2. 同济大学 汽车学院 上海 201804
2. School of Automotive Studies, Tongji University, Shanghai 201804, China
质子交换膜燃料电池(Proton exchange membrane fuel cell,PEMFC)由于其构造简单、能量密度高、能量转化效率高、环境友好等优点,作为化学电源被广泛应用[1-3]。纯Pt具有催化活性高和稳定性好等特点,是质子交换膜燃料电池最常用的催化剂,但是Pt催化剂高昂的成本和较短的使用寿命极大地阻碍了其大规模的商业应用[4],因此在保证催化剂活性的前提下,减少Pt的使用量,提高燃料电池的耐久性就显得尤为重要[5]。
作为质子交换膜燃料电池的阴极发生的氧化还原反应(Oxygen reduction reaction,ORR),在化学反应动力学上速度较慢,成为制约质子交换膜燃料电池性能的关键,因此寻找活性更高、价格更便宜的阴极催化剂就成为研究的重点。在Pt中掺杂非贵金属元素,如Fe、Co、Ni等,有助于提高Pt催化剂的稳定性和催化活性,同时也减少Pt的使用量,降低燃料电池的成本[6]。掺杂过渡金属元素增强Pt催化PEMFC阴极氧化还原反应活性的机理是:减小Pt-Pt键之间的距离(几何效应),形成更多具有5d空轨道的外层电子结构,增强氧的π电子向Pt表面转移(电子效应)[7]。低Pt燃料电池催化剂的研究和应用已有大量的报道[8-14],PtCo/C催化剂表现出较高ORR催化活性而受到广泛关注[15-21]。
许多常用的表征方法如X射线衍射(X-ray diffraction,XRD)、线性扫描伏安法(Linear sweep voltammetry,LSVs)、循环伏安法(Cyclic Voltammetry,C-V)、透射电镜(Transmission electron microscope,TEM)被应用于PtMn(Fe,Co,Ni)/C双金属纳米催化剂的研究[22-25],但是不能获得燃料电池催化剂在工作状态下的结构信息。近几十年来,随着同步辐射光源的建设,XAFS实验技术得到广泛的应用。Ishiguro等[26]利用原位时间分辨XAFS谱,观察到了Pt3M(M=Co,Ni)双金属纳米催化剂在工作状态下Pt-O键的断裂和Pt-Pt的生成。Nagamatsu等[27]通过燃料电池的原位XAFS数据发现催化剂Au@Pt/C和Pd@Pt/C在催化反应过程中Pt-O键的存在抑制了Pt的ORR催化活性。Greco等[28]借助于原位XAFS数据发现增加PtCo/C催化剂的化学和局域结构的有序度并不能影响ORR的催化活性。上海光源BL14W1线站建立的PEMFC原位XAFS实验方法,一方面利用XAFS技术观察催化剂在反应过程中的动态变化,另一方面同步监测催化剂在工作状态下电化学信息,进而找到催化剂的性能变化和结构变化的对应关系[29]。
1 材料与方法 1.1 样品的制备 1.1.1 PtCo/C催化剂的制备采用两步还原法制备PtCo/C,首先纳米Co颗粒通过NaBH4还原CoCl2制备,然后Pt通过乙二醇还原H2PtCl6沉积到Co纳米颗粒上,以XC-72活性碳负载PtCo纳米颗粒制成PtCo/C催化剂。非原位数据采集使用没有经过活化处理的PtCo/C粉末,但是制备成膜电极组件和组装成燃料电池后,在采集燃料电池的原位XAFS数据前,需要经过24 h的活化(100%氢气,反应温度为70 ℃)处理,活化处理过程是在燃料电池里完成。
1.1.2 膜电极组件(Membrane Electrode Accemblies,MEA)的制备取0.04 g的PtCo/C催化剂,并加入0.72 mL H2O,0.372 g的5% Nafion溶液(美国杜邦公司),1.5 g异丙醇,超声分散3 h,然后把Nafion 212膜(美国杜邦公司)在加热平台上加热至80 ℃,把经过超声分散后的溶液喷涂在Nafion膜上,催化剂喷涂面积为20 mmx20 mm。取Pd/C (10 wt%,庄信万丰公司(Johnson Matthey company)) 0.08 g,加入1.44 mL H2O,0.744 g 5% Nafion溶液,3.0 g异丙醇,同样超声分散3 h后,在80 ℃下喷到Nafion 212膜的另一面。将喷好的Nafion膜置于已裁剪的大小和催化剂面积完全相同的炭纸中间,然后在150 ℃和5MPa下热压制备成PtCo/C-Pd/C膜电极组件,同样的方法制备一面是Pt/C (20 wt%,Johnson Matthey company),另一面是Pd/C (10 wt%,Johnson Matthey company)的JM-Pt/C- Pd/C膜电极组件。
1.2 PtCo/C的结构表征使用电感耦合等离子体质谱(Inductively coupled plasma mass spectrometry,ICP-MS)测定PtCo/C的组分和原子比,所用仪器型号为美国热电公司生产的Thermo Elemental XZ-ICP-MS X0186,取两个平行样的平均值。
物理吸附法(Brunauer-Emmet-Teller,BET)用来测定催化剂的比表面积,所用仪器为美国Micromeritics公司生产的ASAP2020。采用液氮作为吸附质。
化学吸附法用来测定活性金属表面积和金属分散度,所用仪器为美国Micromeritics公司生产的AutoChem HP 2950。采用脉冲化学吸附法,以一定剂量的CO(一个脉冲)在相同的时间间隔内注入催化剂表面,直到检测出来的信号峰强度稳定为止。用每一脉冲对应的峰面积乘以总脉冲数,减去检测到的峰面积即为吸收的气体对应的峰面积。
| $\begin{array}{l} D\% = \frac{{2{V_{{\rm{ad}}}}}}{{22.4}} \times \frac{M}{{{M_{{\rm{metal}}}}{\rm{\% }} \times m}} \times 100\% \\ S = \frac{{2{V_{{\rm{ad}}}} \times {N_0} \times \sigma }}{{22.14 \times m \times {M_{{\rm{metal}}}}\% }} \end{array}$ | (1) |
式中:D表示金属分散度;Vad表示吸附的体积;M表示气体分子量;Mmetal%表示金属的质量分数;m表示所用催化剂的质量;S表示金属表面积;N0=6.02x1023;σ为金属原子截面积。
TEM的型号为美国FEI公司生产的Tecnai G2 F20S-TWIN场发射透射电子显微镜(Field Emission Transmission Electron Microscope)。
采用德国Bruker公司生产的D8 Advance X射线衍射仪,对PtCo/C粉末进行XRD测试,X射线管电压为40 kV,管电流为40 mA,步长0.02°,扫描范围(2θ)为20°-90°,积分时间1 s。
在上海光源BL14W1线站采集PtCo/C粉末的非原位XAFS谱,Pt采用透射模式采集XAFS谱,Co采用荧光模式采集XAFS谱。BL14W1线站使用Si(111)双晶单色器来选择X光能量,对于Pt L3边的测量,前电离室气体为90% N2和10% Ar,后电离室气体为75% N2和25% Ar,Co K边的测量,前后电离室气体均为75% N2和25% He。
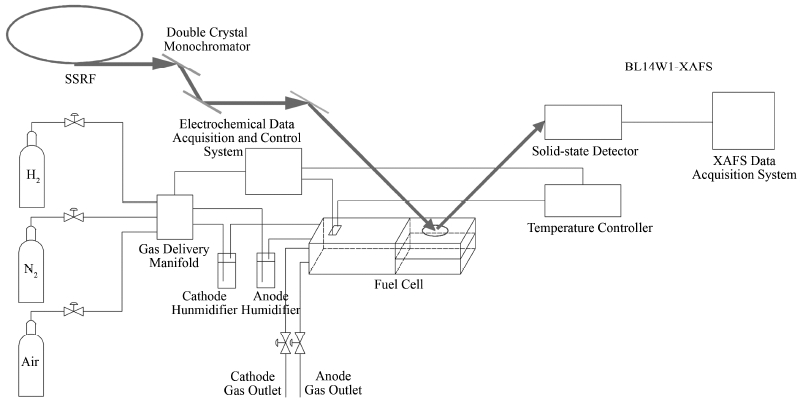
1.3 PtCo/C原位XAFS谱的采集 1.3.1 原位XAFS燃料电池催化剂实验装置在上海光源BL14W1线站搭建的原位燃料电池实验装置,如图 1、2所示。整套装置主要由原位样品池、加热、加湿、气体控制、电化学信号采集几个模块组成,其中样品池位于电离室和固体探测器之间。
|
图 1 上海光源BL14W1线站的原位燃料电池实验装置示意图 Fig. 1 Schematic drawing of the in situ XAFS experiment instrument for fuel cell on beamline BL14W1 Shanghai Synchrotron Radiation Facility (SSRF). |

|
图 2 上海光源BL14W1线站的原位燃料电池实验装置 Fig. 2 Picture of the in situ XAFS experiment instrument for fuel cell on beamline BL14W1 at SSRF. |
两张MEA(PtCo/C-Pd/C和JM-Pt/C-Pd/C)在采集原位数据前,都经过了24 h的活化,反应温度为70 ℃,氢气(20%的氢氦混合气)流量125mL∙min-1,空气流量50 mL∙min-1,氢气和空气在进入燃料电池反应前都经过加湿罐进行加湿。PtCo/C样品的原位XAFS数据采集采用荧光模式,使用32元高纯Ge固体探测器采集PtCo/C的原位XAFS谱,每个数据点的积分时间都为3 s,通过电化学工作站调节工作电压变化,分别采集不同电压下Pt和Co的原位XAFS谱图。
2 结果与分析 2.1 PtCo/C和JM-Pt/C的物理参数比较ICP实验结果显示Pt 的质量百分比为10.3%,Co的质量百分比为0.18%,Pt与Co的原子数比为17:1。如表 1所示,PtCo/C的Pt的质量百分比仅为商业催化剂JM-Pt/C的50%,PtCo/C催化剂粒径更小,但是比表面积小于JM-Pt/C。
| 表 1 PtCo/C和JM-Pt/C物理参数比较 Table 1 Physical parameters PtCo/C and JM-Pt/C comparison. |
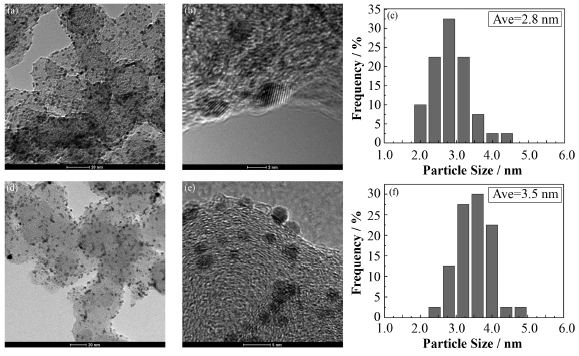
分别采集PtCo/C和JM-Pt/C的TEM成像和高分辨透射电镜(High Resolution TEM,HRTEM)成像,如图 3所示,PtCo/C和JM-Pt/C的粒径分布都比较均匀。使用粒度分析软件Nano Measurer 1.2.5对PtCo/C和JM-Pt/C的纳米颗粒尺寸进行统计分析,PtCo/C平均粒径2.8 nm,小于JM-Pt/C的3.5nm,更小纳米尺寸有利于提高PtCo/C催化ORR的活性。
|
图 3 PtCo/C (a)和JM-Pt/C (d)的TEM图,PtCo/C (b)和JM-Pt/C (e)的HRTEM图,PtCo/C (c)和JM-Pt/C (f)的粒径分布图 Fig. 3 TEM images of PtCo/C (a) and JM-Pt/C (d), HRTEM images of PtCo/C (b) and JM-Pt/C (e), and distribution of nanoparticle size distributions from analysis of TEM images of PtCo/C (c) and JM-Pt/C (f). |
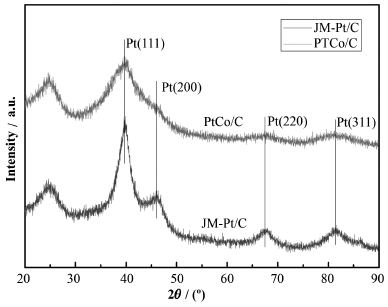
PtCo/C与JM-Pt/C的XRD数据如图 4所示。JM-Pt/C的衍射峰出现在39.8°、46.3°、67.5°、81.3°,分别对应Pt的(111)、(200)、(220)、(311)晶面,表示Pt的面心立方晶格结构[30],PtCo/C的衍射峰相比JM-Pt/C宽化,表明PtCo/C具有更小的纳米颗粒尺寸[31]。PtCo/C与JM-Pt/C的TEM数据证实了这一点。
|
图 4 PtCo/C和JM-Pt/C的XRD数据 Fig. 4 XRD patterns of the PtCo/C and JM-Pt/C. |
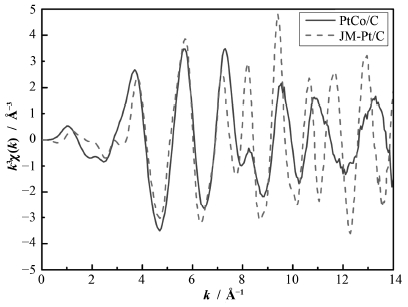
利用Ifeffit软件包对实验获得的EXAFS数据进行处理,结果如表 2所示。图 5是PtCo/C与JM-Pt/C的k空间变换,图 6是PtCo/C和JM-Pt/C的EXAFS拟合后的R空间变换。此时由于PtCo/C的粉末未经还原处理,由PtCo/C的粉末EXAFS的拟合结果可知,Pt与Co并没有成键,存在显著的Co-O和Co-O-Co相互作用,说明PtCo/C中的Co主要以氧化物的形式存在。与JM-Pt/C相比,PtCo/C中Pt的氧化程度更高且具有更短的Pt-Pt金属键长,说明Co的存在影响着催化剂的活性成分Pt的氧化程度,Pt-Pt键长的缩短有可能来自Co的影响和尺寸效应。
| 表 2 PtCo/C和JM-Pt/C的EXAFS拟合结果 Table 2 Structural and electronic structural parameters obtained by EXAFS analysis for PtCo/C and JM-Pt/C catalysts. |
|
图 5 PtCo/C和JM-Pt/C的k空间变换 Fig. 5 k3-weighted EXAFS oscillations χ(k) at Pt L3-edge and their Fourier transforms for PtCo/C and JM-Pt/C. |

|
图 6 PtCo/C和JM-Pt/C的EXAFS拟合结果 (a) PtCo/C的Pt的L3拟合结果,(b) PtCo/C的Co的K边拟合结果,(c) JM-Pt/C的Pt的L3边拟合结果 Fig. 6 Data and fits of the magnitude of Fourier transformed k2-weighted EXAFS spectra of PtCo/C and JM-Pt/C catalysts. (a) PtCo/C of the Pt L3 edge, (b) PtCo/C of the Co K edge, (c) JM-Pt/C of the Pt L3 edge |
如图 7所示,PtCo/C经过24 h的活化处理之后,随着电压逐渐减小,电流密度逐渐增大,PtCo/C和JM-Pt/C中Pt的氧化程度逐渐降低,XANES的线性拟合结果如图 8所示,JM-Pt/C在低电位时出现PtO2含量略有升高。如图 9所示,Co的线性拟合显示,Co的原位XANES是Co和CoO的混合物,在催化反应过程中,随着电压逐渐减小,Co的氧化程度也随之逐渐降低,揭示了在催化反应过程中Pt的d电子向过渡金属Co的转移过程,从而导致Pt的d带空位的增加。这说明Co的存在可以影响Pt的外层电子结构,由于尺寸效应和Co的影响使PtCo/C催化剂具有更短的Pt-Pt键长,从而增强Pt的催化ORR的活性。在低电位时,Co的存在可以抑制PtO2含量的增加,而反应过程中Pt-O键的存在能抑制Pt的催化反应活性[27]。

|
图 7 PtCo/C (a)和JM-Pt/C (b) Pt的原位XANES谱比较 Fig. 7 In situ XANES spectra collected at Pt L3 edge of PtCo/C (a) and JM-Pt/C (b) catalyst with different potentials comparison during the operation of fuel cell. |

|
图 8 PtCo/C (a)和JM-Pt/C (b)催化剂Pt的原位XANES谱数据的线性拟合结果比较 Fig. 8 Linear fitting results of in situ XANES spectra of PtCo/C (a) and JM-Pt/C (b) catalyst comparison. |

|
图 9 PtCo/C催化剂Co的原位XANES谱 Fig. 9 In situ XANES spectra collected at Co K edge of PtCo/C with different potentials during the operation of fuel cell. |
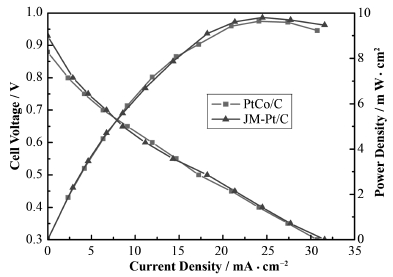
如图 10所示,PtCo/C和JM-Pt/C催化剂从开路电压(Open circuit voltage,OCV)开始,随着电压的逐渐减小,电流密度的逐渐增大,功率密度也随之增大,当电压降到0.4 V左右时,燃料电池的功率密度达到极大值。从JM-Pt/C和PtCo/C的功率曲线可以看出,PtCo/C的催化性能非常接近商业Pt/C催化剂,但是与JM-Pt/C相比,PtCo/C减少了50%的Pt的使用量,因此具有更好的商业应用前景。
|
图 10 PtCo/C和JM-Pt/C催化剂在工作状态下的性能曲线比较 Fig. 10 Performance curve of PtCo/C and JM-Pt/C catalyst comparison in fue cell working condition. |
在Pt中掺杂过渡金属元素Co形成的PtCo/C纳米催化剂,与商业Pt/C催化剂相比,由于Co的存在能够影响Pt的氧化程度,使得金属Pt晶格变小,Pt-Pt键长缩短,导致PtCo/C具有更小的纳米尺寸,减小了Pt-Pt 键之间的距离,从而提高PtCo/C纳米催化剂中Pt的催化活性。过渡金属Co有比Pt更多的d带空位,原位XAFS实验揭示了PtCo/C在催化反应过程中Pt的d电子向过渡金属Co的转移过程,Pt的d带空位的增加,有利于提高Pt催化ORR反应的性能。
| [1] |
David L, Trimm Z, Önsan I. Onboard fuel conversion for hydrogen-fuel-cell-driven venhicles[J].
Catalysis Reviews-Science and Engineering, 2001, 43 (1-2) : 31 –84.
DOI: 10.1081/CR-100104386 ( 0) 0)
|
| [2] |
Ralph T R, Hogarth M P. Catalysis for low temperature fuel cells[J].
Platinum Metals Review, 2002, 46 (3) : 117 –135.
( 0) 0)
|
| [3] |
Varcoe J R, Slade R C T. Prospects for alkaline anion-exchange membranes in low temperature fuel cells[J].
Fuel Cells, 2005, 5 (2) : 187 –200.
DOI: 10.1002/fuce.200400045 ( 0) 0)
|
| [4] |
Schmittinger W, Vahidi A. A review of the main parameters influencing long-term performance and durability of PEM fuel cells[J].
Journal of Power Sources, 2008, 180 (1) : 1 –14.
DOI: 10.1016/j.jpowsour.2008.01.070 ( 0) 0)
|
| [5] |
Borup R, Meyers J, Pivovar B, et al. Scientific aspects of polymer electrolyte fuel cell durability and degradation[J].
Chemical Reviews, 2007, 107 (10) : 3904 –3951.
DOI: 10.1021/cr050182l ( 0) 0)
|
| [6] |
Hector R C, Branko N P. Stability of platinum based alloy cathode catalysts in PEM fuel cells[J].
Journal of Power Sources, 2006, 155 (2) : 253 –263.
DOI: 10.1016/j.jpowsour.2005.05.011 ( 0) 0)
|
| [7] |
Carrette L, Friedrich K A, Stimming U. Fuel cellsfundamentals and applications[J].
Fuel Cells, 2001, 1 (1) : 5 –39.
DOI: 10.1002/1615-6854(200105)1:1<5::AIDFUCE5>3.0.CO;2-G ( 0) 0)
|
| [8] |
Wang G X, Wu H M, David W, et al. Ni@Pt core-shell nanoparticles with enhanced catalytic activity for oxygen reduction reaction[J].
Journal of Alloys and Compounds, 2010, 503 (1) .
DOI: 10.1016/j.jallcom.2010.04.236 ( 0) 0)
|
| [9] |
Laetitia D, Tristan A, Raphael C, et al. Tuning the performance and the stability of porous hollow PtNi/C nanostructures for the oxygen reduction reaction[J].
American Chemical Society Catalysis, 2015, 5 (9) : 5333 –5341.
DOI: 10.1021/acscatal.5b01248 ( 0) 0)
|
| [10] |
Chung Y H, Chung D Y, Jung N, et al. Origin of the enhanced electrocatalysis for thermally controlled nanostructure of bimetallic nanoparticles[J].
The Journal of Physical Chemistry C, 2014, 118 (19) : 9939 –9945.
DOI: 10.1021/jp5019982 ( 0) 0)
|
| [11] |
Kang Y J, Snyder J, Chi M F, et al. Multimetallic core/interlayer/shell nanostructures as advanced electrocatalysts[J].
Nano Letters, 2014, 14 (11) : 6361 –6367.
DOI: 10.1021/nl5028205 ( 0) 0)
|
| [12] |
Duan H M, Hao Q, Xu C X. Nanoporous PtFe alloys as highly active and durable electrocatalysts for oxygen reduction reaction[J].
Journal of Power Sources, 2014, 269 : 589 –596.
DOI: 10.1016/j.jpowsour.2014.07.026 ( 0) 0)
|
| [13] |
Tuaev X, Rudi S, Petkov V, et al. In situ study of atomic structure transformations of Pt-Ni nanoparticle catalysts during electrochemical potential cycling[J].
American Chemical Society Nano, 2013, 7 (7) : 5666 –5674.
DOI: 10.1021/nn402406k ( 0) 0)
|
| [14] |
Kristian N, Yua Y, Lee J M, et al. Synthesis and characterization of Cocore-Ptshell electrocatalyst prepared by spontaneous replacement reaction for oxygen reduction reaction[J].
Electrochimica Acta, 2010, 56 (2) : 1000 –1007.
DOI: 10.1016/j.electacta.2010.09.073 ( 0) 0)
|
| [15] |
Lin R, Cao C H, Zhao T T, et al. Synthesis and application of coreeshell Co@Pt/C electrocatalysts for proton exchange membrane fuel cells[J].
Journal of Power Sources, 2013, 223 : 190 –198.
DOI: 10.1016/j.jpowsour.2012.09.073 ( 0) 0)
|
| [16] |
Chaisubanana N, Tantavichet N. Pulse reverse electrodeposition of Pt-Co alloys onto carbon cloth electrodes[J].
Journal of Alloys and Compounds, 2013, 559 : 69 –75.
DOI: 10.1016/j.jallcom.2013.01.079 ( 0) 0)
|
| [17] |
Tian F, Anderson A B. Theoretical study of early steps in corrosion of Pt and Pt/Co alloy electrodes[J].
The Journal of Physical Chemistry C, 2008, 112 (47) : 18566 –18571.
DOI: 10.1021/jp807094m ( 0) 0)
|
| [18] |
Ohyagi S, Sasaki T. Durability of a PEMFC Pt-Co cathode catalyst layer during voltage cycling tests under supersaturated humidity conditions[J].
Electrochimica Acta, 2013, 102 : 336 –341.
DOI: 10.1016/j.electacta.2013.04.060 ( 0) 0)
|
| [19] |
Murthi V S, Urian R C, Mukerjee S. Oxygen reduction kinetics in low and medium temperature acid environment:correlation of water activation and surface properties in supported Pt and Pt alloy electrocatalysts[J].
The Journal of Physical Chemistry B, 2004, 108 (30) : 11011 –11023.
DOI: 10.1021/jp048985k ( 0) 0)
|
| [20] |
Jia Q Y, Liang W T, Bates M K, et al. Activity descriptor identification for oxygen reduction on platinum-based bimetallic nanoparticles:in situ observation of the linear composition-strain-activity relationship[J].
American Chemical Society Nano, 2015, 9 (1) : 387 –400.
DOI: 10.1021/nn506721f ( 0) 0)
|
| [21] |
Loukrakpam R, Luo J, He T, et al. Nanoengineered PtCo and PtNi catalysts for oxygen reduction reaction:an assessment of the structural and electrocatalytic properties[J].
The Journal of Physical Chemistry C, 2011, 115 (5) : 1682 –1694.
DOI: 10.1021/jp109630n ( 0) 0)
|
| [22] |
Patrick B, Ham H C, Yang S H, et al. Atomic structure and composition of "Pt3Co" nanocatalysts in fuel cells:an aberration-corrected STEM HAADF study[J].
Chemistry of Materials, 2013, 25 (4) : 530 –535.
DOI: 10.1021/cm3029164 ( 0) 0)
|
| [23] |
Wang Y J, Zhao N N, Fang B Z, et al. Carbon-supported Pt-based alloy electrocatalysts for the oxygen reduction reaction in polymer electrolyte membrane fuel cells:particle size, shape, and composition manipulation and their impact to activity[J].
American Chemical Society Chemical Reviews, 2015, 115 (9) : 3433 –3467.
DOI: 10.1021/cr500519c ( 0) 0)
|
| [24] |
Spanos I, Kirkensgaard J J K, Mortensen K, et al. Investigating the activity enhancement on PtxCo1-x alloys induced by a combined strain and ligand effect[J].
Journal of Power Sources, 2014, 245 : 908 –914.
DOI: 10.1016/j.jpowsour.2013.07.023 ( 0) 0)
|
| [25] |
Shirlaine K, Jennifer L, Michael F, et al. Structure-activity-stability relationships of Pt-Co alloy electrocatalysts in gas-diffusion electrode layers[J].
The Journal of Physical Chemistry C, 2007, 111 (9) : 3744 –3752.
DOI: 10.1021/jp067269a ( 0) 0)
|
| [26] |
Ishiguro N, Kityakarn S, Tada M, et al. Rate enhancements in structural transformations of Pt-Co and PtNi bimetallic cathode catalysts in polymer electrolyte fuel cells studied by in situ time-resolved X-ray absorption fine structure[J].
The Journal of Physical Chemistry C, 2014, 118 (29) : 15874 –15883.
DOI: 10.1021/jp504738p ( 0) 0)
|
| [27] |
Nagamatsu S, Arai T, Iwasawa Y, et al. Potentialdependent restructuring and hysteresis in the structural and electronic transformations of Pt/C, Au(Core)-Pt(Shell)/C, and Pd(Core)-Pt(Shell)/C cathode catalysts in polymer electrolyte fuel cells characterized by in situ X-ray absorption fine structure[J].
The Journal of Physical Chemistry C, 2013, 117 (25) : 13094 –13107.
DOI: 10.1021/jp402438e ( 0) 0)
|
| [28] |
Greco G, Witkowska A, Cicco A D, et al. Local ordering changes in Pt-Co nanocatalyst induced by fuel cell working conditions[J].
The Journal of Physical Chemistry C, 2012, 116 (23) : 12791 –12802.
DOI: 10.1021/jp2099569 ( 0) 0)
|
| [29] |
尚明丰, 黄宇营, 王建强, 等. 用于质子交换膜燃料电池催化剂结构研究的原位XAFS实验方法[J].
物理化学学报, 2015, 31 (8) : 1609 –1614.
SHANG Mingfeng, HUANG Yuying, WANG Jianqiang, et al. In situ XAFS experimental methods for catalyst structure of proton exchange membrane fuel cell[J]. Acta Physico-Chimica Sinica, 2015, 31 (8) : 1609 –1614. DOI: 10.3866/PKU.WHXB201505252 (  0) 0)
|
| [30] |
Lee M H, Do J S. Kinetics of oxygen reduction reaction on Corich core-Ptrich shell/C electrocatalysts[J].
Journal of Power Sources, 2009, 188 (2) : 353 –358.
DOI: 10.1016/j.jpowsour.2008.12.051 ( 0) 0)
|
| [31] |
Xion L, Kanna A M, Manthiram A. Pt-M (M=Fe,Co,Ni, and Cu) electrocatalysts synthesized by an aqueous route for proton exchange membrane fuel cells[J].
Electrochemistry Communication, 2002, 4 (11) : 898 –903.
DOI: 10.1016/S1388-2481(02)00485-X ( 0) 0)
|


