脑卒中是仅次于心脏病的全球第二大死因,也是导致成人残疾的主要原因[1-2],其中缺血性脑卒中是最常见的类型[2]。研究表明,缺血性脑卒中损害脑白质是卒中复发及长期神经功能缺损的重要危险因素[3]。脑白质主要由有髓神经纤维组成,胼胝体是脑内最大和最致密的白质纤维束,研究发现急性缺血性脑卒中后非缺血区域的胼胝体存在继发性脱髓鞘损伤[4-5]。继发性脱髓鞘会导致神经传导速度减慢或中断,严重影响中枢神经功能,引发长期的感觉、运动和认知障碍,是脑卒中患者远期预后不良的重要原因[3]。目前针对急性缺血性脑卒中继发性脱髓鞘尚无有效的干预措施。
迷走神经电刺激(vagus nerve stimulation,VNS)作为一种新型的神经调控技术,是偏头痛、阿尔茨海默病、创伤性脑损伤和神经病理性疼痛[6-8]的潜在治疗方法。2011年Nature首次报道VNS具有诱导大脑可塑性、重塑神经环路的作用[9];2021年Lancet报道VNS联合康复训练改善了脑梗死后遗症期患者上肢运动障碍,显示VNS的神经调控作用在缺血性脑卒中后遗症期具有重要作用[10]。另外,VNS可以通过抑制神经元凋亡[11]、抑制氧化应激[12]、调控小胶质细胞极化及神经炎症[13]改善急性缺血性脑卒中的功能。本课题组前期研究发现VNS可促进缺血性脑卒中恢复期大鼠髓鞘再生[14]。但VNS对急性缺血性脑卒中继发性脱髓鞘的影响尚不清楚。本研究拟通过大脑中动脉阻塞(middle cerebral artery occlusion,MCAO)建立脑缺血再灌注(ischemia/reperfusion,I/R)大鼠模型,观察VNS对急性期I/R大鼠继发性脱髓鞘改变的影响。
1 材料和方法 1.1 实验动物SPF级健康雄性Sprague-Dawley大鼠78只,7~8周龄,体重250~280 g,购自重庆医科大学实验动物中心[实验动物生产许可证号:SCXK(渝)2022-0010]。动物饲养于SPF级动物房,室温(23±1)℃,明暗周期12 h/12 h,自由进食和饮水。本实验遵循重庆医科大学实验动物伦理相关规定,大鼠适应性饲养1周后进行实验。
1.2 实验材料RIPA裂解液(强)、BCA蛋白浓度测定试剂盒(增强型)、脱脂奶粉、化学发光试剂(上海碧云天生物技术股份有限公司),SDS-PAGE上样缓冲液、SDS-PAGE凝胶制备试剂盒(上海雅酶生物医药科技有限公司),MCAO线栓(北京西浓科技有限公司),髓鞘相关糖蛋白(myelin-associated glycoprotein,MAG)抗体(美国CST公司),髓鞘碱性蛋白(myelin basic protein,MBP)抗体(英国Abcam公司),β肌动蛋白抗体、HRP标记的羊抗鼠二抗、HRP标记的羊抗兔二抗(武汉三鹰生物技术有限公司),PVDF膜(美国Millipore公司),2.5%戊二醛固定液、中性树胶(北京Biosharp生物公司),固蓝染色液(北京雷根生物技术有限公司),2% 2, 3, 5-氯化三苯基四氮唑(2, 3, 5-triphenyltetrazolium chloride,TTC)染色液(北京索莱宝科技有限公司),锇酸、醋酸铀、柠檬酸铅(北京中镜科仪技术有限公司)。
1.3 动物分组与I/R模型的建立选取36只大鼠随机分为假手术组和I/R处理后1、3、7 d组(I/Rd1组、I/R d3组、I/R d7组),每组9只;另选取42只大鼠随机分为假手术组、I/R组和I/R+VNS组,每组14只。除假手术组外,其他组大鼠均采用MCAO线栓法构建I/R模型[15],具体方法如下:采用2%戊巴比妥钠(50 mg/kg)腹腔注射麻醉大鼠,将大鼠固定于动物手术板上,颈部备皮后做颈正中切口,暴露左颈总动脉、颈外动脉、颈内动脉,结扎左颈总动脉,于结扎远心端剪一小口并插入硅胶线栓,造成大脑局灶性缺血,缺血120 min后移除线栓,完成血管阻塞后的复灌注。假手术组大鼠仅插入尼龙线约6 mm,其他手术操作同I/R组。
1.4 VNS处理I/R+VNS组大鼠使用华佗牌电子针疗仪SDZ-V进行VNS。参照文献[16]在缺血阻塞后30 min,小心分离出左侧迷走神经,将VNS刺激电极放置在左侧迷走神经处给予60 min重复电刺激。刺激参数:0.5 mA方波,波宽0.5 ms,频率20 Hz,每次连续30 s,每隔5 min 1次。刺激结束后将大鼠放置在电热毯上,体温维持在37 ℃,直至苏醒。苏醒后,将大鼠单独分笼,自由进食、饮水。连续干预3 d。在术后3 d内(观察期),对假手术组、I/R组和I/R+VNS组所有大鼠进行严密的监测和护理,其中I/R组和I/R+VNS组各有1只大鼠在观察期内死亡,假手术组有1只大鼠因行为学异常未纳入后续实验和分析。
1.5 髓鞘相关蛋白检测取大鼠非缺血侧胼胝体保存于液氮进行各项指标检测。取保存于液氮中的非缺血侧胼胝体脑组织,研磨后加入PBS制成匀浆,加入RIPA裂解液,移至离心管内,置于4 ℃离心机中以9 660×g离心15 min,取上清,用BCA法定量蛋白质。取30 μg蛋白质样本,加入4倍体积的上样缓冲液,混合均匀,80 V电压下进行电泳分离;湿法转膜后用5%脱脂奶粉溶液封闭2 h,TBST清洗,加MBP、MAG、β肌动蛋白抗体4 ℃摇床孵育过夜;TBST清洗,加入二抗室温孵育1 h;洗膜后加入化学发光试剂,曝光显影后,读取蛋白质条带灰度值。
1.6 髓鞘的透射电子显微镜观察将大鼠处死后立即进行生理盐水灌注,然后取脑组织,浸没在戊二醛溶液中,用手术刀取若干大小约1 mm3的非缺血侧胼胝体组织块。将分离好的组织浸泡于2.5%戊二醛溶液中4 ℃固定24 h,用1%锇酸进行再固定,脱水、包埋后切片、展片,再捞至铜网,用醋酸铀在避光条件下染色12 min,随后用柠檬酸铅室温染色1~2 min。用透射电子显微镜观察并采集图像,测量髓鞘厚度并计算g-ratio。g-ratio为轴突截面积与神经纤维总截面积的比值,用于量化髓鞘的厚度和包裹效率。
1.7 髓鞘固蓝染色将大鼠脑组织常规脱水、浸蜡、包埋后连续切片;水浴展片,烤片,常规脱蜡至水,用固蓝染色液染色后,采用Luxol分化液分色,再放入70%乙醇中分色30 s至灰、白质清晰,蒸馏水冲洗;脱水、透明,中性树脂封片,光学显微镜下观察并拍照。
1.8 TTC染色I/R处理3 d后断头处死大鼠,将取出的新鲜脑组织置于皿中(-20 ℃,0.5 h),用手术刀片以2 mm的厚度从额叶到枕叶做冠状位连续切片;随后加入2% TTC染色液,放入37 ℃恒温箱中孵育20 min,每5 min将脑组织翻一次面;染色完成后将脑组织切片移入4%多聚甲醛溶液中固定1 h,用高分辨率相机拍照。
1.9 统计学处理应用GraphPad Prism 9.0软件进行统计学分析。数据均以x±s表示,两组间比较采用独立样本t检验;多组间比较采用单因素方差分析,并运用Tukey法进行多重检验。检验水准(α)为0.05。
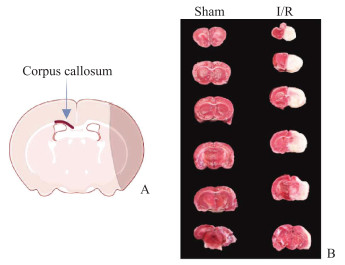
2 结果 2.1 I/R模型的建立图 1A红色标注为非缺血侧胼胝体,即实验取材部位(图片用BioRender.com制作)。图 1B为TTC染色显示的脑梗死情况(白色区域)。与假手术组(0%)相比,I/R组大鼠脑梗死体积增加[(34.14±1.93)%,P<0.01]。
|
图 1 TTC染色检测大鼠脑梗死 Fig 1 Cerebral infarct of rats detected by TTC staining A: Experimental sampling site (the diagram was drawn by BioRender.com); B: TTC staining to visualize the volume of cerebral infarction. TTC: 2, 3, 5-triphenyltetrazolium chloride; I/R: Ischemia/reperfusion. |
2.2 I/R后非缺血侧胼胝体发生继发性脱髓鞘改变且在第3天损伤最严重
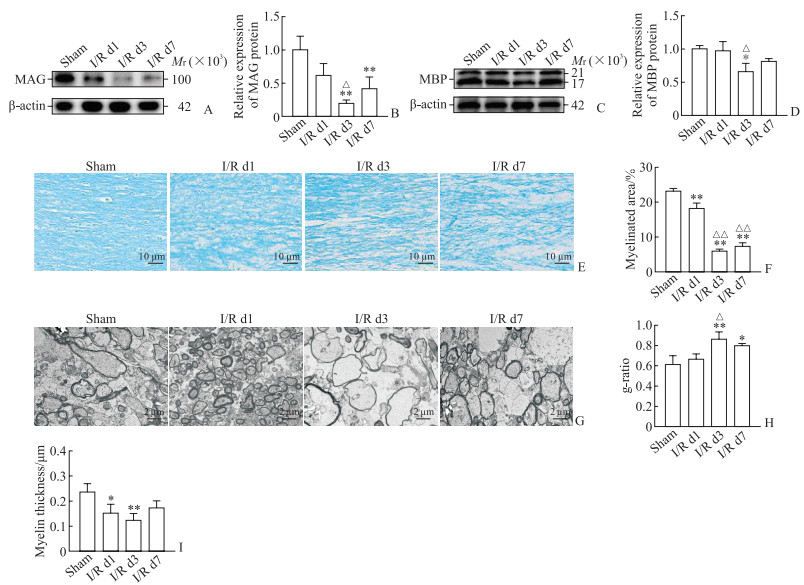
采用蛋白质印迹法检测I/R后非缺血侧胼胝体中髓鞘相关蛋白MAG、MBP的表达情况。与假手术组相比,I/R后第1天MAG蛋白表达水平下降,但差异无统计学意义(P>0.05);第3天进一步下降,与假手术组相比差异有统计学意义(P<0.01);在第7天时MAG蛋白表达水平有所恢复,但仍低于假手术组(P<0.01,图 2A、2B)。与假手术组相比,MBP蛋白表达水平在I/R后第1天无明显变化;第3天下降(P<0.05);第7天有所恢复,与假手术组相比差异无统计学意义(P>0.05,图 2C、2D)。固蓝染色结果如2E、2F所示,与假手术组相比,I/R后非缺血侧胼胝体的固蓝染色强度下降,提示髓鞘完整性破坏,且在第3天下降最明显(P<0.01);在第7天有所恢复,但仍低于假手术组(P<0.01)。透射电子显微镜观察结果如图 2G~2I所示,与假手术组相比,I/R后大鼠非缺血侧胼胝体的g-ratio升高,髓鞘厚度下降,且在第3天变化最明显(均P<0.01)。后续实验在I/R后第3天对大鼠进行取材。
|
图 2 I/R后大鼠非缺血侧胼胝体随时间的变化趋势 Fig 2 Change trend of corpus callosum on non-ischemic side of rats after I/R A: MAG expression detected by Western blotting; B: Results of quantitative analysis of MAG expression; C: MBP expression detected by Western blotting; D: Results of quantitative analysis of MBP expression; E: Luxol fast blue staining of the corpus callosum tissue; F: Results of quantitative analysis of myelinated area; G: Transmission electron microscopy observation of the corpus callosum tissue; H: Statistical analysis of g-ratio (the ratio of the cross-sectional area of axons to the total cross-sectional area of nerve fibers); I: Statistical analysis of myelin thickness. *P < 0.05, **P < 0.01 vs sham group; △P < 0.05, △△P < 0.01 vs I/R d1 group. n=3, x±s. I/R: Ischemia/reperfusion; MAG: Myelin-associated glycoprotein; MBP: Myelin basic protein. |
2.3 VNS可改善I/R后非缺血侧胼胝体的继发性脱髓鞘改变
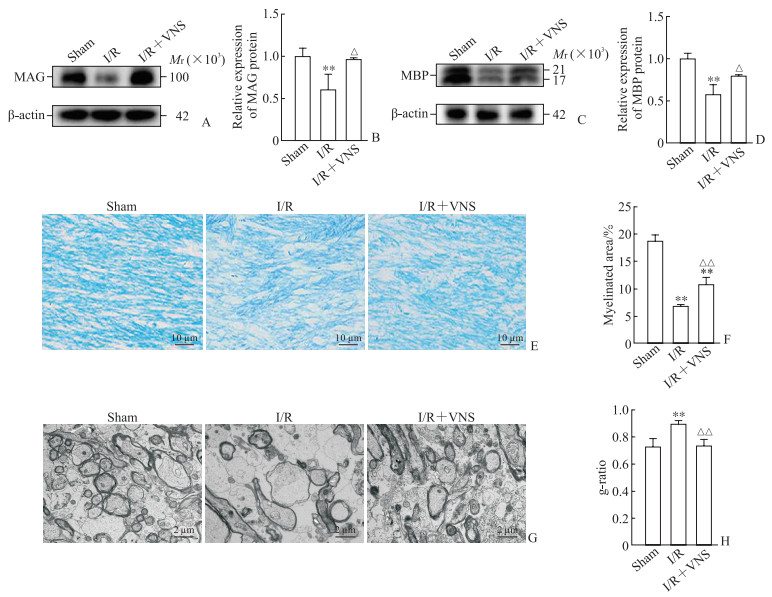
如图 3A~3D所示,与假手术组相比,I/R后第3天大鼠非缺血侧胼胝体组织中髓鞘相关蛋白MAG和MBP表达水平均下降(均P<0.01);而经VNS干预后MAG和MBP表达水平均较I/R组升高(均P<0.05),且与假手术组相比差异无统计学意义(均P>0.05)。如图 3E、3F所示,I/R后第3天非缺血侧胼胝体的固蓝染色强度降低(P<0.01),表明髓鞘完整性受到损害;而经VNS干预后固蓝染色强度增加(P<0.01),但仍低于假手术组(P<0.01)。如图 3G、3H所示,与假手术组相比,I/R后第3天g-ratio增高(P<0.01),经VNS干预后g-ratio下降(P<0.01),表明I/R后非缺血侧胼胝体髓鞘厚度变薄,而VNS干预可改善继发性脱髓鞘改变。
|
图 3 VNS干预对I/R后第3天大鼠非缺血侧胼胝体脱髓鞘的影响 Fig 3 Effect of VNS intervention on demyelination of non-ischemic side corpus callosum of rats on day 3 after I/R A: MAG expression detected by Western blotting; B: Results of quantitative analysis of MAG expression; C: MBP expression detected by Western blotting; D: Results of quantitative analysis of MAG expression; E: Luxol fast blue staining of the corpus callosum tissue; F: Results of quantitative analysis of myelinated area; G: Transmission electron microscopy observation of the corpus callosum tissue; H: Statistical analysis of g-ratio (the ratio of the cross-sectional area of axons to the total cross-sectional area of nerve fibers). **P < 0.01 vs sham group; △P < 0.05, △△P < 0.01 vs I/R group. n=3 (Fig 3B and Fig 3D) or 5 (Fig 3F and Fig 3H), x±s. VNS: Vagus nerve stimulation; I/R: Ischemia/reperfusion; MAG: Myelin-associated glycoprotein; MBP: Myelin basic protein. |
3 讨论
本研究显示大鼠I/R后非缺血侧胼胝体出现继发性脱髓鞘改变,具体表现为髓鞘相关蛋白MAG、MBP表达水平下降、髓鞘完整性受到损害、髓鞘厚度变薄,这些改变在I/R后第3天最严重,在第7天有所恢复,但未恢复至正常水平。VNS干预可提高大鼠I/R后髓鞘相关蛋白MAG、MBP的表达水平、减轻髓鞘完整性的损害、增加髓鞘厚度,促进I/R后继发性脱髓鞘改变的恢复。
缺血性脑卒中不仅导致灰质损伤,还会因低血流导致脑白质损伤。脑白质主要由轴突、产生髓鞘的少突胶质细胞、星形胶质细胞和少突胶质前体细胞组成[17]。与灰质相比,白质血流量低且侧支循环较少,尤其是深部脑白质。此外,少突胶质细胞,尤其是少突胶质前体细胞对缺血诱导的氧化应激、兴奋性毒性和炎症高度敏感,因此缺血后易发生少突胶质前体细胞死亡和白质损伤。上述因素共同导致了缺血性脑卒中后白质损伤的高发生率[18]。白质损伤可引起脑卒中后认知功能障碍、感觉运动障碍及情绪行为障碍等多重神经功能损害,严重影响患者的生活质量和独立生活能力,白质损伤的严重程度与脑卒中患者的长期运动缺陷和认知能力下降相关[19]。
脱髓鞘是白质损伤的主要病理改变,其特征是少突胶质细胞死亡和髓鞘破坏[20],引起轴突变性和神经元丢失,进而导致各种神经功能的损害[21],改善脑卒中后继发性脱髓鞘能减轻大鼠认知障碍[5]。髓鞘是包裹在神经元轴突外的重要保护层,其主要成分包括由少突胶质细胞分泌的鞘磷脂和髓鞘蛋白,对于神经元的健康和电脉冲的快速传导至关重要,有助于支持中枢神经系统的功能,如认知功能、学习及记忆等[21]。少突胶质细胞是中枢神经系统中唯一产生髓鞘的细胞,对轴突完整性的维持和髓鞘再生至关重要[22-23];缺血、缺氧所致的氧化应激、炎症和兴奋性毒性是少突胶质细胞发生损伤的潜在因素,少突胶质细胞死亡是缺血性脑卒中后脱髓鞘的主要机制[22-24]。总之,白质作为中枢神经系统内髓鞘富集区域,其功能完整性高度依赖于少突胶质细胞-髓鞘-轴突单元的结构稳定。胼胝体是大脑中最大的白质束,是主要的半球间连合纤维,负责感觉、运动和认知信息的整合,也是白质卒中和血管性痴呆的主要损伤部位[25]。基于此,本研究选取非缺血侧胼胝体作为I/R后继发性脱髓鞘的研究部位。本研究观察到大鼠I/R后非缺血侧胼胝体发生脱髓鞘改变,具体表现为髓鞘相关蛋白MAG、MBP的表达水平下降、髓鞘完整性损害、髓鞘厚度变薄,这与文献报道[4, 5, 26]一致。此外,本研究中I/R后第3天脱髓鞘最严重、第7天有所恢复,这与Liu等[27]的研究结果相符,其原因可能是缺血性脑卒中后24 h内少突胶质细胞持续丢失,而到卒中后7 d时少突胶质细胞的数量开始增加,对脱髓鞘起到了内源性修复作用[28-29]。
MBP是中枢神经系统髓鞘中含量丰富的蛋白质,占中枢神经系统髓磷脂总蛋白质的30%[30]。MBP与正常神经系统发育和神经退行性疾病密切相关,常用于检测中枢神经系统的脱髓鞘疾病。在MBP基因缺陷模型小鼠中,中枢神经系统出现髓鞘结构异常[30-31]。MAG是一种分子量为100 000的Ⅰ型跨膜糖蛋白,选择性地定位于轴突周围的施万细胞膜和少突胶质细胞膜上,既是维持髓鞘轴突所需的配体,也是介导少突胶质细胞分化、维持和存活的轴突信号的受体,在胶质-轴突的相互作用和髓鞘形成中发挥着重要的作用[32]。有研究证明,MAG基因敲除小鼠表现出功能性认知和运动缺陷,有髓轴突周围区域表现出结构异常[33]。在含有MAG的基质上培养少突胶质细胞既增加了细胞存活率,又增加了髓鞘样膜的形成[34]。总之,在缺乏MAG的情况下少突胶质细胞不能有效地形成髓鞘。本研究结果表明,I/R后MBP、MAG表达减少,进一步证实缺血性脑卒中后会发生继发性脱髓鞘。
VNS已获美国FDA批准用于治疗特定的神经和精神疾病,最初用于治疗顽固性癫痫,后续成为抑郁、帕金森病、阿尔茨海默病、丛集性偏头痛等疾病的替代疗法[35]。研究显示,VNS联合康复训练可改善缺血性脑卒中后遗症期患者上肢运动障碍,提示VNS在缺血性脑卒中后遗症期具有神经调控作用[10]。本课题组前期研究证实,VNS可改善脑卒中亚急性期患者的运动功能[36]。白质约占大脑体积的一半[37],脑卒中后白质损伤与患者长期运动、感觉和认知功能损害密切相关[23]。目前尚无研究探索VNS对脑卒中后白质继发性脱髓鞘的影响。本研究发现,VNS可提高大鼠I/R模型髓鞘相关蛋白MAG、MBP的表达水平、减轻髓鞘完整性的损害、增加髓鞘厚度,促进I/R后继发性脱髓鞘改变的恢复。本课题组前期研究发现,3周的VNS干预可促进恢复期脑卒中大鼠髓鞘再生[14]。本研究采用急性期脑卒中大鼠模型,发现VNS干预可减轻急性期脑卒中大鼠的继发性脱髓鞘改变,这为VNS在脑卒中不同阶段治疗中的应用提供了依据。I/R后小胶质细胞和星形胶质细胞过度激活[23],诱导神经炎症,从而抑制少突胶质前体细胞的增殖、分化,并诱导少突胶质细胞的凋亡和死亡[28],最终导致脱髓鞘并影响髓鞘的修复过程。而VNS可通过抑制小胶质细胞和星形胶质细胞的激活[38]减轻神经炎症[13, 39],改善I/R后少突胶质细胞的功能,进而减少白质的继发性脱髓鞘改变。此外,VNS可通过激活蓝斑核释放去甲肾上腺素并作用于小胶质细胞、星形胶质细胞和神经元上的β2-肾上腺素能受体,减轻神经炎症[40-44]。综上所述,VNS干预可改善急性期缺血性脑卒中大鼠的继发性脱髓鞘改变,其机制可能包括抑制小胶质细胞和星形胶质细胞的激活和增加[38]、抑制神经炎症[13, 39]、增加谷氨酸能突触的数量和活性[38]、增强小胶质细胞对受损少突胶质细胞和髓鞘碎片的清除能力[45]、调控去甲肾上腺素信号及调节突触可塑性等。
本研究有以下不足之处:(1)仅观察了VNS对I/R后非缺血侧胼胝体继发性脱髓鞘的影响,还需进一步观察VNS对其他白质区域的影响;(2)本研究表明VNS可改善I/R后继发性脱髓鞘改变,但未进一步探讨其具体机制。在下一步的研究中拟从炎症、少突胶质细胞凋亡或死亡、氧化应激等多角度深入探讨VNS对I/R后继发性脱髓鞘的保护机制,并寻找VNS干预继发性性脱髓鞘的分子靶点。
| [1] |
HILKENS N A, CASOLLA B, LEUNG T W, et al. Stroke[J]. Lancet, 2024, 403(10446): 2820-2836. DOI:10.1016/s0140-6736(24)00642-1 |
| [2] |
WALTER K. What is acute ischemic stroke?[J]. JAMA, 2022, 327(9): 885. DOI:10.1001/jama.2022.1420 |
| [3] |
LIU S, JIN R, XIAO A Y, et al. Inhibition of CD147 improves oligodendrogenesis and promotes white matter integrity and functional recovery in mice after ischemic stroke[J]. Brain Behav Immun, 2019, 82: 13-24. DOI:10.1016/j.bbi.2019.07.027 |
| [4] |
ZUO M, GUO H, WAN T, et al. Wallerian degeneration in experimental focal cortical ischemia[J]. Brain Res Bull, 2019, 149: 194-202. DOI:10.1016/j.brainresbull.2019.04.023 |
| [5] |
WAN T, ZHU W, ZHAO Y, et al. Astrocytic phagocytosis contributes to demyelination after focal cortical ischemia in mice[J]. Nat Commun, 2022, 13(1): 1134. DOI:10.1038/s41467-022-28777-9 |
| [6] |
BOHOTIN C, SCHOLSEM M, MULTON S, et al. Vagus nerve stimulation in awake rats reduces formalin-induced nociceptive behaviour and fos-immunoreactivity in trigeminal nucleus caudalis[J]. Pain, 2003, 101(1/2): 3-12. DOI:10.1016/s0304-3959(02)00301-9 |
| [7] |
MAUSKOP A. Vagus nerve stimulation relieves chronic refractory migraine and cluster headaches[J]. Cephalalgia, 2005, 25(2): 82-86. DOI:10.1111/j.1468-2982.2005.00611.x |
| [8] |
SMITH D C, MODGLIN A A, ROOSEVELT R W, et al. Electrical stimulation of the vagus nerve enhances cognitive and motor recovery following moderate fluid percussion injury in the rat[J]. J Neurotrauma, 2005, 22(12): 1485-1502. DOI:10.1089/neu.2005.22.1485 |
| [9] |
ENGINEER N D, RILEY J R, SEALE J D, et al. Reversing pathological neural activity using targeted plasticity[J]. Nature, 2011, 470(7332): 101-104. DOI:10.1038/nature09656 |
| [10] |
DAWSON J, LIU C Y, FRANCISCO G E, et al. Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): a randomised, blinded, pivotal, device trial[J]. Lancet, 2021, 397(10284): 1545-1553. DOI:10.1016/S0140-6736(21)00475-X |
| [11] |
JIANG Y, LI L, LIU B, et al. Vagus nerve stimulation attenuates cerebral ischemia and reperfusion injury via endogenous cholinergic pathway in rat[J]. PLoS One, 2014, 9(7): e102342. DOI:10.1371/journal.pone.0102342 |
| [12] |
JIANG Y, LI L, TAN X, et al. miR-210 mediates vagus nerve stimulation-induced antioxidant stress and anti-apoptosis reactions following cerebral ischemia/reperfusion injury in rats[J]. J Neurochem, 2015, 134(1): 173-181. DOI:10.1111/jnc.13097 |
| [13] |
LIU Y L, WANG S R, MA J X, et al. Vagus nerve stimulation is a potential treatment for ischemic stroke[J]. Neural Regen Res, 2023, 18(4): 825-831. DOI:10.4103/1673-5374.350698 |
| [14] |
LONG L, ZANG Q, JIA G, et al. Transcutaneous auricular vagus nerve stimulation promotes white matter repair and improves dysphagia symptoms in cerebral ischemia model rats[J]. Front Behav Neurosci, 2022, 16: 811419. DOI:10.3389/fnbeh.2022.811419 |
| [15] |
BELAYEV L, ALONSO O F, BUSTO R, et al. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model[J]. Stroke, 1996, 27(9): 1616-1623. DOI:10.1161/01.str.27.9.1616 |
| [16] |
SUN R, SONG Y, LI S, et al. Levo-tetrahydropalmatine attenuates neuron apoptosis induced by cerebral ischemia-reperfusion injury: involvement of c-Abl activation[J]. J Mol Neurosci, 2018, 65(3): 391-399. DOI:10.1007/s12031-018-1063-9 |
| [17] |
WANG S S, SHULTZ J R, BURISH M J, et al. Functional trade-offs in white matter axonal scaling[J]. J Neurosci, 2008, 28(15): 4047-4056. DOI:10.1523/JNEUROSCI.5559-05.2008 |
| [18] |
WANG Y, LIU G, HONG D, et al. White matter injury in ischemic stroke[J]. Prog Neurobiol, 2016, 141: 45-60. DOI:10.1016/j.pneurobio.2016.04.005 |
| [19] |
KISSELA B, LINDSELL C J, KLEINDORFER D, et al. Clinical prediction of functional outcome after ischemic stroke: the surprising importance of periventricular white matter disease and race[J]. Stroke, 2009, 40(2): 530-536. DOI:10.1161/STROKEAHA.108.521906 |
| [20] |
XU J, ZHAO J, WANG R, et al. Shh and Olig2 sequentially regulate oligodendrocyte differentiation from hiPSCs for the treatment of ischemic stroke[J]. Theranostics, 2022, 12(7): 3131-3149. DOI:10.7150/thno.69217 |
| [21] |
VARAS R, ORTIZ F C. Neuroinflammation in demyelinating diseases: oxidative stress as a modulator of glial cross-talk[J]. Curr Pharm Des, 2019, 25(45): 4755-4762. DOI:10.2174/1381612825666191216125725 |
| [22] |
HUANG S, REN C, LUO Y, et al. New insights into the roles of oligodendrocytes regulation in ischemic stroke recovery[J]. Neurobiol Dis, 2023, 184: 106200. DOI:10.1016/j.nbd.2023.106200 |
| [23] |
SHI H, HU X, LEAK R K, et al. Demyelination as a rational therapeutic target for ischemic or traumatic brain injury[J]. Exp Neurol, 2015, 272: 17-25. DOI:10.1016/j.expneurol.2015.03.017 |
| [24] |
XIE Y, ZHANG X, XU P, et al. Aberrant oligodendroglial LDL receptor orchestrates demyelination in chronic cerebral ischemia[J]. J Clin Invest, 2021, 131(1): e128114. DOI:10.1172/JCI128114 |
| [25] |
MARIN M A, CARMICHAEL S T. Mechanisms of demyelination and remyelination in the young and aged brain following white matter stroke[J]. Neurobiol Dis, 2019, 126: 5-12. DOI:10.1016/j.nbd.2018.07.023 |
| [26] |
JIA J, ZHENG L, YE L, et al. CD11c+microglia promote white matter repair after ischemic stroke[J]. Cell Death Dis, 2023, 14(2): 156. DOI:10.1038/s41419-023-05689-0 |
| [27] |
LIU C, HAN S, ZHENG J, et al. EphA4 regulates white matter remyelination after ischemic stroke through Ephexin-1/RhoA/ROCK signaling pathway[J]. Glia, 2022, 70(10): 1971-1991. DOI:10.1002/glia.24232 |
| [28] |
ZHANG R, CHOPP M, ZHANG Z G. Oligodendrogenesis after cerebral ischemia[J]. Front Cell Neurosci, 2013, 7: 201. DOI:10.3389/fncel.2013.00201 |
| [29] |
MCIVER S R, MUCCIGROSSO M, GONZALES E R, et al. Oligodendrocyte degeneration and recovery after focal cerebral ischemia[J]. Neuroscience, 2010, 169(3): 1364-1375. DOI:10.1016/j.neuroscience.2010.04.070 |
| [30] |
BOGGS J M. Myelin basic protein: a multifunctional protein[J]. Cell Mol Life Sci, 2006, 63(17): 1945-1961. DOI:10.1007/s00018-006-6094-7 |
| [31] |
CARRÉ J L, GOETZ B D, O'CONNOR L T, et al. Mutations in the rat myelin basic protein gene are associated with specific alterations in other myelin gene expression[J]. Neurosci Lett, 2002, 330(1): 17-20. DOI:10.1016/s0304-3940(02)00709-7 |
| [32] |
QUARLES R H. Myelin-associated glycoprotein (MAG): past, present and beyond[J]. J Neurochem, 2007, 100(6): 1431-1448. DOI:10.1111/j.1471-4159.2006.04319.x |
| [33] |
WEISS M D, HAMMER J, QUARLES R H. Oligodendrocytes in aging mice lacking myelin-associated glycoprotein are dystrophic but not apoptotic[J]. J Neurosci Res, 2000, 62(6): 772-780. DOI:10.1002/1097-4547(20001215)62:6<772::AID-JNR3>3.0.CO;2-X |
| [34] |
GARD A L, MAUGHON R H, SCHACHNER M. In vitro oligodendrogliotrophic properties of cell adhesion molecules in the immunoglobulin superfamily: myelin-associated glycoprotein and N-CAM[J]. J Neurosci Res, 1996, 46(4): 415-426. DOI:10.1002/(SICI)1097-4547(19961115)46:4<415::AID-JNR3>3.0.CO;2-J |
| [35] |
KUMAR A, BUNKER M T, AARONSON S T, et al. Durability of symptomatic responses obtained with adjunctive vagus nerve stimulation in treatment-resistant depression[J]. Neuropsychiatr Dis Treat, 2019, 15: 457-468. DOI:10.2147/NDT.S196665 |
| [36] |
WU D, MA J, ZHANG L, et al. Effect and safety of transcutaneous auricular vagus nerve stimulation on recovery of upper limb motor function in subacute ischemic stroke patients: a randomized pilot study[J]. Neural Plast, 2020, 2020: 8841752. DOI:10.1155/2020/8841752 |
| [37] |
SAMPAIO-BAPTISTA C, JOHANSEN-BERG H. White matter plasticity in the adult brain[J]. Neuron, 2017, 96(6): 1239-1251. DOI:10.1016/j.neuron.2017.11.026 |
| [38] |
BACHMANN H, VANDEMOORTELE B, VERMEIRSSEN V, et al. Vagus nerve stimulation enhances remyelination and decreases innate neuroinflammation in lysolecithin-induced demyelination[J]. Brain Stimul, 2024, 17(3): 575-587. DOI:10.1016/j.brs.2024.04.012 |
| [39] |
ZHANG L, LIU Y, WANG S, et al. Vagus nerve stimulation mediates microglia M1/2 polarization via inhibition of TLR4 pathway after ischemic stroke[J]. Biochem Biophys Res Commun, 2021, 577: 71-79. DOI:10.1016/j.bbrc.2021.09.004 |
| [40] |
BASSI G S, DIAS D P M, FRANCHIN M, et al. Modulation of experimental arthritis by vagal sensory and central brain stimulation[J]. Brain Behav Immun, 2017, 64: 330-343. DOI:10.1016/j.bbi.2017.04.003 |
| [41] |
KRAHL S E, CLARK K B, SMITH D C, et al. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation[J]. Epilepsia, 1998, 39(7): 709-714. DOI:10.1111/j.1528-1157.1998.tb01155.x |
| [42] |
DE KEYSER J, LAUREYS G, DEMOL F, et al. Astrocytes as potential targets to suppress inflammatory demyelinating lesions in multiple sclerosis[J]. Neurochem Int, 2010, 57(4): 446-450. DOI:10.1016/j.neuint.2010.02.012 |
| [43] |
LAUREYS G, GERLO S, SPOOREN A, et al. β2-adrenergic agonists modulate TNF-α induced astrocytic inflammatory gene expression and brain inflammatory cell populations[J]. J Neuroinflammation, 2014, 11: 21. DOI:10.1186/1742-2094-11-21 |
| [44] |
LAUREYS G, CLINCKERS R, GERLO S, et al. Astrocytic β2-adrenergic receptors: from physiology to pathology[J]. Prog Neurobiol, 2010, 91(3): 189-199. DOI:10.1016/j.pneurobio.2010.01.011 |
| [45] |
KOTTER M R, SETZU A, SIM F J, et al. Macrophage depletion impairs oligodendrocyte remyelination following lysolecithin-induced demyelination[J]. Glia, 2001, 35(3): 204-212. DOI:10.1002/glia.1085 |
 2025, Vol. 46
2025, Vol. 46


