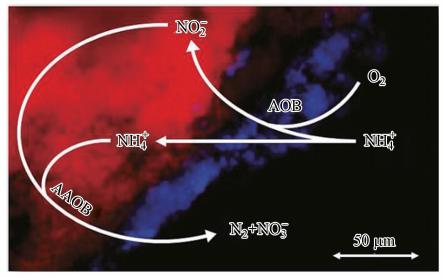 |
| 图 5 AAOB与AOB的空间协同作用示意图(背景为颗粒污泥切片荧光原位杂交(fluorescence in situ hybridization,FISH)图片; 其中AAOB探针AMX368用Cy3标记(红色),AOB探针NSO190、NOS1225用Cy5标记(蓝色),NOB探针Ntspa662、NIT1035用Fluos标记(绿色)) Fig. 5 The spatial synergism of AAOB and AOB |
(3)AAOB与硝化菌基于底物和DO的竞争作用
AOB和AAOB都以氨作为基质,然而DO作为一个重要的影响因子可以决定系统中的优势菌种.由于AAOB无需氧气所以在缺氧条件下AAOB占主导作用.但这并不意味着AOB会在系统中消失,因为AOB有多种代谢方式,这有益于他们在不利的条件下生存(Bock et al.,1995). 有趣的是,据报道Nitrosomonas europaea也可以按照厌氧氨氧化方式进行代谢,但其竞争基质的能力弱于AAOB(Schmidt and Bock,1998).
氧对厌氧氨氧化活性具有极强抑制作用.据Jetten等(Jetten et al., 1998)报道微量的溶解氧(>0.5%)就会抑制厌氧氨氧化过程.而Strous等(Strous et al., 1997)研究表明,只有在充入惰性气体,将水中的溶解氧完全吹脱时,AAOB才具备降解氨及亚硝酸盐的能力,也就是说只有在严格无氧的条件下才能检测到厌氧氨氧化活性,且该研究进一步表明,溶解氧抑制是可逆的.此外AAOB与AOB相比其倍增时间长且细胞产率低.以上这些说明,当AOB与AAOB在好氧条件下进行竞争,AOB会获得更多的基质和空间.
3.2.3 AAOB与反硝化菌的种间关系(1)AAOB与反硝化菌基于底物的协同作用
在自然生态系统中,如海洋表面,由于氧气比较充足,氨可被氧化为硝酸盐,在水的流动作用下硝酸盐被传输到海洋深处的缺氧区,在缺氧区反硝化菌可将硝酸盐还原成亚硝酸盐,为AAOB提供必要基质. 为了衡量厌氧氨氧化作用对海洋氮循环的贡献大小,对格陵兰东、西海岸的沉积物、杜尔塞海湾的缺氧水体以及黑海水域做了相关研究.发现由厌氧氨氧化作用所产生的N2占总N2的量分别为:1%~35%(Rysgaard et al., 2004)、19%~35%(Dalsgaard et al., 2003)和40%(Kuypers et al., 2003).
在人工生态系统中,AAOB可以和反硝化菌共存.在以反硝化污泥当做种泥来启动厌氧氨氧化反应器研究中发现AAOB和反硝化菌都以亚硝酸盐当做基质,但能够决定脱氮过程的主要因素是C/N比和TOC负荷(Sumino et al., 2006).Tal等(Tal et al., 2006)以硝化污泥和反硝化污泥当做种泥来富集厌氧氨氧化,结果表明用反硝化污泥富集的厌氧氨氧化活性较高(以联氨氧化活性(Schalk et al., 1998)表征约0.16 μmol · beed-1 · h-1),说明AAOB和反硝化菌的协同作用要强于AOB.
(2)AAOB与反硝化菌基于底物和有机物的竞争作用
反硝化菌和AAOB都以亚硝酸盐作为基质,但反硝化菌的另一种基质有机物能够抑制厌氧氨氧化活性.当COD/NO-2-N比值较低时,以厌氧氨氧化方式去除氮的过程不会有显著的影响(Güven et al., 2005),甚至AAOB能够利用有机物作为基质.但当COD/NO-2-N比值较高时,亚硝酸盐将会被反硝化菌所利用,从而抑制AAOB(Furukawa et al., 2009).值得注意的是反硝化菌是异养菌,其生长速率和细胞产量都比AAOB高,自养的AAOB逐渐被淘洗从而使厌氧氨氧化的脱氮能力降低(Lackner et al., 2008),这意味着如果反应器一直在不利于AAOB的条件下运行,反硝化菌的数量将会超过AAOB的数量,而AAOB在整个菌群中的比例将会随之降低(Tang et al., 2010).但相关研究表明在少量有机物存在的条件下AAOB可以执行另一种代谢途径:利用有机物作为电子供体将硝酸盐还原为氨,即异化硝酸盐还原为氨(dissimilatory nitrate reduction to ammonia,DNRA)(Kartal et al., 2008). 图 6所示为少量有机物存在条件下,AAOB与反硝化菌的竞争关系.
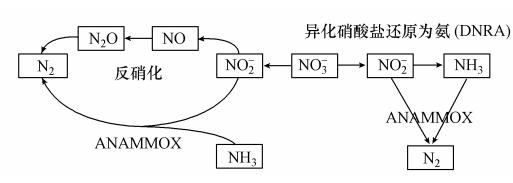 |
| 图 6 有机物存在下AAOB与反硝化菌的竞争关系 Fig. 6 The competition between AAOB and denitrifier in the presence of organic matter |
Güven 等(Güven et al., 2005)研究表明,有机酸能被AAOB转化,而对于丙酸来说,其消耗速率较高为0.8 nmol · min-1 · mg protein-1,而葡萄糖、甲酸、丙酸则对厌氧氨氧化的活性无明显影响.进一步研究发现AAOB以亚硝酸盐或硝酸盐作为电子受体的情况下可将丙酸转化为CO2.且对长达150 d系统中的微生物进行种群结构的分析发现AAOB数量和反硝化菌的数量并没有显著变化,这意味着AAOB与反硝化菌相比显示出对丙酸的竞争优势.在膜曝气生物膜系统中,当COD/N>2时,厌氧氨氧化活性降低,异养菌成为优势菌属(Lackner et al., 2008).当COD高于300 mg · L-1时,厌氧氨氧化活性丧失,甚至从系统中消失(Chamchoi et al., 2008). Molinuevo等(Molinuevo et al., 2009)研究表明,当COD高于290 mg · L-1时,厌氧氨氧化作用被反硝化作用所取代.如若发生上述这些情况,通常采用AAOB细胞固定化技术(Furukawa et al., 2009)或额外投加AAOB(Tang et al., 2011a)的方法来解决.
3.2.4 AAOB与厌氧甲烷氧化菌的种间关系据报道氨、溶解性甲烷、亚硝酸盐(硝酸盐)通常共存于缺氧生态环境中(Luesken et al., 2011a).而根据化学计量反应式(1)(2),吉布斯自由能变皆小于零,说明反应可自发正向进行,因此可以推测自然界中存在以NO-2和NO-3为电子受体的厌氧甲烷氧化过程.但经过长时间的调查和研究都未曾在自然界中寻其踪迹(Strous and Jetten, 2004).直至1991年,Smith等(Smith et al., 1991)利用原位自然梯度示踪实验在受污染的地下水环境中发现了厌氧甲烷氧化耦合反硝化的现象,但一直未能获得其富集培养物.直至2006年Raghoebarsing 等(Raghoebarsing et al., 2006),用Twentekanaal河的缺氧沉积物作为接种污泥,在实验室条件下获得了以硝态氮作为电子受体的厌氧甲烷氧化富集培养物,并将该过程命名为反硝化型甲烷厌氧氧化(Denitrification-dependent anaerobic methane oxidation,DAMO). 值得注意的是,当系统中同时存在NO-2和NO-3时,反硝化型厌氧甲烷氧化过程优先利用亚硝酸盐作为电子受体(Raghoebarsing et al., 2006),因此又将该过程称为依赖亚硝酸盐型的厌氧甲烷氧化(nitrite-dependent anaerobic methane oxidation,N-DAMO).
在自然生态系统中,N-DAMO主要分布于淡水水域(Kojima et al., 2012),且在淡水沉积物和淡水水域中也发现了AAOB的存在,这是否能够说明N-DAMO和AAOB可以在相同的生境下共存呢?通过对N-DAMO与AAOB的生理学和生态学的研究表明,N-DAMO与AAOB很可能在含有甲烷、氨、氮氧化物的缺氧生态环境中共存(Zhu et al., 2010).
Wang等(Wang et al., 2012),在研究水稻土(0~100 cm)中N-DAMO与AAOB的共存和分布时发现N-DAMO与AAOB共存于水稻土中,且两者均在30~60 cm处分布较多.Yang 等(Yang et al., 2012),对青藏高原的两个盐湖(盐度分别为32和84 g · L-1)的表层沉积物进行研究,发现N-DAMO和AAOB可以共存于天然盐碱环境中.Zhu 等(Zhu et al., 2010),对湿地生态系统中的厌氧氨氧化和厌氧甲烷氧化作用进行了详细的综述,并推测淡水湿地非常适合AAOB和N-DAMO的共同生长.
在人工生态系统中,同样也发现了N-DAMO与AAOB共存的现象.Luesken等(Luesken et al., 2011a),以N-DAMO富集污泥(70%~80%)作为种泥,在SBR反应器中进行N-DAMO和AAOB的混合培养.161 d后,混合培养物中N-DAMO和AAOB的数量各占50%左右,且该系统能有效的同步去除氨、溶解性甲烷及亚硝酸盐,其亚硝酸盐转化速率为0.1 kg · m-3 · d-1(17.2 mmol · d-1).而Zhu 等(Zhu et al., 2011a),则以实际规模的厌氧氨氧化反应器中的颗粒污泥作为种泥,进行N-DAMO和AAOB的混合培养研究.研究表明AAOB颗粒污泥作为接种污泥进行N-DAMO和AAOB的混合培养是可行的.同时他们也证明了实际规模的厌氧氨氧化反应器(Luesken et al., 2011b)中的N-DAMO的确为NO-2(或NO-3)及甲烷的去除做了贡献.
关于N-DAMO与AAOB的协同与竞争作用的机制研究较少.如 图 6所示,为AAOB与N-DAMO的竞争与协同作用关系.从图中可以看出,N-DAMO与AAOB都在缺氧条件下生存且都以亚硝酸盐作为电子受体,即他们在同一个体系中共同竞争亚硝酸盐.且Jetten等(Jetten et al.,2008)表明如果系统中氨含量过高,则N-DAMO将会失去与AAOB的竞争能力,这说明AAOB与N-DAMO相比具有更强的亚硝酸盐亲和能力.此外,N-DAMO反应过程中所产生的CO2可作为AAOB的无机碳源,而当NO-2浓度较低时,AAOB反应过程中产生的NO-3也可作为N-DAMO的电子受体.
厌氧消化工艺的主要终产物为氨和甲烷.气相中的甲烷易被收集和纯化用作能源物质,但液相中的甲烷却很难被回收,尤其是在低温条件下.如果不对溶解性甲烷进行适当处理,其将会缓慢释放到空气中造 成温室效应.因此N-DAMO与AAOB混合培养技术 用于处理厌氧消化工艺出水具有非常光明的前景.
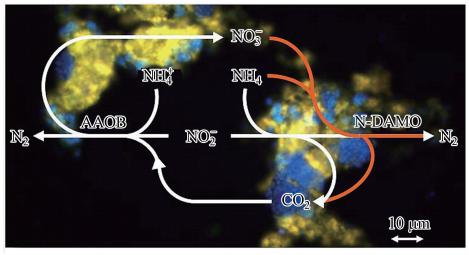 |
| 图 7 AAOB与N-DAMO的竞争与协同作用示意图(背景为荧光原位杂交(fluorescence in situ hybridization,FISH)图片; 其中AAOB探针AMX368用Cy5标记(蓝色),N-DAMO探针DBACT1027用Cy3标记(黄色)) Fig. 7 The competition and synergism between the AAOB and N-DAMO |
厌氧氨氧化工艺作为一种具有能耗低、无需碳源等优点的新兴工艺,受到越来越多学者的研究和关注.但执行厌氧氨氧化过程的AAOB生长缓慢、细胞产率低,且易受环境条件的影响,使得该工艺应用受到严重阻碍.为了更好的将厌氧氨氧化工艺投入实际生产规模中,对有利于AAOB种群竞争的因素进行相关研究是相当必要的.而想要促进厌氧氨氧化生长,提高厌氧氨氧化活性,必须优化营养条件和环境条件.如 图 8所示为各竞争因素对AAOB种群结构的影响.通过控制运行参数可对种群结构进行调整,使相应的菌种介入为AAOB提供基质,而将AAOB的竞争 者从反应器中淘汰.因此研究AAOB与其他细菌的协同竞争关系相当重要.
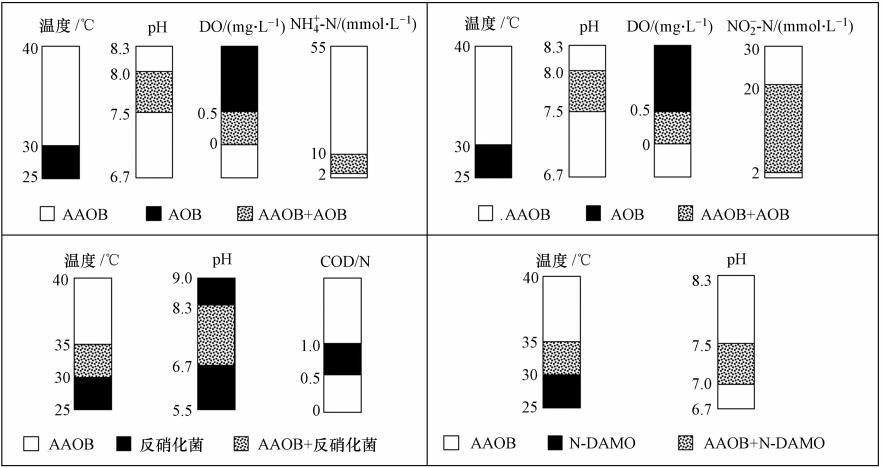 |
| 图 8 竞争因素对AAOB种群结构的影响(Raghoebarsing et al.,2006;郑平等,2004) Fig. 8 The impact of competitive factors on AAOB population structure |
对今后AAOB与其他细菌的协同竞争关系的相关研究,建议学者们从如下几个方面进行.
1)由于厌氧氨氧化的纯培物至今未能获得,因此阻碍了AAOB的群体感应系统和种内竞争的机理研究,为更加深入的了解AAOB种内关系建议加强AAOB的纯培养研究.
2)目前只对C and idatus Kuenenia stuttgartiensis和C and idatus Scalindua profunda的基因组进行了研究,且表现出明显的区别.而其他的厌氧氨氧化菌的序列测定仍没有报道,是否其他厌氧氨氧化菌也拥有某些特殊功能基因,而这些基因又能够潜在的影响AAOB种内关系,这对AAOB的种内关系的研究来说具有非常重要的意义.
3)由于N-DAMO的研究才刚刚起步,所以目前对N-DAMO与AAOB混合培养的研究甚少,以至于对N-DAMO与AAOB的协同竞争关系仍然不明确,而厌氧氨氧化-甲烷厌氧氧化联合工艺对处理厌氧消化工艺出水非常有效,因此在今后厌氧消化工艺的研究中可以将其推广以致应用.
| [1] | Arau jo J C,Campos A C,Correa M M,et al.2011.Anammox bacteria enrichment and characterization from municipal activated sludge[J].Water Science & Technology,64(7):1428-1434 |
| [2] | Blackburne R,Yuan Z G,Keller J.2008.Partial nitrification to nitrite using low dissolved oxygen concentration as the main selection factor[J].Biodegradation,19(2):303-312 |
| [3] | Bock E,Schmidt I,Stüven R,et al.1995.Nitrogen loss caused by denitrifying Nitrosomonas cells using ammonium or hydrogen as electron-donors and nitrite as electron-acceptor[J].Archives of Microbiology,163(1):16-20 |
| [4] | Broda E.1977.Two kinds of lithotrophs missing in nature[J].Zeitschrift Fur Allgemeine Mikrobiologie,17(6):491-493 |
| [5] | Chamchoi N,Nitisoravut S,Schmidt J E.2008.Inactivation of ANAMMOX communities under concurrent operation of anaerobic ammonium oxidation (ANAMMOX) and denitrification[J].Bioresource Technology,99(9):3331-3336 |
| [6] | Dalsgaard T,Canfield D E,Petersen J,et al.2003.N2 production by the anammox reaction in the anoxic water column of Golfo Dulce,Costa Rica[J].Nature,422(6932):606-608 |
| [7] | De Clippeleir H,Defoirdt T,Vanhaecke L,et al.2011.Long-chain acylhomoserine lactones increase the anoxic ammonium oxidation rate in an OLAND biofilm[J].Applied Microbiology and Biotechnology,90(4):1511-1519 |
| [8] | de Souza N.2012.Single-cell methods[J].Nature Methods,9:35 |
| [9] | Ding S,Zheng P,Lu H F,et al.2013.Ecological characteristics of anaerobic ammonia oxidizing bacteria[J].Applied Microbiology and Biotechnology,97(5):1841-1849 |
| [10] | Egli K,Fanger U,Alvarez P J J,et al.2001.Enrichment and characterization of an anammox bacterium from a rotating biological contactor treating ammonium-rich leachate[J].Archives of Microbiology,175(3):198-207 |
| [11] | Fuchsman C A,Staley J T,Oakley B B,et al.2012.Free-living and aggregate-associated Planctomycetes in the Black Sea[J].FEMS Microbiology Ecology,80(2):402-416 |
| [12] | Furukawa K,Inatomi Y,Qiao S,et al.2009.Innovative treatment system for digester liquor using anammox process[J].Bioresource Technology,100(22):5437-5443 |
| [13] | Galán A,Molina V,Thamdrup B,et al.2009.Anammox bacteria and the anaerobic oxidation of ammonium in the oxygen minimum zone off northern Chile[J].Deep-Sea Research Part II-Topical Studies in Oceanography,56(16):1021-1031 |
| [14] | Güven D,Dapena A,Kartal B,et al.2005.Propionate oxidation by and methanol inhibition of anaerobic ammonium-oxidizing bacteria[J].Applied and Environmental Microbiology,71(2):1066-1071 |
| [15] | Hong Y G,Li M,Cao H L,et al.2011.Residence of habitat-specific anammox bacteria in the deep-sea subsurface sediments of the South China Sea:analyses of marker gene abundance with physical chemical parameters[J].Microbial Ecology,62(1):36-47 |
| [16] | Hu B L,Zheng P,Tang C J,et al.2010.Identification and quantification of anammox bacteria in eight nitrogen removal reactors[J].Water Research,44(17):5014-5020 |
| [17] | Isaka K,Date Y,Sumino T,et al.2006.Growth characteristic of anaerobic ammonium-oxidizing bacteria in an anaerobic biological filtrated reactor[J].Applied Microbiology and Biotechnology,70(1):47-52 |
| [18] | Jetten M S M,Camp H J M,Keltjens J T M,et al.2008.Two impossible microbes with global implications:the nitrite-dependent anaerobic oxidation of methane and ammonium//Drake H L,Liu S J,Liu H L.Microbes and the Environment:Perspective and Challenges.Beijing:Science Press.15-22 |
| [19] | Jetten M S M,Strous M,van de Pas-Schoonen K T,et al.1998.The anaerobic oxidation of ammonium[J].FEMS Microbiology Reviews,22(5):421-437 |
| [20] | Joss A,Salzgeber D,Eugster J,et al.2009.Full-scale nitrogen removal from digester liquid with partial nitritation and anammox in one SBR[J].Environmental Science & Technology,43(14):5301-5306 |
| [21] | Kartal B,Rattray J,van Niftrik L A,et al.2007.Candidatus "Anammoxoglobus propionicus" a new propionate oxidizing species of anaerobic ammonium oxidizing bacteria[J].Systematic and Applied Microbiology,30(1):39-49 |
| [22] | Kartal B,van Niftrik L,Rattray J,et al.2008.Candidatus 'Brocadia fulgida':an autofluorescent anaerobic ammonium oxidizing bacterium[J].FEMS Microbiology Ecology,63(1):46-55 |
| [23] | Kartal B,Kuenen J G,van Loosdrecht M C M.2010.Sewage treatment with anammox[J].Science,328(5979):702-703 |
| [24] | Kojima H,Tsutsumi M,Ishikawa K,et al.2012.Distribution of putative denitrifying methane oxidizing bacteria in sediment of a freshwater lake,Lake Biwa[J].Systematic and Applied Microbiology,35(4):233-238 |
| [25] | Kuypers M M M,Sliekers A O,Lavik G,et al.2003.Anaerobic ammonium oxidation by anammox bacteria in the Black Sea[J].Nature,422(6932):608-611 |
| [26] | Lackner S,Terada A,Smets B F.2008.Heterotrophic activity compromises autotrophic nitrogen removal in membrane-aerated biofilms:Results of a modeling study[J].Water Research,42(4/5):1102-1112 |
| [27] | Lam P,Jensen M M,Lavik G,et al.2007.Linking crenarchaeal and bacterial nitrification to anammox in the Black Sea[J].Proceedings of the National Academy of Sciences of the United States of America,104(17):7104-7109 |
| [28] | Li H,Chen S,Mu B Z,et al.2010.Molecular detection of anaerobic ammonium-oxidizing (Anammox) bacteria in high-temperature petroleum reservoirs[J].Microbial Ecology,60(4):771-783 |
| [29] | Liang Z,Liu J X,Li J.2009.Decomposition and mineralization of aquatic humic substances (AHS) in treating landfill leachate using the Anammox process[J].Chemosphere,74(10):1315-1320 |
| [30] | Liu S T,Gong Z,Yang F L,et al.2008a.Combined process of urea nitrogen removal in anaerobic Anammox co-culture reactor[J].Bioresource Technology,99(6):1722-1728 |
| [31] | Liu S T,Yang F L,Gong Z,et al.2008b.Application of anaerobic ammonium-oxidizing consortium to achieve completely autotrophic ammonium and sulfate removal[J].Bioresource Technology,99(15):6817-6825 |
| [32] | Luesken F A,Sánchez J,van Alen T A,et al.2011a.Simultaneous nitrite-dependent anaerobic methane and ammonium oxidation processes[J].Applied and Environmental Microbiology,77(19):6802-6807 |
| [33] | Luesken F A,Zhu B L,van Alen T A,et al.2011b.pmoA primers for detection of anaerobic methanotrophs[J].Applied and Environmental Microbiology,77(11):3877-3880 |
| [34] | Meyer R L,Risgaard-Petersen N,Allen D E.2005.Correlation between anammox activity and microscale distribution of nitrite in a subtropical mangrove sediment[J].Applied and Environmental Microbiology,71(10):6142-6149 |
| [35] | Molinuevo B,Cruz Garcia M,Karakashev D,et al.2009.Anammox for ammonia removal from pig manure effluents:Effect of organic matter content on process performance[J].Bioresource Technology,100(7):2171-2175 |
| [36] | Moore T A,Xing Y P,Lazenby B,et al.2011.Prevalence of anaerobic ammonium-oxidizing bacteria in contaminated groundwater[J].Environmental Science & Technology,45(17):7217-7225 |
| [37] | Mulder A,Vandegraaf A A,Robertson L A,et al.1995.Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor[J].FEMS Microbiology Ecology,16(3):177-184 |
| [38] | Nielsen M,Bollmann A,Sliekers O,et al.2005.Kinetics,diffusional limitation and microscale distribution of chemistry and organisms in a CANON reactor[J].FEMS Microbiology Ecology,51(2):247-256 |
| [39] | Philips S,Wyffels S,Sprengers R,et al.2002.Oxygen-limited autotrophic nitrification/denitrification by ammonia oxidisers enables upward motion towards more favourable conditions[J].Applied Microbiology and Biotechnology,59(4/5):557-566 |
| [40] | Raghoebarsing A A,Pol A,van de Pas-Schoonen K T,et al.2006.A microbial consortium couples anaerobic methane oxidation to denitrification[J].Nature,440(7086):918-921 |
| [41] | Rich J J,Dale O R,Song B,et al.2008.Anaerobic ammonium oxidation (Anammox) in Chesapeake Bay sediments[J].Microbial Ecology,55(2):311-320 |
| [42] | Rothrock M J Jr,Vanotti M B,Szögi A A,et al.2011.Long-term preservation of anammox bacteria[J].Applied Microbiology and Biotechnology,92(1):147-157 |
| [43] | Rysgaard S,Glud R N,Risgaard-Petersen N,et al.2004.Denitrification and anammox activity in Arctic marine sediments[J].Limnology and Oceanography,49(5):1493-1502 |
| [44] | Schalk J,Oustad H,Kuenen J G,et al.1998.The anaerobic oxidation of hydrazine:a novel reaction in microbial nitrogen metabolism[J].FEMS Microbiology Letters,158(1):61-67 |
| [45] | Schmid M,Twachtmann U,Klein M,et al.2000.Molecular evidence for genus level diversity of bacteria capable of catalyzing anaerobic ammonium oxidation[J].Systematic and Applied Microbiology,23(1):93-106 |
| [46] | Schmid M,Walsh K,Webb R,et al.2003.Candidatus "Scalindua brodae",sp.nov.,Candidatus "Scalindua wagneri",sp.nov.,two new species of anaerobic ammonium oxidizing bacteria[J].Systematic and Applied Microbiology,26(4):529-538 |
| [47] | Schmidt I,Bock E.1998.Anaerobic ammonia oxidation by cell-free extracts of Nitrosomonas eutropha[J].Antonie Van Leeuwenhoek,73(3):271-278 |
| [48] | Shen L D,Hu A H,Jin R C,et al.2012.Enrichment of anammox bacteria from three sludge sources for the startup of monosodium glutamate industrial wastewater treatment system[J].Journal of Hazardous Materials,199-200:193-199 |
| [49] | Siegrist H,Salzgeber D,Eugster J,et al.2008.Anammox brings WWTP closer to energy autarky due to increased biogas production and reduced aeration energy for N-removal[J].Water Science & Technology,57(3):383-388 |
| [50] | Sliekers A O,Derwort N,Campos-Gomez J L,et al.2002.Completely autotrophic nitrogen removal over nitrite in one single reactor[J].Water Research,36(10):2475-2482 |
| [51] | Sliekers A O,Haaijer S,Schmid M,et al.2004.Nitrification and Anammox with urea as the energy source[J].Systematic and Applied Microbiology,27(3):271-278 |
| [52] | Smith R L,Howes B L,Garabedian S P.1991.In situ measurement of methane oxidation in groundwater by using natural-gradient tracer tests[J].Applied and Environmental Microbiology,57(7):1997-2004 |
| [53] | Strous M,van Gerven E,Kuenen J G,et al.1997.Effects of aerobic and microaerobic conditions on anaerobic ammonium-oxidizing (Anammox) sludge[J].Applied and Environmental Microbiology,63(6):2446-2448 |
| [54] | Strous M,Heijnen J J,Kuenen J G,et al.1998.The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms[J].Applied Microbiology and Biotechnology,50(5):589-596 |
| [55] | Strous M,Fuerst J A,Kramer E H M,et al.1999a.Missing lithotroph identified as new planctomycete[J].Nature,400(6743):446-449 |
| [56] | Strous M,Kuenen J G,Jetten M S M.1999b.Key physiology of anaerobic ammonium oxidation[J].Applied and Environmental Microbiology,65(7):3248-3250 |
| [57] | Strous M,Jetten M S M.2004.Anaerobic oxidation of methane and ammonium[J].Annual Review of Microbiology,58:99-117 |
| [58] | Strous M,Pelletier E,Mangenot S,et al.2006.Deciphering the evolution and metabolism of an anammox bacterium from a community genome[J].Nature,440(7085):790-794 |
| [59] | Sumino T,Isaka K,Ikuta H,et al.2006.Nitrogen removal from wastewater using simultaneous nitrate reduction and anaerobic ammonium oxidation in single reactor[J].Journal of Bioscience and Bioengineering,102(4):346-351 |
| [60] | Tal Y,Watts J E M,Schreier H J.2006.Anaerobic ammonium-oxidizing (anammox) bacteria and associated activity in fixed-film biofilters of a marine recirculating aquaculture system[J].Applied and Environmental Microbiology,72(4):2896-2904 |
| [61] | Tang C J,Zheng P,Wang C H,et al.2010.Suppression of anaerobic ammonium oxidizers under high organic content in high-rate Anammox UASB reactor[J].Bioresource Technology,101(6):1762-1768 |
| [62] | Tang C J,Zheng P,Chen T T,et al.2011a.Enhanced nitrogen removal from pharmaceutical wastewater using SBA-ANAMMOX process[J].Water Research,45(1):201-210 |
| [63] | Tang C J,Zheng P,Wang C H,et al.2011b.Performance of high-loaded ANAMMOX UASB reactors containing granular sludge[J].Water Research,45(1):135-144 |
| [64] | Thamdrup B.2012.New pathways and processes in the global nitrogen cycle[J].Annual Review of Ecology,Evolution,and Systematics,43(1):407-428 |
| [65] | Toh S K,Ashbolt N J.2002.Adaptation of anaerobic ammonium-oxidising consortium to synthetic coke-ovens wastewater[J].Applied Microbiology and Biotechnology,59(2/3):344-352 |
| [66] | Tsushima I,Ogasawara Y,Kindaichi T,et al.2007.Development of high-rate anaerobic ammonium-oxidizing (anammox) biofilm reactors[J].Water Research,41(8):1623-1634 |
| [67] | van de Vossenberg J,Woebken D,Maalcke W J,et al.2013.The metagenome of the marine anammox bacterium ‘Candidatus Scalindua profunda' illustrates the versatility of this globally important nitrogen cycle bacterium[J].Environmental Microbiology,15(5):1275-1289 |
| [68] | van der Star W R L,Abma W R,Blommers D,et al.2007.Startup of reactors for anoxic ammonium oxidation:Experiences from the first full-scale anammox reactor in Rotterdam[J].Water Research,41(18):4149-4163 |
| [69] | van der Star W R L,Miclea A I,van Dongen U G J M,et al.2008.The membrane bioreactor:a novel tool to grow anammox bacteria as free cells[J].Biotechnology and Bioengineering,101(2):286-294 |
| [70] | Vandegraaf A A,Mulder A,De Bruijn P,et al.1995.Anaerobic oxidation of ammonium is a biologically mediated process[J].Applied and Environmental Microbiology,61(4):1246-1251 |
| [71] | Wang Y,Zhu G B,Harhangi H R,et al.2012.Co-occurrence and distribution of nitrite-dependent anaerobic ammonium and methane-oxidizing bacteria in a paddy soil[J].FEMS Microbiology Letters,336(2):79-88 |
| [72] | Ward B B,Glover H E,Lipschultz F.1989.Chemoautotrophic activity and nitrification in the oxygen minimum zone off peru[J].Deep-Sea Research Part A-Oceanographic Research Papers,36(7):1031-1051 |
| [73] | Woebken D,Lam P,Kuypers M M M,et al.2008.A microdiversity study of anammox bacteria reveals a novel Candidatus Scalindua phylotype in marine oxygen minimum zones[J].Environmenal Microbiology,10(11):3106-3119 |
| [74] | Yamamoto T,Takaki K,Koyama T,et al.2008.Long-term stability of partial nitritation of swine wastewater digester liquor and its subsequent treatment by Anammox[J].Bioresource Technology,99(14):6419-6425 |
| [75] | Yang J,Jiang H C,Wu G,et al.2012.Co-occurrence of nitrite-dependent anaerobic methane oxidizing and anaerobic ammonia oxidizing bacteria in two Qinghai-Tibetan saline lakes[J].Frontiers of Earth Science,6(4):383-391 |
| [76] | Yoshinaga I,Amano T,Yamagishi T,et al.2011.Distribution and diversity of anaerobic ammonium oxidation (anammox) bacteria in the sediment of a eutrophic freshwater lake,Lake Kitaura,Japan[J].Microbes and Environments,26(3):189-197 |
| [77] | 郑平,徐向阳,胡宝兰.2004.新型生物脱氮理论与技术[M].北京:科学出版社 |
| [78] | Zhu B L,Sanchez J,van Alen T A,et al.2011a.Combined anaerobic ammonium and methane oxidation for nitrogen and methane removal[J].Biochemical Society Transactiona,39(6):1822-1825 |
| [79] | Zhu G B,Jetten M S M,Kuschk P,et al.2010.Potential roles of anaerobic ammonium and methane oxidation in the nitrogen cycle of wetland ecosystems[J].Applied Microbiology and Biotechnology,86(4):1043-1055 |
| [80] | Zhu G B,Wang S Y,Feng X J,et al.2011b.Anammox bacterial abundance,biodiversity and activity in a constructed wetland[J].Environmental Science & Technology,45(23):9951-9958 |
 2014, Vol.34
2014, Vol.34


