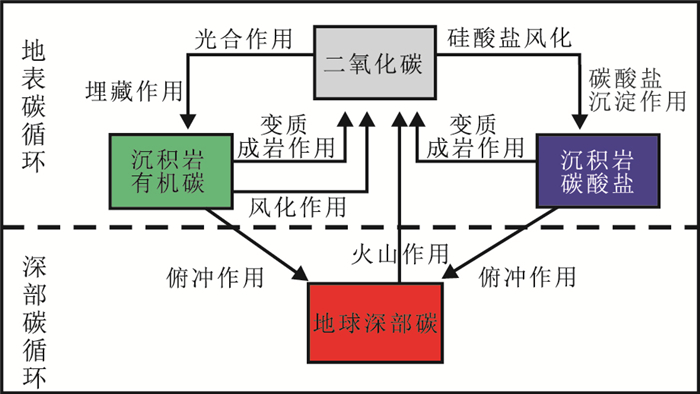
碳循环过程通常可以划分为两种亚循环过程 (图 1) :一是在固体地球外部的大气圈、水圈、生物圈 (包括人类活动) 以及浅地表层内的,循环周期较短的 (万年尺度内) 地表碳循环;二是地球内部的岩石圈、软流圈、地幔和地核之间的,循环周期更加漫长的 (百万年以上) 地球深部碳循环。地表碳循环明显影响气候变化、生态环境等与人类生存息息相关的科学问题,一直是地球环境科学研究的重点领域,倍受人们的关注。但近些年来越来越多的研究表明,地球深部碳循环过程对地表碳循环产生了重要影响,是影响地表碳循环的主要原因 (Hicks and Secco, 1997;Kerrick and Connolly, 1998;Massonne and Kopp, 2004;El Korh et al., 2009;Dasgupta and Hirschmann, 2010)。据有关地球碳储量的初步估算,地球深部碳循环涉及的碳含量占整个地球系统碳循环的90%以上 (Javoy et al., 1982;Javoy,1997;Hilton et al., 2002)。而实验岩石学研究表明,碳在地幔主要矿物中的溶解度都很低 (Keppler et al., 2003;Panero and Kabbes, 2008;Shcheka et al., 2006),这就说明地球内部大规模的碳储量主要以单质碳 (金刚石或石墨)、碳酸盐矿物、碳化合物和碳氢化合物等形式存在于地壳、地幔和地核的各种岩石中 (Deines,2002;Hazen and Schiffries, 2013),这些含碳的物质在固体地球形成和演化过程中起到重要作用,也是地表碳循环的主要物质来源 (Hicks and Secco, 1997;Lee et al., 2012)。因此,研究地质尺度的深部碳循环对研究地表碳循环具有重要的指示意义。
|
图 1 深部碳循环模式图 (修改自Berner,2003) Figure 1 The deep carbon cycle model (modified after Berner, 2003) |
从板块构造角度来说,地球表面的含碳物质主要是通过板块俯冲作用被带入到深部地球。俯冲过程中的变质脱碳反应以及各类岩浆作用,又把一部分地球内部的含碳物质 (以CO2为主) 喷发到地表,直接参与地表碳循环演化过程 (Berner,2003) (图 1)。板块从形成到俯冲消亡过程中,都伴随有碳转换过程的发生。新生洋壳在洋中脊扩张生长过程中,由于洋脊变质作用和热液蚀变作用,可以使新生洋壳基性岩乃至超基性岩发生水化和碳酸盐化作用,将海水中的CO2以碳酸盐形式沉淀在被改造的洋壳基性岩和超基性岩中 (Alt and Teagle, 1999;Sleep and Zahnle, 2001;Kelley et al., 2005;Slagle and Goldberg, 2011;Rosenbauer et al., 2012)。随着洋底向海沟一侧不断扩张,洋壳火成岩继续发生水化和碳酸盐化作用。同时,部分大陆壳风化产物以及海底生物作用形成的钙质碳酸盐,可以在洋壳火成岩上部沉淀出含有大量碳酸盐的沉积物乃至纯碳酸盐层,使更多的地表系统的碳以碳酸盐形式进入到俯冲循环系统 (Plank and Langmuir, 1998)。Dasgupta (2013)综合前人对洋壳蛇绿岩套碳酸盐化过程的研究,认为每年大约有 (5.4~8.8)×1013 g的碳通过碳酸盐化过程进入新生洋壳并被俯冲带入深部地球系统。深部碳循环过程中,碳从地球深部返回到地表系统的主要方式是通过俯冲带变质过程和各类岩浆作用的碳释放,其中以岛弧岩浆作用、洋中脊岩浆作用及地幔柱岩浆作用为主。Burton等 (2013)总结了新生代以来已知所有的火山岩碳释放通量,认为不同源区的岩浆作用的碳释放量 (5.4×108 g) 只占俯冲进入地球深部碳总量[(5.4~8.8)×1013 g]的很小一部分。由此认为地球系统新生代以来俯冲进入地幔的碳基本都被保留在地幔中,且地幔中碳含量是逐渐增加的。换句话说,新生代以来,全球地表碳含量是随着俯冲作用进行而明显减少的。此结论似乎与新生代以来地球上多次由于CO2温室效应导致的全球变暖事件不一致,这说明人类过度的碳排放可能是导致温室效应的主要原因。Lee等 (2012)则提出,新生代以来白垩纪到早第三季全球变暖事件有可能是全球大规模的大洋板片向大陆俯冲过程,形成的陆缘弧岩浆作用对大陆壳风化碳酸岩的加热脱碳作用导致的全球大气大规模温室效应。事实上,大量岩石学观察和实验岩石学研究都证实地表系统中的碳可以在深俯冲板片中以碳酸盐形式稳定存在并被带入深部地球系统,地表的碳循环与深部碳循环有着不可分割的成因联系 (Zhang and Liou, 1994;Zhu and Ogasawara, 2002;Dasgupta et al., 2004;Yaxley and Brey, 2004;Kawamoto,2006;Poli et al., 2009;Keshav and Gudfinnsson, 2010)。最近,Kelemen和Manning (2015)基于钙质碳酸盐的高温高压溶解度实验认为俯冲带中绝大部分含碳相在变质作用过程中,可能会被俯冲带流体溶解带出到岛弧区,而返回地表。由此可见,与俯冲作用相关的地球深部碳循环对地表系统地质尺度上全球规模的气候变化起到了至关重要的控制和影响。
目前看来,与俯冲带相关的深部碳循环的关键科学问题应该包括以下几个方面:
1 俯冲带变质过程中含碳物质相的转变及其物理化学条件已有研究结果表明,俯冲带深部碳循环涉及到的含碳物质主要包括固体的碳酸盐矿物,单质石墨、金刚石,各种碳氢化合物,C-O-H流体及各种含碳熔体等 (表 1)。除了像石墨/金刚石的相转变反应已有确切的高温高压实验确定外,其他的含碳物质相在俯冲带中的稳定温压条件及转换关系都不清楚 (图 2,表 1)。它们在俯冲带中的稳定性怎样?它们之间是如何相互转化的?这些问题是关系到含碳相物质的稳定性,是目前深部碳循环研究的最基础的问题之一,需要开展深入的研究工作。
|
|
表 1 俯冲带中可能的含碳相及其稳定性研究现状 (修改自Hazen and Schiffries, 2013) Table 1 Research status of the possible carbon bearing phase and its stability in subduction zone (modified after Hazen and Schiffries, 2013)) |

|
图 2 俯冲带深部碳循环示意图及相关含碳相的稳定性 Figure 2 The deep carbon cycle and the stability of carbon bearing material in subduction zone |
通常认为,浅层地壳中分布最广的钙质碳酸盐矿物 (方解石和文石) 在俯冲递进变质过程中往往通过流体作用或者固相反应分解消耗,高温高压实验结果和地球深部来源的岩石样品中仅存的菱镁矿包体都证明只有菱镁矿等铁镁碳酸盐矿物可以在地幔深度稳定存在 (图 2) (Irving and Wyllie, 1975;Biellmann et al., 1993;Yang et al., 1993;Zhang and Liou, 1994;Wang et al., 1996;Fiquet et al., 2002;Isshiki et al., 2004;Brenker et al., 2007;Tao et al., 2013)。但相关钙质碳酸盐在俯冲带中是如何通过流体作用或者固相反应分解消耗,相应的岩石学和高温高压实验研究还不是很完善 (Luth,1995;Knoche et al., 1999;Ono et al., 2005)。俯冲带白云石在上地幔的温压条件下可以分解为菱镁矿和文石,因而前人提出将CaMg (CO3)2 (白云石)=MgCO3 (菱镁矿)+CaCO3 (文石) 反应作为继柯石英/石英和石墨/金刚石后的第3个超高压变质标志反应 (Luth,2001),但有关这个变质反应的稳定上限一直存有争论 (Hammouda et al., 2011)。笔者最近的工作表明,这个变质分解反应压力明显受白云石中Fe2+替代Mg2+的控制,在极端富铁端员中,这个反应发生的条件有可能达不到柯石英的稳定范围 (Tao et al., 2014)。随着板块进一步俯冲到地幔深度,菱镁 (铁) 矿等也要分解,通常认为它们会被还原为单质的碳 (石墨或金刚石),但具体的变质反应及其控制因素 (如氧逸度?) 都在探讨中 (Zhu and Ogasawara, 2002;Stagno et al., 2011;Tao et al., 2013)。另外,近些年来有关CH4和其他碳氢化合物的高温高压实验和理论计算研究均表明,俯冲带乃至深部地幔中可能存在大量的无机成因的碳氢化合物 (McCollom and Seewald, 2007;Frost and McCammon, 2008;Poli et al., 2009;McCollom,2013;Sephton and Hazen, 2013;Sverjensky et al., 2014),这同时也得到了大量岩石学观察的证实,如在高压-超高压变质岩和地幔岩石中发现大量原生的CH4和其他碳氢化合物的气液包裹体 (Fu et al., 2003;Song et al., 2009;Arai et al., 2012;Herms et al., 2012)。笔者最近在西南天山俯冲带榴辉岩中发现了碳氢化合物的流体包裹体,并通过高温高压实验证实这些碳氢化合物都是来自含铁碳酸盐在变质流体中通过溶解、歧化作用形成的无机产物 (Tao et al., 2017)。这些在俯冲带中通过无机作用合成的碳氢化合物对于探讨油气的成因、前陆盆地油气资源 (如CH4水合物) 勘查都具有重要意义。然而迄今为止,关于俯冲带中无机碳氢化合物的可能成因及其转换关系和稳定性都不是很明确,需要进一步的研究。
2 俯冲带脱碳机制 2.1 纯变质反应脱碳这是经典的变质反应过程,通常认为蚀变洋壳在俯冲带变质过程中,由于温度、压力升高,碳酸盐矿物与硅酸盐矿物会发生变质反应,并释放出CO2,这一过程通常也伴随有大量流体H2O的排出。Kerrick和Connolly (2001a, 2001b) 较早开始利用洋壳成分开展相平衡理论模拟工作,在Peacock和Wang (1999)提出来的有关冷俯冲和热俯冲模型基础上,通过相模拟计算提出洋壳俯冲过程中在不同的地热梯度情况下的脱水和脱CO2是不耦合的,是沿着高温地热梯度的俯冲在弧前带可以发生脱水反应,而沿着低温地热梯度的俯冲则不会发生脱水反应。对于脱CO2反应只有沿着高温地热梯度的俯冲才能发生,也就是说脱水反应往往发生在脱CO2反应之前。目前观察到的脱CO2的反应如不纯的白云岩在大理岩中经常发生的变质反应:K (AlSi3O8) (钾长石)+3 (白元石)+H2O=金云母+3CaCO3 (方解石)+3CO2 (Bucher and Frey, 1994)。Kerrick和Caldeira (1998)曾用这个变质反应估算喜马拉雅造山带的脱CO2反应过程。Ague (2000)利用变质脱碳反应:5Dolomite+8 quartz+H2O=Tremolite+3Calcite+7CO2,来探讨由绿片岩相到角闪岩相转变过程中CO2的释放量。在西南天山冷俯冲带中,笔者观察到的主要脱碳变质反应为:Glaucophane+Dolomite+Zoisite=Magnesite+Coesite+Omphacite+Garnet+H2O (Zhang et al., 2002),这个变质反应过程中没有发生CO2释放,这也进一步表明在低温冷俯冲变质过程中可能没有纯变质脱CO2反应的发生。有关不同地温梯度的俯冲带变质过程中纯变质脱碳反应占有多大比例尚不清楚。同时,笔者最近在西南天山冷俯冲带中发现伴随着折返升温过程,可能会有可观的CO2释放。
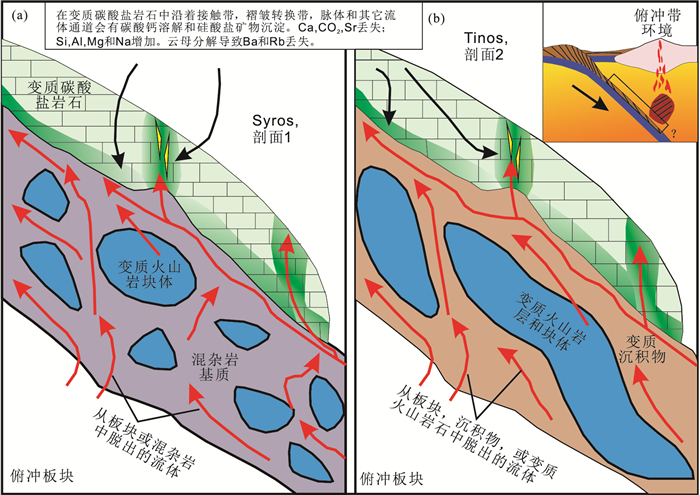
2.2 流体溶解脱碳 (流体渗透作用)由于俯冲带中观察到的纯变质脱碳反应产生的CO2的量很有限,无法解释在岛弧火山喷发过程中出现的大量CO2,这就使人们想到俯冲过程中可能存在一些其他脱碳机制。目前比较流行的解释是俯冲带中大量CO2可能是通过流体的溶解过程被带到地表的。该观点最早是基于玄武岩的理论相平衡模拟计算,Kerrick等 (1998, 2001a, 2001b) 提出,在冷俯冲带中如日本东北部的太平洋板块俯冲带中几乎没有脱水作用,而在热的俯冲带中如日本西南的菲律宾板块俯冲带中,在弧前区有流体释放。但此类冷俯冲带中有限的CO2释放也无法解释火山岩中大量CO2存在的事实,因而提出可能存在俯冲带中脱水流体溶解碳酸岩这种可能的脱碳机制,后期大量的CO2释放到岛弧岩浆房中。近年来,Frezzotti等 (2014)在阿尔卑斯冷俯冲带发现了金刚石与流体包裹体伴生出现在石榴子石斑晶中,并提出金刚石是在变质流体中结晶而成的,进一步证实流体可以溶解碳 (Frezzotti et al., 2011;2014)。最具说服力的证据是有关希腊高压变质带的岩石学研究,Ague和Nicolescu (2014)观察到了钙质大理岩中相当部分的碳酸盐被变质流体溶解后带走的通道 (蚀变带),而提出了在俯冲带中大部分碳酸岩可能被变质流体溶解后带入岛弧区,从而也解释了在俯冲带中单纯由变质反应释放出来的CO2不足以形成弧岩浆中的CO2的量的问题 (图 3)。这一岩石学观察也得到了Kelemen和Manning (2015)高温高压下钙质碳酸盐 (方解石) 的高溶解度实验的确认。然而,前述岩石学和高温高压实验模拟研究都只是关注了钙质碳酸盐溶解脱碳作用,但是根据前述讨论,我们知道,一般超高压俯冲带碳酸盐化洋壳中稳定的碳酸盐可能已经经过变质作用转变为富镁碳酸盐 (白云石或者菱镁矿),所以应当深入研究一下深俯冲高温高压条件下富镁碳酸盐的溶解度及其对俯冲带流体溶解脱碳的影响。
|
图 3 俯冲带流体溶解脱碳模式图 (据Ague and Nicolescu, 2014) Figure 3 The diagram to show the CO2 release by fluid dissolution in subduction zone (modified after Ague and Nicolescu, 2014) |
如果深俯冲碳酸盐化板片的地温梯度超过碳酸盐化蛇绿岩 (橄榄岩、玄武岩、泥质岩) 的熔融温度,那么俯冲带中含碳相将会以熔融作用脱出俯冲板片,交代深部地幔或者返回地表。岩石学和高压实验都证实,部分随洋壳或者陆壳俯冲的碳酸盐化岩石 (橄榄岩、玄武岩、泥质岩) 在地球深部可能会发生部分熔融作用。近年来,关于碳酸化硅酸盐的熔融、相关系和元素分配得到较多关注,其中碳酸盐化橄榄岩的部分熔融研究最多,且最为深入 (Yaxley and Brey, 2004;Dasgupta et al., 2007;Brey et al., 2009;Ghosh et al., 2009;Keshav and Gudfinnsson, 2010;Tumiati et al., 2013),其次是碳酸盐化的榴辉岩和泥质岩的部分熔融研究 (Hammouda,2003;Dasgupta et al., 2004;Dasgupta et al., 2005;Dasgupta et al., 2006;Thomsen and Schmidt, 2008a,2008b;Grassi and Schmidt, 2010;Litasov and Ohtani, 2010;Grassi and Schmidt, 2011;Tsuno and Dasgupta, 2011;Grassi et al., 2012;Kiseeva et al., 2012;Tsuno et al., 2012;Kiseeva et al., 2013)。深俯冲碳酸盐化蛇绿岩在地球深部产生的碳酸盐熔体和硅酸盐熔体对地幔的交代作用,可能是导致地球内部化学成分 (碳酸岩成因)、地球物理性质 (地震低速带) 不均一性的主要原因 (Dasgupta,2013)。Litasov等 (2012)认为,随着俯冲带进入地球内部的碱金属 (Na、K) 碳酸盐可以明显降低碳酸化硅酸盐的液相线温度。最近,Thomson等 (2016)利用高温高压实验并结合金刚石包体的岩石学观察,发现一般情况下深俯冲的碳酸盐化玄武岩系统会在300~700 km的区间发生碳酸盐熔融。也就说在此深度,俯冲碳酸盐化榴辉岩会产生熔融障碍,碳酸盐不会在此深度以下继续稳定存在,而是以碳酸盐熔体脱出俯冲板片。综上,我们可以继续关注富含K和Na的碳酸盐化泥质变质岩以及富Fe和Mg的碳酸盐化橄榄岩在高温高压下的熔融行为,及其对俯冲带脱碳作用和地幔交代作用的贡献。
2.4 氧化还原反应脱碳前人研究俯冲带中碳酸盐的稳定性质时,一般都只局限于讨论温度和压力对碳酸岩矿物稳定性的影响 (Zhang and Liou, 1994,1996;Isshiki et al., 2004;Poli et al., 2009)。然而,由于碳酸盐中含有C以及Fe、Mn等变价元素,使碳酸盐的稳定性也受到氧逸度的明显影响 (Forst and McCammon, 2008;Tao et al., 2013)。前人通过氧逸度变化的高温高压实验证明,碳酸盐化俯冲板片如果进入深部地幔,俯冲板片中的碳酸盐及其熔体在进入250 km深度的金属饱和氧逸度 (IW buffer:铁-氧化亚铁氧逸度缓冲剂) 条件下,可以被还原成金刚石而稳定下来 (Rohrbach and Schmidt, 2011;Stagno et al., 2011)。而富含金刚石的深部地幔减压上涌到上部相对氧化的环境下,则被氧化成碳酸岩熔体被洋中脊和地幔柱岩浆作用带出地表 (Rohrbach and Schmidt, 2011;Stagno et al., 2013)。然而,迄今为止,关于氧逸度对俯冲带中碳酸盐的稳定性乃至脱碳作用的影响的研究却没有更多的工作 (Connolly,1995;Galvez et al., 2013;Stagno et al., 2013)。此问题的解决将涉及两个关键的科学问题,一是俯冲带氧逸度演化的确定,二是氧逸度对俯冲带中各种含碳相关系的影响。这些问题的进一步探究都可能为俯冲带中脱碳机制、含碳流体的演化以及俯冲带石墨 (金刚石) 和碳氢化合物的成因具有重要意义。
基于岛弧岩浆中常见的氧化性CO2-H2O流体事实以及岛弧岩浆流体主要来自俯冲脱水流体的假设 (Tatsumi and Eggins, 1995),前人通过对比不同地幔源区氧逸度的限定结果,间接的认为俯冲板片氧化性物质 (H2O) 的加入是引起地幔楔具有较高氧逸度的原因 (Wood and Virgo, 1989;Parkinson and Arculus, 1999)。然而,Lee等 (2010)通过对地幔楔橄榄岩进行Zn/Fe体系的氧逸度计算时认为,地幔楔氧逸度可能没有前人所认为的那么高,前人所得到的较高的地幔楔氧逸度可能是地幔包体在浅层地表的后期分异作用所致。Song等 (2009)通过对祁连山造山带地幔楔方辉橄榄岩橄榄石中CH4包裹体的岩石学和同位素研究发现,这些碳氢化合物可能是来自壳源还原性的俯冲带流体。近来有在西南天山俯冲带榴辉岩和泥质片岩中发现了普遍存在的石墨 (Lü et al., 2009,2013) 和一些碳氢化合物的流体包裹体 (Tao et al., 2017) 的报道。这些事实都表明俯冲带氧化还原状态并不一定像前人所认为的那样都是相对氧化的。也许部分俯冲带具有相对低的氧逸度并能使俯冲带中的碳酸盐还原形成石墨乃至碳氢化合物。因此,通过俯冲带变质岩石原位限定俯冲带进变质过程中氧化还原环境就变得非常重要。
前人也提出了一些氧逸度计用以估计地壳岩石的氧逸度,但是这些氧逸度计都限定于某些特殊的体系或者矿物组合,如变质铁矿 (Frost, 1979a, 1979b) 或者铁铝榴石-磁铁矿-矽线石矿物组合 (Anovitz et al., 1993),极大地限制了这些氧逸度计的应用范围。Donohue和Essene (2000)发展了石榴子石-绿帘石氧逸度计和热力学数据,随后这个氧逸度计被用于计算苏鲁地体青龙山富绿帘石榴辉岩的氧逸度 (fO2 > HM+2.5) (Mattinson et al., 2004) 和北祁连缝合带线理化/块状榴辉岩的氧逸度 (线理化榴辉岩:fO2 > FMQ+2;块矿榴辉岩:fO2 > FMQ+4) (Cao et al., 2011)。然而,由于高压榴辉岩中绿帘石一般是退变质成因,致使用此氧逸度计计算得到的榴辉岩氧逸度值区间一般都很大,只能代表俯冲带变质岩石后期退变的氧逸度。到目前为止,还没有很好的氧逸度计可以原位的限定出俯冲带进变质过程中的氧逸度演化轨迹。
如果俯冲带的氧逸度并不一定是前人所认为的那么高,或者说俯冲带的氧逸度有可能低到将俯冲带碳酸盐还原成无机碳氢化合物,那么俯冲带中富含碳氢化合物的还原性CH4-H2O流体肯定和前人所认为的富含CO2的氧化性的CO2-H2O是不同的。这种低氧逸度条件可能将俯冲带中不易迁移的碳酸盐转变成易迁移的碳氢化合物流体,从而使俯冲带中碳以还原性流体脱出去进而交代地幔楔产生岛弧岩浆作用。那么俯冲带中这些还原性流体是如何形成和演化的?这些还原性流体有什么特殊性质?俯冲带中是否存在无机成因的碳氢化合物?它们对俯冲带中成矿元素的迁移有什么意义?这些问题的回答都需要进一步的研究。
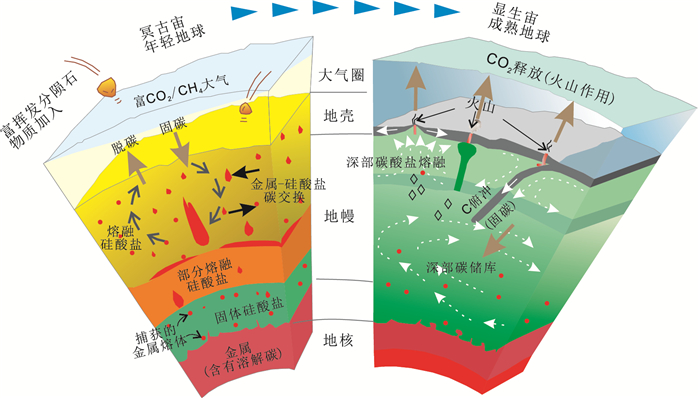
3 俯冲带深部碳循环和地幔交代作用地球形成初期经历了熔融金属地核的分离过程,残余硅酸盐岩浆洋在冥古代也分离成靠近熔融金属地核的固体硅酸盐,中部部分熔融硅酸盐以及上部的熔融硅酸盐区域。在硅酸盐地幔中也弥散分布着部分熔融的金属熔体 (图 4)。在冥古代年轻地球表面,也围绕着原始的富集CO2和CH4的大气层,此时的深部碳循环是通过原始大气和上部熔融硅酸盐地幔平衡交换作用完成的。在同一深度层面,无论地幔硅酸盐熔体、硅酸盐固体以及熔融金属地核其成分和物理属性应当是均匀的。随着冥古代年轻地球逐步变冷,到显生宙成熟地球之后,最主要的变化是产生与板块构造相关的俯冲作用和岩浆作用。岩浆作用从原始地幔部分熔融抽离熔融组成,导致地幔岩石熔融区域和非熔融区域产生水平层面的不均一。最为重要的是,岩浆作用产生的大洋和大陆壳被地表各个圈层改造 (风化,蚀变) 之后,其物理化学属性会发生明显的变化,之后这些蚀变板片伴随着俯冲作用重新进入地幔,对地幔岩石进行交代,导致地幔岩石在水平层面物理化学属性的明显的不均一化。在这里我们将重点关注俯冲带深部碳循环作用对地幔的交代作用及其对地幔不均一性的影响。
|
左图为早期地球岩浆洋阶段涉及到碳循环的深部过程;右图为显生宙成熟地球板块构造框架下的碳循环过程 图 4 深部碳循环模式图 (修改自Dasgupta,2013) Figure 4 Deep carbon cycle model (modified after Dasgupta, 2013) |
碳同位素地球化学特征被广泛用来揭示地球系统中不同含碳相的起源和演化历史。由于高温可以明显降低稳定同位素的平衡分馏作用,所以长期以来,前人都认为地球内部 (高温环境下) 的碳同位素分馏效应是可以忽略的。然而,综合来自地幔岩石中的含碳相 (碳酸岩、金伯利岩碳酸岩、金刚石及火山释放CO2) 的碳同位素特征,则发现地幔碳同位素变化区间可以高达40‰,表现出明显的不均一性 (Deines,2002;Cartigny et al., 2014)。如果用地幔主要的含碳相 (火成碳酸岩、金伯利岩碳酸盐、金刚石及火山CO2释放) 代表地幔全碳,所获得的地幔碳具有约为-5‰的碳同位素均值。同时,地幔捕掳体也表现出与此相同的碳同位素均值。然而,地幔矿物和岩石的溶解残余物质却表现出明显亏损的碳同位素特征 (-22‰) (Deines,2002)。来自不同地质背景下的各类玄武岩中地幔捕掳体的碳同位素显示在-5‰和-25‰有2个主要峰值。Cartigny等 (2014)综合对比了全球数以千计的金刚石的碳同位素特征,发现其具有从-42‰~5‰的碳同位素变化区间,并认为这些金刚石的碳同位素不均一性反映了岩石圈地幔的分馏过程。前人提出一些引起地幔碳同位素不均一的解释:如俯冲加碳作用 (Jaques et al., 1989;Bulanova et al., 2010);岩浆脱碳作用 (Galimov,1991;Javoy,1997;Deines,2002), 含碳相质量分馏 (Maruoka et al., 2004) 及地核分异作用 (Grady et al., 2004;Wood et al., 2013)。直到现在,真正引起地幔碳同位素不均一的原因依然存在较大的争议。
3.2 俯冲带深部碳循环对地幔镁同位素的影响前人研究发现,未经扰动的地幔岩石 (比如新鲜的MORB、OIB等) 都具有相对一致和稳定的Mg同位素值 (-0.25‰±0.07‰,2SD) (Teng et al., 2007, 2010;Lai et al., 2015),也就是说岩浆熔融和分异过程应该对地幔Mg同位素分异没有大的影响。然而,最近的研究也发现,地幔中局部区域也存在一些相对低Mg同位素不均一性区域 (Yang et al., 2012;张洪铭和李曙光, 2012, Li et al., 2015)。综合分析发现,地球上各种储库中只有地表沉积碳酸盐具有非常低的Mg同位素值 (Li et al., 2015)。同时,对比经历过不同变质脱水作用的变质岩石的Mg同位素特征,发现俯冲带变质脱水作用对Mg同位素具有非常小的影响 (Wang et al., 2014a),因此可以认为局部地幔低Mg同位素值是在俯冲再循环的地表沉积岩交代作用产生的 (Li et al., 2015)。例如,Yang等 (2012)和Huang等 (2015b)分别报道了华北和华南克拉通上具有相对较低的δ26Mg值的新生代玄武岩,结合其他的地球化学特征,提出这些具有低Mg同位素值的玄武岩可能是由于太平洋俯冲循环碳酸盐交代作用引起的。Wang等 (2014b)在大别-苏鲁大陆俯冲造山带中也识别出了被变质沉积碳酸盐交代并降低了δ26Mg值的变质榴辉岩,并认为这些榴辉岩进入深部地幔后,将成为引起地幔Mg同位素不均一的重要交代介质。关于深俯冲碳酸盐对地幔Mg同位素的交代影响作用,我们需要弄清以下几个问题:俯冲带中不同来源的碳酸盐 (如沉积碳酸盐和蚀变碳酸盐) 是否都可以交代地幔?俯冲流体对碳酸盐的溶解作用对其Mg同位素的分馏是否有影响?俯冲熔融作用对Mg同位素的影响?
3.3 俯冲带深部碳循环对地幔氧逸度的影响前人通过改变氧逸度的高温高压实验认为,碳酸盐化俯冲洋壳板片如果进入深部地幔,俯冲板片中的碳酸盐及其熔体在进入250 km深度的金属饱和氧逸度 (IW buffer:铁-氧化亚铁氧逸度缓冲剂) 条件下,可以被还原成金刚石稳定下来 (Rohrbach and Schmidt, 2011;Stagno et al., 2011)。这里我们想要强调的一个问题是:如果地表的碳酸盐伴随俯冲作用进入深部地幔,其中的碳酸盐被还原成金刚石,那么从另外一个角度来说,会有多少地幔还原相 (Fe2+或者Fe0) 会被碳酸盐氧化?也就是说,如何理解俯冲带碳酸盐对地幔岩石氧逸度的影响。最近,Xu等 (2017) 在华北克拉通中央造山带西缘一处火成碳酸岩中发现了一些再循环的榴辉岩捕虏体,在榴辉岩的石榴子石中发现了一些富含Fe3+ (Fe2O3含量约为17.8%) 的超硅石榴子石 (Si#约为3.18),其中Fe3+含量 (Fe2O3含量约为17.8%) 明显高于前人认为的上地幔乃至过渡带低于5%含量 (McCammon et al., 2005),也就是说,地幔中也许存在由于俯冲作用导致的极度富Fe3+的氧逸度不均一的区域。随后我们设计高温高压实验标定了适用于富Fe3+超硅石榴子石压力计,新的压力计显示此天然富Fe3+的超硅石榴子石至少来自380 km以下的深部上地幔。控制氧逸度和碳酸盐交代实验表明,氧逸度并不是控制这种富Fe3+超硅石榴子石的唯一因素,碳酸盐对地幔岩石的交代作用可能也是形成富Fe3+的超硅石榴子石的主要原因。由此,笔者提出在深俯冲碳酸盐可能和富Fe2+的地幔岩石发生氧化还原作用形成富Fe3+的超硅石榴石和金刚石,对地幔氧化还原环境不均一性产生明显的影响。也就是说,通过岩石学和高温高压实验结合,笔者认为深俯冲的碳酸盐和地幔岩石的氧化还原作用可以将地幔岩石氧化,提高地幔岩石氧逸度,从而导致地幔氧逸度的不均一性。关于深俯冲碳酸盐对地幔岩石的氧化还原作用,需要更多的来自地幔深部金刚石包裹体或者地幔捕虏体的岩石学研究以及相应的高温高压实验模拟研究来确定。
致谢: 成文过程中曾得到李曙光院士的帮助和指导,欧阳自远院士、王成善院士的支持和推荐在通报上组织“深部碳循环”专辑,在此一并致谢!
| [] | Ague J J. 2000. Release of CO2 from carbonate rocks during regional metamorphism of lithologically heterogeneous crust. Geology, 12: 1123–1126. |
| [] | Ague J J, Nicolescu S. 2014. Carbon dioxide released from subduction zones by fluid-mediated reactions. Nature Geoscience, 7(5): 355–360. DOI:10.1038/ngeo2143 |
| [] | Alt J C, Teagle D A H. 1999. The uptake of carbon during alteration of ocean crust. Geochimica et Cosmochimica Acta, 63(10): 1527–1535. DOI:10.1016/S0016-7037(99)00123-4 |
| [] | Anovitz L M, Essene E J, Metz G W, Bohlen S R, Westrum Jr E F, Hemingway B S. 1993. Heat capacity and phase equilibria of almandine, Fe3Al2Si3O12. Geochimica et Cosmochimica Acta, 51(17): 4191–4204. |
| [] | Arai S, Ishimaru S, Mizukami T. 2012. Methane and propane micro-inclusions in olivine in titanoclinohumite-bearing dunites from the Sanbagawa high-P metamorphic belt, Japan:Hydrocarbon activity in a subduction zone and Ti mobility. Earth and Planetary Science Letters, 353-354: 1–11. DOI:10.1016/j.epsl.2012.07.043 |
| [] | Berner R A. 2003. The long-term carbon cycle, fossil fuels and atmospheric composition. Nature, 426(6964): 323–326. DOI:10.1038/nature02131 |
| [] | Biellmann C, Gillet P, Guyot F, Peyronneau J, Reynard B. 1993. Experimental evidence for carbonate stability in the earth's lower mantle. Earth and Planetary Science Letters, 118(1-4): 31–41. DOI:10.1016/0012-821X(93)90157-5 |
| [] | Brenker F E, Vollmer C, Vincze L, Vekemans B, Szymanski A, Janssens K, Szaloki I, Nasdala L, Joswig W, Kaminsky F. 2007. Carbonates from the lower part of transition zone or even the lower mantle. Earth and Planetary Science Letters, 260(1-2): 1–9. DOI:10.1016/j.epsl.2007.02.038 |
| [] | Brey G P, Bulatov V K, Girnis A V. 2009. Influence of water and fluorine on melting of carbonated peridotite at 6 and 10 GPa. Lithos, 112(S1): 249–259. |
| [] | Bucher K, Frey M. 1994. Petrogenesis of Metamorphic rocks. Springer, New York, 318 |
| [] | Bulanova G P, Walter M J, Smith C B, Kohn S C, Armstrong L S, Blundy J, Gobbo L. 2010. Mineral inclusions in sublithospheric diamonds from Collier 4 kimberlites pipe, Juina, Brazil:Subducted protoliths, carbonated melts and primary kimberlite magmatism. Contributions to Mineralogy and Petrology, 160(4): 489–510. DOI:10.1007/s00410-010-0490-6 |
| [] | Burton M R, Sawyer G M, Granieri D. 2013. Deep carbon emissions from volcanoes. Reviews in Mineralogy and Geochemistry, 75(1): 323–354. DOI:10.2138/rmg.2013.75.11 |
| [] | Byrnes A P, Wyllie P J. 1981. Subsolidus and melting relations for the join CaCO3-MgCO3 at 10 Kbar. Geochimica et Cosmochimica Acta, 45(3): 321–328. DOI:10.1016/0016-7037(81)90242-8 |
| [] | Cao Y, So ng, S G, Niu Y L, Jung H, Jin Z M. 2011. Variation of mineral composition, fabric and oxygen fugacity from massive to foliated eclogites during exhumation of subducted ocean crust in the North Qilian suture zone, NW China. Journal of Metamorphic Geology, 29(7): 699–720. DOI:10.1111/j.1525-1314.2011.00937.x |
| [] | Cartigny P, Palot M, Thomassot E, Harris J W. 2014. Diamond formation:A stable isotope perspective. Annual Review of Earth and Planetary Sciences, 42: 699–732. DOI:10.1146/annurev-earth-042711-105259 |
| [] | Connolly J A D. 1995. Phase diagram methods for graphitic rocks and application to the system C-O-H-FeO-TiO2-SiO2. Contributions to Mineralogy and Petrology, 119(1): 94–116. DOI:10.1007/BF00310720 |
| [] | Dasgupta R, Hirschmann M M, Withers A C. 2004. Deep global cycling of carbon constrained by the solidus of anhydrous, carbonated eclogite under upper mantle conditions. Earth and Planetary Science Letters, 227(1-2): 73–85. DOI:10.1016/j.epsl.2004.08.004 |
| [] | Dasgupta R, Hirschmann M M, Dellas N. 2005. The effect of bulk composition on the solidus of carbonated eclogite from partial melting experiments at 3 GPa. Contributions to Mineralogy and Petrology, 149(3): 288–305. DOI:10.1007/s00410-004-0649-0 |
| [] | Dasgupta R, Hirschmann M M, Stalker K. 2006. Immiscible transition from carbonate-rich to silicate-rich melts in the 3 GPa melting interval of eclogite+CO2 and genesis of silica-undersaturated ocean island lavas. Journal of Petrology, 47(4): 647–671. |
| [] | Dasgupta R, Hirschmann M M, Smith N D. 2007. Partial melting experiments of peridotite+CO2 at 3 GPa and genesis of alkalic ocean island basalts. Journal of Petrology, 48(11): 2093–2124. DOI:10.1093/petrology/egm053 |
| [] | Dasgupta R, Hirschmann M M. 2010. The deep carbon cycle and melting in Earth's interior. Earth and Planetary Science Letters, 298(1-2): 1–13. DOI:10.1016/j.epsl.2010.06.039 |
| [] | Dasgupta R. 2013. Ingassing, storage, and outgassing of terrestrial carbon through geologic time. Reviews in Mineralogy and Geochemistry, 75(1): 183–229. DOI:10.2138/rmg.2013.75.7 |
| [] | Deines P. 2002. The carbon isotope geochemistry of mantle xenoliths. Earth-Science Reviews, 58(3-4): 247–278. DOI:10.1016/S0012-8252(02)00064-8 |
| [] | Dobrzhinetskaya L F. 2012. Microdiamonds-Frontier of ultrahigh-pressure metamorphism:A review. Gondwana Research, 21(1): 207–223. DOI:10.1016/j.gr.2011.07.014 |
| [] | Donohue C L, Essene E J. 2000. An oxygen barometer with the assemblage garnet-epidote. Earth and Planetary Science Letters, 181(3): 459–472. DOI:10.1016/S0012-821X(00)00219-3 |
| [] | El Korh A, Schmidt S T, Ulianov A, Potel S. 2009. Trace element partitioning in HP-LT metamorphic assemblages during subduction-related metamorphism, Ile de Groix, France; a Detailed LA-ICPMS Study. Journal of Petrology, 50(6): 1107–1148. DOI:10.1093/petrology/egp034 |
| [] | Fiquet G, Guyot F, Kunz M, Matas J, Andrault D, Hanfland M. 2002. Structural refinements of magnesite at very high pressure. The American Mineralogist, 87(8-9): 1261–1265. DOI:10.2138/am-2002-8-927 |
| [] | Franzolin E, Merlini M, Poli S, Schmidt M W. 2012. The temperature and compositional dependence of disordering in Fe-bearing dolomites. American Mineralogist, 97(10): 1676–1684. DOI:10.2138/am.2012.4126 |
| [] | Frezzotti M L, Selverstone J, Sharp Z D, Compagnoni R. 2011. Carbonate dissolution during subduction revealed by diamond-bearing rocks from the Alps. Nature Geosicence, 4(10): 703–706. DOI:10.1038/ngeo1246 |
| [] | Frezzotti M L, Huizenga J M, Compagnoni R, Selverstone J. 2014. Diamond formation by carbon saturation in C-O-H fluids during cold subduction of oceanic lithosphere. Geochimica et Cosmochimica Acta, 143: 68–86. DOI:10.1016/j.gca.2013.12.022 |
| [] | Frost B R. 1979a. Metamorphism of iron-formation; parageneses in the system Fe-Si-C-O-H. Economic Geology, 74(4): 775–785. DOI:10.2113/gsecongeo.74.4.775 |
| [] | Frost B R. 1979b. Mineral equilibria involving mixed-volatiles in a C-O-H fluid phase. The stabilities of graphite and siderite. American Journal of Science, 279(9): 1033–1059. |
| [] | Frost D J, McCammon C A. 2008. The redox state of Earth's mantle. Annual Review of Earth and Planetary Sciences, 36: 389–420. DOI:10.1146/annurev.earth.36.031207.124322 |
| [] | Fu B, Touret J L R, Zheng Y F, Jahn B M. 2003. Fluid inclusions in granulites, granulitized eclogites and garnet clinopyroxenites from the Dabie-Sulu terranes, eastern China. Lithos, 70(3-4): 293–319. DOI:10.1016/S0024-4937(03)00103-8 |
| [] | Galimov E M. 1991. Isotope fractionation related to kimberlite magmatism and diamond formation. Geochimica et Cosmochimica Acta, 55(6): 1697–1708. DOI:10.1016/0016-7037(91)90140-Z |
| [] | Galvez M E, Beyssac O, Martinez I, Benzerara K, Chaduteau C, Malvoisin B, Malavieille J. 2013. Graphite formation by carbonate reduction during subduction. Nature Geoscience, 6(6): 473–477. DOI:10.1038/ngeo1827 |
| [] | Ghosh S, Ohtani E, Litasov K D, Terasaki H. 2009. Solidus of carbonated peridotite from 10 to 20 GPa and origin of magnesiocarbonatite melt in the Earth's deep mantle. Chemical Geology, 262(1-2): 17–28. DOI:10.1016/j.chemgeo.2008.12.030 |
| [] | Grady M M, Verchovsky A B, Wright I P. 2004. Magmatic carbon in Martian meteorites:Attempts to constrain the carbon cycle on Mars. International Journal of Astrobiology, 3(2): 117–124. DOI:10.1017/S1473550404002071 |
| [] | Grassi D, Schmidt M W. 2010. Melting of carbonated pelites at 8~13 GPa:Generating K-rich carbonatites for mantle metasomatism. Contributions to Mineralogy and Petrology, 162(1): 169–191. |
| [] | Grassi D, Schmidt M W. 2011. The melting of carbonated pelites from 70 to 700 km depth. Journal of Petrology, 52(4): 765–789. DOI:10.1093/petrology/egr002 |
| [] | Grassi D, Schmidt M W, Günther D. 2012. Element partitioning during carbonated pelite melting at 8, 13 and 22 GPa and the sediment signature in the EM mantle components. Earth and Planetary Science Letters, 327-328: 84–96. DOI:10.1016/j.epsl.2012.01.023 |
| [] | Hammouda T. 2003. High-pressure melting of carbonated eclogite and experimental constraints on carbon recycling and storage in the mantle. Earth and Planetary Science Letters, 214(1-2): 357–368. DOI:10.1016/S0012-821X(03)00361-3 |
| [] | Hammouda T, Andrault D, Koga K, Katsura T, Martin A M. 2011. Ordering in double carbonates and implications for processes at subduction zones. Contributions to Mineralogy and Petrology, 161(3): 439–450. DOI:10.1007/s00410-010-0541-z |
| [] | Hazen R M, Schiffries C M. 2013. Why deep carbon?. Reviews in Mineralogy and Geochemistry, 75(1): 1–6. DOI:10.2138/rmg.2013.75.1 |
| [] | Herms P, John T, Bakker R J, Schenk V. 2012. Evidence for channelized external fluid flow and element transfer in subducting slabs (Raspas Complex, Ecuador). Chemical Geology, 310-311: 79–96. DOI:10.1016/j.chemgeo.2012.03.023 |
| [] | Hicks T L, Secco R A. 1997. Dehydration and decomposition of pyrophyllite at high pressures:Electrical conductivity and X-ray diffraction studies to 5 GPa. Canadian Journal of Earth Sciences, 34(6): 875–882. DOI:10.1139/e17-071 |
| [] | Hilton D R, Fischer T P, Marty B. 2002. Noble gases and volatile recycling at subduction zones. Reviews in Mineralogy and Geochemistry, 47(1): 319–370. DOI:10.2138/rmg.2002.47.9 |
| [] | Huang J, Li S G, Xiao Y L, Ke S, Li W Y, Tian Y. 2015. Origin of low δ26Mg Cenozoic basalts from South China Block and their geodynamic implications. Geochimica et Cosmochimica Acta, 164: 298–317. DOI:10.1016/j.gca.2015.04.054 |
| [] | Irving A J, Wyllie P J. 1975. Subsolidus and melting relationships for Calcite, Magnesite and the join CaCO3-MgCO3 to 36 kb. Geochimica et Cosmochimica Acta, 39(1): 35–53. DOI:10.1016/0016-7037(75)90183-0 |
| [] | Isshiki M, Irifune T, Hirose K, Ono S, Ohishi Y, Watanuki T, Nishibori E, Takata M, Sakata M. 2004. Stability of magnesite and its high-pressure form in the lowermost mantle. Nature, 427(6969): 60–63. DOI:10.1038/nature02181 |
| [] | Jaques A L, Hall A E, Sheraton J W, Smith C B, Sun S S, Drew R M, Foudoulis C, Ellingsen K. 1989. Composition of crystalline inclusions and C-isotopic composition of Argyle and Ellendale diamonds. In:Kimberlites and Related Rocks:Proceedings of 4th International Kimberlite Conference. Geological Society of Australia Special Publication: 966–989. |
| [] | Javoy M, Pineau F, and Allègre C J. 1982. Carbon geodynamic cycle. Nature, 300(5888): 171–173. DOI:10.1038/300171a0 |
| [] | Javoy M. 1997. The major volatile elements of the Earth:Their origin, behavior, and fate. Geophysical Research Letters, 24(2): 177–180. DOI:10.1029/96GL03931 |
| [] | Kawamoto T. 2006. Hydrous phases and water transport in the subducting slab. Reviews in Mineralogy and Geochemistry, 62: 273–289. DOI:10.2138/rmg.2006.62.12 |
| [] | Kelemen P B, Manning C E. 2015. Reevaluating carbon fluxes in subduction zones, what goes down, mostly comes up. Proceedings of the Natioal Academy of Sciences of the United States of America, 112(30): E3997–E4006. DOI:10.1073/pnas.1507889112 |
| [] | Kelley D S, Karson J A, Früh-Green G L, Yoerger D R, Shank T M, Butterfield D A, Hayes J M, Schrenk M O, Olson E J, Proskurowski G, Jakuba M, Bradley A, Larson B, Ludwig K, Glickson D, Buckman K, Bradley A S, Brazelton W J, Roe K, Elend M J, Delacour A, Bernasconi S M, Lilley M D, Baross J A, Summons R E, Sylva S P. 2005. A serpentinite-hosted ecosystem:The lost city hydrothermal field. Science, 307(5714): 1428–1434. DOI:10.1126/science.1102556 |
| [] | Keppler H, Wiedenbeck M, Shcheka S S. 2003. Carbon solubility in olivine and the mode of carbon storage in the Earth's mantle. Nature, 424(6947): 414–416. DOI:10.1038/nature01828 |
| [] | Kerrick D M, Caldeira K. 1998. Metamorphic CO degassing from orogenic belts. Chemical Geology, 145: 213–232. DOI:10.1016/S0009-2541(97)00144-7 |
| [] | Kerrick D M, Connolly J A D. 1998. Subduction of ophicarbonates and recycling of CO2 and H2O. Geology, 26(4): 375–378. DOI:10.1130/0091-7613(1998)026<0375:SOOARO>2.3.CO;2 |
| [] | Kerrick D M, Connolly J A D. 2001a. Metamorphic devolatilization of subducted marine sediments and the transport of volatiles into the Earth's mantle. Nature, 411(6835): 293–296. DOI:10.1038/35077056 |
| [] | Kerrick D M, Connolly J A D. 2001b. Metamorphic devolatilization of subducted oceanic metabasalts:Implications for seismicity, arc magmatism and volatile recycling. Earth and Planetary Science Letters, 189(1-2): 19–29. DOI:10.1016/S0012-821X(01)00347-8 |
| [] | Keshav S, Gudfinnsson G H. 2010. Experimentally dictated stability of carbonated oceanic crust to moderately great depths in the Earth:Results from the solidus determination in the system CaO-MgO-Al2O3-SiO2-CO2. Journal of Geophysical Research, 115(B5): B05205. |
| [] | Kiseeva E S, Yaxley G M, Hermann J, Litasov K D, Rosenthal A, Kamenetsky V S. 2012. An experimental study of carbonated eclogite at 3. 5-5.5 GPa-implications for silicate and carbonate metasomatism in the cratonic mantle. Journal of Petrology, 53(4): 727–759. |
| [] | Kiseeva E S, Litasov K D, Yaxley G M, Ohtani E, Kamenetsky V S. 2013. Melting and phase relations of carbonated eclogite at 9~21 GPa and the petrogenesis of alkali-rich melts in the deep mantle. Journal of Petrology, 54(8): 1555–1583. DOI:10.1093/petrology/egt023 |
| [] | Knoche R, Sweeney R J, Luth R W. 1999. Carbonation and decarbonation of eclogites:The role of garnet. Contributions to Mineralogy and Petrology, 135(4): 332–339. DOI:10.1007/s004100050515 |
| [] | Kolesnikov A, Kutcherov V G, Goncharov A F. 2009. Methane-derived hydrocarbons produced under upper-mantle conditions. Nature Geoscience, 2(8): 566–570. DOI:10.1038/ngeo591 |
| [] | Lai Y J, Von Strandmann P A E P, Dohmen R, Takazawa E, Elliott T. 2015. The influence of melt infiltration on the Li and Mg isotopic composition of the Horoman Peridotite Massif. Geochimica et Cosmochimica Acta, 164: 318–332. DOI:10.1016/j.gca.2015.05.006 |
| [] | Lee C T A, Luffi P, Le Roux V, Dasgupta R, Albaréde F, Leeman W P. 2010. The redox state of arc mantle using Zn/Fe systematics. Nature, 468(7324): 681–685. DOI:10.1038/nature09617 |
| [] | Lee C T A, Shen B, Slotnick B S, Liao K, Dickens G R, Yokoyama Y, Lenardic A, Dasgupta R, Jellinek M, Lackey J S, Schneider T, Tice M M. 2012. Continental arc-island arc fluctuations, growth of crustal carbonates, and long-term climate change. Geosphere, 9(1): 21–36. |
| [] | Li S G. 2015. Tracing deep carbon recycling by Mg isotopes. Earth Science Frontiers, 22(5): 143–159. |
| [] | Litasov K, Ohtani E. 2010. The solidus of carbonated eclogite in the system CaO-Al2O3-MgO-SiO2-Na2O-CO2 to 32 GPa and carbonatite liquid in the deep mantle. Earth and Planetary Science Letters, 295(1-2): 115–126. DOI:10.1016/j.epsl.2010.03.030 |
| [] | Litasov K D, Shatskiy A, Ohtani E, Yaxley G M. 2012. Solidus of alkaline carbonatite in the deep mantle. Geology, 41(1): 79–82. |
| [] | Luth R W. 1995. Experimental determination of the reaction dolomite+2coesite=diopside+2CO2 to 6 GPa. Contributions to Mineralogy and Petrology, 122(1-2): 152–158. DOI:10.1007/s004100050118 |
| [] | Luth R W. 2001. Experimental determination of the reaction aragonite+magnesite=dolomite at 5 to 9 GPa. Contributions to Mineralogy and Petrology, 141(2): 222–232. DOI:10.1007/s004100100238 |
| [] | Lü Z, Zhang L F, Du J X, Kurt B. 2009. Petrology of coesite-bearing eclogite from Habutengsu valley, western Tianshan, NW China and its tectonometamorphic implications. Jounal of Metamorphic Geology, 27: 773–787. DOI:10.1111/jmg.2009.27.issue-9 |
| [] | Lü Z, Bucher K, Zhang L F. 2013. Omphacite-bearing calcite marble and associated coesite-bearing pelitic schist from the meta-ophiolitic belt of Chinese western Tianshan. Journal of Asian Earth Sciences, 76: 37–47. DOI:10.1016/j.jseaes.2013.07.034 |
| [] | Maruoka T, Kurati G, Dobosi G, Koeberl C. 2004. Isotopic composition of carbon in diamonds of diamondites:Record of mass fractionation in the upper mantle. Geochimica et Cosmochimica Acta, 68(7): 1635–1644. DOI:10.1016/j.gca.2003.10.007 |
| [] | Massonne H J, Kopp J. 2004. A low-variance mineral assemblage with talc and phengite in an eclogite from the saxonian erzgebirge, Central Europe, and its P-T Evolution. Journal of Petrology, 46(2): 355–375. DOI:10.1093/petrology/egh079 |
| [] | Mattinson C G, Zhang R Y, Tsujimori T, Liou J G. 2004. Epidote-rich talc-kyanite-phengite eclogites, Sulu terrane, eastern China:P-T-fO2 estimates and the significance of the epidote-talc assemblage in eclogite. American Mineralogist, 89(11-12): 1772–1783. DOI:10.2138/am-2004-11-1224 |
| [] | McCammon C A. 2005. Mantle oxidation state and oxygen fugacity: Constraints on mantle chemistry, structure, and dynamics. In: Van Der Hilst R D, Bass J D, Matas J, Trampert J, eds. Earth's Deep Mantle: Structure, Composition, and Evolution. Washington, DC: American Geophysical Union, 219-240 |
| [] | McCollom T M, Seewald J S. 2007. Abiotic synthesis of organic compounds in deep-sea hydrothermal environments. Chemical Reviews, 107(2): 382–401. DOI:10.1021/cr0503660 |
| [] | McCollom T M. 2013. Laboratory simulations of abiotic hydrocarbon formation in earth's deep subsurface: Reviews in Mineralogy and Geochemistry, 75(1): 467-494 |
| [] | Milkov A V. 2005. Molecular and stable isotope compositions of natural gas hydrates:A revised global dataset and basic interpretations in the context of geological settings. Organic Geochemistry, 36(5): 681–702. DOI:10.1016/j.orggeochem.2005.01.010 |
| [] | Morlidge M, Pawley A, Droop G. 2006. Double carbonate breakdown reactions at high pressures:An experimental study in the system CaO-MgO-FeO-MnO-CO2. Contributions to Mineralogy and Petrology, 152: 365–373. DOI:10.1007/s00410-006-0112-5 |
| [] | Ono S, Kikegawa T, Ohishi Y, Tsuchiya J. 2005. Post-aragonite phase transformation in CaCO3 at 40 GPa. The American Mineralogist, 90(4): 667–671. DOI:10.2138/am.2005.1610 |
| [] | Pan C C, Yu L P, Liu J Z, Fu J M. 2006. Chemical and carbon isotopic fractionations of gaseous hydrocarbons during abiogenic oxidation. Earth and Planetary Science Letters, 246(1-2): 70–89. DOI:10.1016/j.epsl.2006.04.013 |
| [] | Panero W R, Kabbes J E. 2008. Mantle-wide sequestration of carbon in silicates and the structure of magnesite Ⅱ. Geophysical Research Letters, 35(14): L14307. DOI:10.1029/2008GL034442 |
| [] | Peacock S M, Wang K. 1999. Seismic consequences of warm versus coll subduction metamorphism:Examples from Southwest and Northeast Japan. Science, 286: 937–939. DOI:10.1126/science.286.5441.937 |
| [] | Parkinson I J, Arculus R J. 1999. The redox state of subduction zones:Insights from arc-peridotites. Chemical Geology, 160(4): 409–423. DOI:10.1016/S0009-2541(99)00110-2 |
| [] | Plank T, Langmuir C H. 1998. The chemical composition of subducting sediment and its consequences for the crust and mantle. Chemical Geology, 145(3-4): 325–394. DOI:10.1016/S0009-2541(97)00150-2 |
| [] | Poli S, Franzolin E, Fumagalli P, Crottini A. 2009. The transport of carbon and hydrogen in subducted oceanic crust:An experimental study to 5 GPa. Earth and Planetary Science Letters, 278(3-4): 350–360. DOI:10.1016/j.epsl.2008.12.022 |
| [] | Rohrbach A, Schmidt M W. 2011. Redox freezing and melting in the Earth's deep mantle resulting from carbon-iron redox coupling. Nature, 472(7342): 209–212. DOI:10.1038/nature09899 |
| [] | Rosenbauer R J, Thomas B, Bischoff J L, Palandri J. 2012. Carbon sequestration via reaction with basaltic rocks:Geochemical modeling and experimental results. Geochimica et Cosmochimica Acta, 89: 116–133. DOI:10.1016/j.gca.2012.04.042 |
| [] | Sephton M A, Hazen R M. 2013. On the origins of deep hydrocarbons. Reviews in Mineralogy and Geochemistry, 75(1): 449–465. DOI:10.2138/rmg.2013.75.14 |
| [] | Shcheka S S, Wiedenbeck M, Frost D J, Keppler H. 2006. Carbon solubility in mantle minerals. Earth and Planetary Science Letters, 245(3-4): 730–742. DOI:10.1016/j.epsl.2006.03.036 |
| [] | Slagle A L, Goldberg D S. 2011. Evaluation of ocean crustal Sites 1256 and 504 for long-term CO2 sequestration. Geophysical Research Letters, 38(16): L16307. |
| [] | Sleep N H, Zahnle K. 2001. Carbon dioxide cycling and implications for climate on ancient Earth. Journal of Geophysical Research, 106(E1): 1373–1399. DOI:10.1029/2000JE001247 |
| [] | Song S G, Su L, Niu Y L, Lai Y, Zhang L F. 2009. CH4 inclusions in orogenic harzburgite:Evidence for reduced slab fluids and implication for redox melting in mantle wedge. Geochimica et Cosmochimica Acta, 73(6): 1737–1754. DOI:10.1016/j.gca.2008.12.008 |
| [] | Stagno V, Tange Y, Miyajima N, McCammon C A, Irifune T, Frost D J. 2011. The stability of magnesite in the transition zone and the lower mantle as function of oxygen fugacity. Geophysical Research Letters, 38(19): L19309. |
| [] | Stagno V, Ojwang D O, McCammon C A, Frost D J. 2013. The oxidation state of the mantle and the extraction of carbon from Earth's interior. Nature, 493(7430): 84–88. DOI:10.1038/nature11679 |
| [] | Sverjensky D A, Stagno V, Huang F. 2014. Important role for organic carbon in subduction-zone fluids in the deep carbon cycle. Nature Geoscience, 7: 909–913. DOI:10.1038/ngeo2291 |
| [] | Tao R, Liu X, Zhang L, Liu J, Stagno V, and Fei Y. 2017. Natural formation and synthesis of abiotic hydrocarbons from carbonates at high pressure and temperature. under review. |
| [] | Tao R B, Fei Y W, Zhang L F. 2013. Experimental determination of siderite stability at high pressure American Mineralogist, 98(8-9): 1565-1572 |
| [] | Tao R B, Zhang L F, Fei Y W, Liu Q. 2014. The effect of Fe on the stability of dolomite at high pressure:Experimental study and petrological observation in eclogite from southwestern Tianshan, China. Geochimica et Cosmochimica Acta, 143: 253–267. DOI:10.1016/j.gca.2014.02.031 |
| [] | Tatsumi Y, Eggins S. 1995. Subduction Zone Magmatism.Cambridge:Blackwell ScienceTeng F Z, Wadhwa M, Helz R T. 2007. Investigation of magnesium isotope fractionation during basalt differentiation:Implications for a chondritic composition of the terrestrial mantle. Earth and Planetary Science Letters, 261(1-2): 84–92. |
| [] | Teng F Z, Li W Y, Ke S, Marty B, Dauphas N, Huang S C, Wu F Y, Pourmand A. 2010. Magnesium isotopic composition of the Earth and chondrites. Geochimica Et Cosmochimica Acta, 74(14): 4150–4166. DOI:10.1016/j.gca.2010.04.019 |
| [] | Thomsen T B, Schmidt M W. 2008a. The biotite to phengite reaction and mica-dominated melting in fluid+carbonate-saturated pelites at high pressures. Journal of Petrology, 49(10): 1889–1914. DOI:10.1093/petrology/egn051 |
| [] | Thomsen T B, Schmidt M W. 2008b. Melting of carbonated pelites at 25-5.0 GPa, silicate-carbonatite liquid immiscibility, and potassium-carbon metasomatism of the mantle. Earth and Planetary Science Letters, 267(1-2): 17–31. DOI:10.1016/j.epsl.2007.11.027 |
| [] | Thomson A R, Walter M J, Kohn S C, Brooker R A. 2016. Slab melting as a barrier to deep carbon subduction. Nature, 529: 76–83. DOI:10.1038/nature16174 |
| [] | Tsuno K, Dasgupta R. 2011. Melting phase relation of nominally anhydrous, carbonated pelitic-eclogite at 25-3.0 GPa and deep cycling of sedimentary carbon. Contributions to Mineralogy and Petrology, 161(5): 743–763. DOI:10.1007/s00410-010-0560-9 |
| [] | Tsuno K, Dasgupta R, Danielson L, Righter K. 2012. Flux of carbonate melt from deeply subducted pelitic sediments:Geophysical and geochemical implications for the source of Central American volcanic arc. Geophysical Research Letters, 39(16): L16307. |
| [] | Tumiati S, Fumagalli P, Tiraboschi C, Poli S. 2013. An experimental study on COH-bearing peridotite up to 32 GPa and implications for crust-mantle recycling. Journal of Petrology, 54(3): 453–479. DOI:10.1093/petrology/egs074 |
| [] | Wang A L, Pasteris J D, Meyer H O A, Dele-Duboi M L. 1996. Magnesite-bearing inclusion assemblage in natural diamond. Earth and Planetary Science Letters, 141(1-4): 293–306. DOI:10.1016/0012-821X(96)00053-2 |
| [] | Wang S J, Teng F Z, Li S G, Hong J A. 2014a. Magnesium isotopic systematics of mafic rocks during continental subduction. Geochimica et Cosmochimica Acta, 143: 34–48. DOI:10.1016/j.gca.2014.03.029 |
| [] | Wang S J, Teng F Z, Li S G. 2014b. Tracing carbonate-silicate interaction during subduction using magnesium and oxygen isotopes. Nature communications, 5: 5328. DOI:10.1038/ncomms6328 |
| [] | Wood B J, Virgo D. 1989. Permian mantle oxidation state:Ferric iron contents of Iherzolite spinels by 57 Fe Mössbauer spectroscopy and resultant oxygen fugacities. Geochimica et Cosmochimica Acta, 53(6): 1277–1291. DOI:10.1016/0016-7037(89)90062-8 |
| [] | Wood B J, Li J, Shahar A. 2013. Carbon in the core:Its influence on the properties of core and mantle. Reviews in Mineralogy and Geochemistry, 75(1): 231–250. DOI:10.2138/rmg.2013.75.8 |
| [] | Wyllie P J. 1965. Melting relationships in the system CaO-MgO-CO2-H2O, with petrological applications. Journal of Petrology, 6(1): 101–123. DOI:10.1093/petrology/6.1.101 |
| [] | Xu C, Kynicky′ J, Tao R, Liu X, Zhang L, Pohanka M, Song W, Fei Y. 2017. Recovery of an oxidized majorite from Earth's deep asthenosphere: A window into the oxidation state and convection of the mantle: Science Advance, in Press |
| [] | Yang J J, Godard G, Kienast J R, Lu Y Z, Sun J X. 1993. Ultrahigh-pressure (60 Kbar) magnesite-bearing garnet peridotites from Northeastern Jiangsu, China. Journal of Geology, 101(5): 541–554. DOI:10.1086/648248 |
| [] | Yang W, Teng F Z, Zhang H F, Li S G. 2012. Magnesium isotopic systematics of continental basalts from the North China craton:Implications for tracing subducted carbonate in the mantle. Chemical Geology, 328: 185–194. DOI:10.1016/j.chemgeo.2012.05.018 |
| [] | Yaxley G M, Brey G P. 2004. Phase relations of carbonate-bearing eclogite assemblages from 25 to 5.5 GPa:Implications for petrogenesis of carbonatites. Contributions to Mineralogy and Petrology, 146(5): 606–619. DOI:10.1007/s00410-003-0517-3 |
| [] | Zhang L F, Ellis D J, Williams S, Jiang W B. 2002. Ultra-high pressure metamorphism in western Tianshan, China Part Ⅱ evidence from magnesite in eclogite. American Mineralogist, 87(7): 861–866. DOI:10.2138/am-2002-0708 |
| [] | Zhang R Y, Liou J G. 1994. Significance of magnesite paragenesis in ultrahigh-pressure metamorphic rocks. American Mineralogist, 79(3-4): 397–400. |
| [] | Zhang R Y, Liou J G. 1996. Coesite inclusions in dolomite from eclogite in the southern Dabie Mountains, China; the significance of carbonate minerals in UHPM rocks. American Mineralogist, 81(1-2): 181–186. DOI:10.2138/am-1996-1-222 |
| [] | Zhu Y F, Ogasawara Y. 2002. Carbon recycled into deep earth:Evidence from dolomite dissociation in subduction-zone rocks. Geology, 30(10): 947–950. DOI:10.1130/0091-7613(2002)030<0947:CRIDEE>2.0.CO;2 |
| [] | 张洪铭, 李曙光. 2012. 深部碳循环及同位素示踪:回顾与展望. 中国科学 (D辑), 42(10): 1459–1472. |
 2017, Vol. 36
2017, Vol. 36

