2. 山东农业大学作物生物学国家重点实验室, 山东泰安 271018;
3. 山东农业大学园艺作物生物学农业部重点开放实验室, 山东泰安 271018
2. State Key Laboratory of Crop Biology, Tai'an 271018, China;
3. Horticultural Crop Biology, Key Laboratory of the Ministry of Agriculture, Tai'an 271018, China
穴盘育苗是蔬菜生产的重要环节,基质种类、配比、有机质含量及腐植酸含量直接影响育成苗的质量。腐植酸(humic acids简写为HA)是一类成分复杂的天然有机物质[1-2],主要存在于褐煤、风化煤、泥炭中,其含量丰富[3],易提取,且成本低。许多研究报道来源不同的腐植酸及其复合肥可以促进不同种类、不同培养环境下植物的生长发育[4-10]。有研究提出,腐植酸可通过改善土壤某些营养物质的吸收利用从而显著促进植株生长[11-12];也有研究认为,腐植酸可以促进植物光合、呼吸作用、提高抗逆性,直接影响植物代谢[13-15]。目前,关于基质中添加腐植酸的研究鲜有报道,其适宜用量及效果也还缺乏数据。本试验在蔬菜育苗基质中添加不同量的腐植酸,研究基质中腐植酸含量对番茄穴盘苗的硝酸还原酶活性及光合参数的影响,以期深入理解腐植酸促进番茄穴盘苗生长的生理基础,从而为腐植酸在穴盘育苗上的应用提供科学依据。
1 材料与方法 1.1 试验材料本试验于2015年4月~5月在山东农业大学南校区园艺试验站进行。供试番茄(Lycopersicon esculentum Mill.)品种‘红粉冠军’由郑州郑研种苗科技有限公司选育。供试基质为泰安生产的‘春风’蔬菜专用育苗基质,草炭、蛭石、珍珠岩体积比4:3:2,有机质≥45%,pH值5.8~6.5,EC值1.0~1.5 mS/cm。经实测基质容重为0.46 g/cm3,pH值为6.16,EC值1.37 mS/cm,孔隙度为82%,腐植酸含量为54.5 g/L(约11.85 %)。供试腐植酸为山东创新腐植酸科技有限公司生产的‘创新腐植酸’,纯度60%,黑色粉末状,水分含量6.59 %,CEC为3.99 meq/g,总酸性基4.61 meq/g,羧基1.75 meq/g,酚羟基2.88 meq/g。育苗穴盘为50孔塑料盘(53 cm × 28 cm × 5.5 cm),每孔容积55 cm3。
1.2 试验设计在预备试验基础上,基质中折合纯腐植酸量添加设5个处理,分别为:0、10、20、30、40 g/L(依次记为CK、T1、T2、T3、T4),每处理3次重复,每个重复3个穴盘。腐植酸和基质充分混匀,平衡2 h后装盘。番茄种子浸种催芽后播种,待植株5叶1心时(子叶展开后29~31天)进行生长与生理指标测定。试验期间环境条件:4月~5月昼/夜平均气温为18.4~32.3℃和14.2~22.2℃,昼/夜平均空气湿度为33%~93%和61%~95.5%,昼间平均光强为2795~43852 lux,累积光强为67070~1052447 lux。
1.3 测定项目及方法 1.3.1 形态指标用直尺测量株高(基质表面到生长点之间的距离);用游标卡尺测量幼苗茎粗(子叶节下1 cm处);在茎基部将番茄幼苗地上部和根系分开,纯净水洗净,吸水纸拭干水分,分别做好标记置于烘箱内105℃杀青15 min,75℃烘干至恒重,称其干重;根冠比=根干重/地上部干重;壮苗指数=(茎粗/株高+根干重/地上部干重)×全株干重(g)。
1.3.2 根系活力及叶片硝酸还原酶(NR)活性根系活力用TTC还原法测定[19]。叶片硝酸还原酶活性用活体法测定[20]。
1.3.3 叶片光合色素含量及光合参数的测定叶绿素、类胡萝卜素含量用无水乙醇:丙酮=1:1提取法测定。用Ciras-2型便携式光合作用测定系统(英国PP-Systems公司生产)于晴天上午9:00~11:00,测定见光一致的第2、3片展开的功能叶的净光合速率(Pn)、气孔导度(Gs)、蒸腾速率(Tr)、细胞间隙CO2浓度(Ci)。测定条件:光强800 μmol/(m2·s)、温度28℃、空气中CO2浓度380 μmol/mol。
1.3.4 叶绿素荧光参数的测定选取受光条件一致的第2、3片展开功能叶,暗适应30 min,用FMS2型调制式荧光仪(英国Hansatech生产)测定相应光强下的荧光参数。
1.4 数据处理采用Microsoft Excel 2010软件进行数据处理和作图,采用DPS 7.55软件进行统计分析,采用Duncan法进行差异显著性检验(P<0.05)。
2 结果与分析 2.1 腐植酸添加量对番茄幼苗生长的影响由表 1可以看出,当处理番茄幼苗长到5叶1心时,其株高、茎粗、地上部干重和根干重值都以T2处理最高,与其他处理差异显著。T1和T2处理幼苗株高分别比CK高出9.5%和14.3%,处理间差异显著(P<0.05);T3处理与CK差异不显著;T4处理比CK差。幼苗的茎粗以T2处理最大,T4处理最小,处理间差异显著。幼苗地上部干重T2处理的显著高于CK及其他处理,T4处理显著低于CK,T1、T3处理与CK差异不显著。根干重T1、T2处理的显著高于CK和T3,T3处理与CK无显著差异,但T4处理显著低于CK。
| 表1 不同腐植酸添加量番茄幼苗生长状况 Table 1 Growth of tomato seedlings affected by humic acid addition |
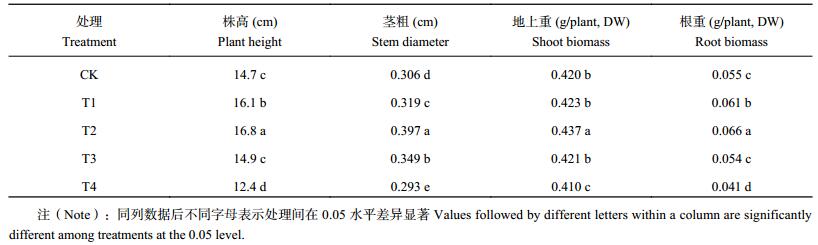 |
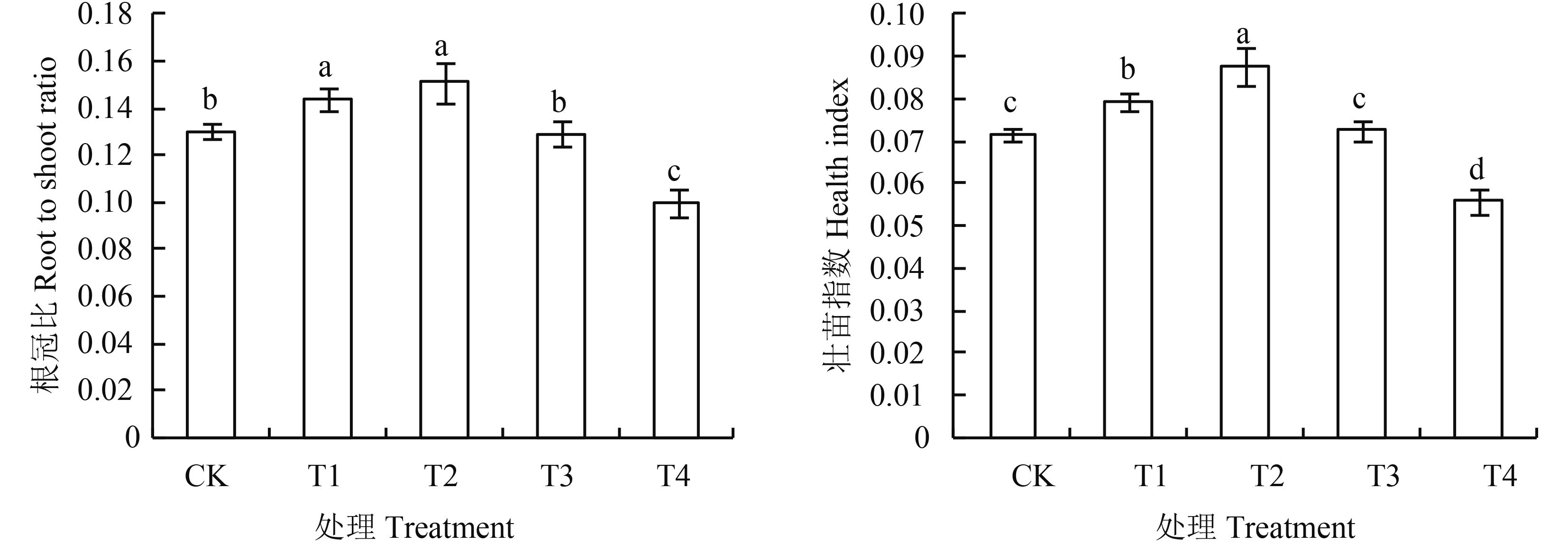
由图 1可知,随基质腐植酸添加量的增加,根冠比和壮苗指数呈现出先升高后降低的趋势。T1、T2处理根冠比显著高于CK,T3处理与CK差异不显著,T4处理则显著低于CK;通过计算壮苗指数大小依次为T2 > T1 > T3 > CK > T4,其中最大值为T2处理(0.088),比CK高22%,其次是T1,比CK高10.3%,均与CK差异显著;最小值T4处理(0.056)较CK低22.6%,差异显著;T3与CK差异不显著(P<0.05)。
 |
|
图1
腐植酸添加量对番茄幼苗根冠比和壮苗指数的影响
Fig. 1
Effects of humic acid addition on root to shoot ratio and health index of tomato seedlings
[注(Note):方柱上不同字母表示处理间在0.05水平差异显著 Different letters above the bars are significantly different among treatments at the 0.05 level.] |
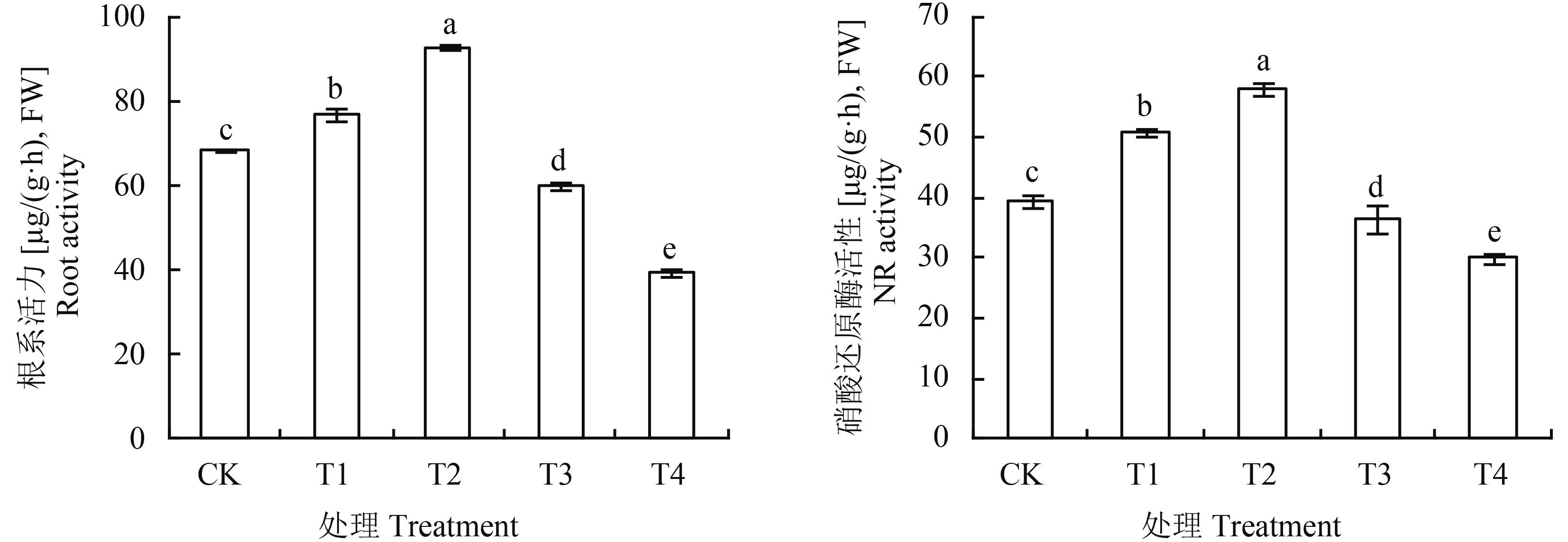
由图 2可知,基质中腐植酸添加量对番茄幼苗根系活力有明显的影响,T1、T2处理根系活力显著高于CK,分别提高了12.0%、35.5%,以T2处理的活力最强;腐植酸添加量再增加的T3、T4处理番茄幼苗根系活力呈下降趋势,显著低于CK。腐植酸添加处理对番茄叶片NR活性的影响与根系活力变化趋势相似,随着腐植酸添加量的增加,NR活性呈先升高后下降的趋势,4个处理中T2处理NR活性最高,较CK高29.1%,T4处理最低,较CK低23.8%,各处理间差异显著。
 |
|
图2
腐植酸添加量对番茄根系活力和叶片硝酸还原酶活性(NR)的影响
Fig. 2
Effects of humic acid addition on root activity and NR activity of tomato leaves
[注(Note):方柱上不同字母表示处理间在0.05水平差异显著 Different letters above the bars are significantly different among treatments at the 0.05 level.] |
由表 2可以看出,适当增加基质中腐植酸含量,可提高番茄叶片光合色素含量,但腐植酸含量过高,则会降低光合色素含量。番茄幼苗叶片叶绿素a、b,叶绿素总量和类胡萝卜素含量均以T2处理最高,与CK差异显著;处理T1和T3的幼苗叶片叶绿素a、b,叶绿素总量和类胡萝卜素含量虽低于T2处理,但也均高于CK,差异显著;而T4处理的则显著低于CK。叶绿素a/叶绿素b值是CK、T3、T4处理较大,但3个处理间差异不显著;T1次之,T2最低,与其它3个处理差异显著。处理T1、T2叶绿素a/叶绿素b值较低是由于增施腐植酸后(与CK相比)叶绿素b含量增加幅度(19.2%和37.5%)大于叶绿素a增加幅度(1.1%和9.1%)所致。
| 表2 腐植酸添加量对番茄幼苗叶片光合色素含量的影响 Table 2 Effects of humic acid addition on the photosynthetic pigments of tomato seedlings leaves |
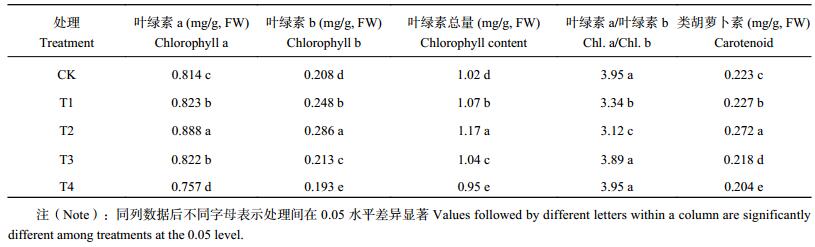 |
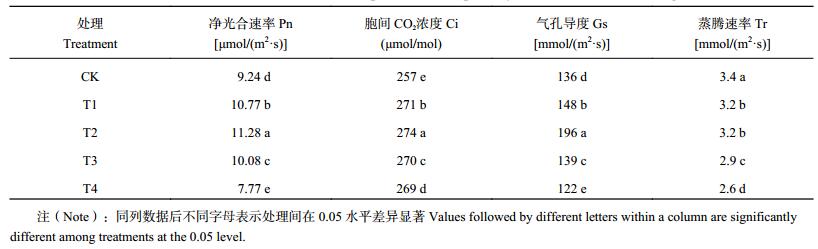
由表 3可知,随腐植酸添加量的增加,番茄叶片Pn先升高后降低,T1、T2、T3处理分别比CK高16.6%、22.1%、9.1%,T4处理比CK低15.9%,且差异显著;番茄叶片Gs的变化趋势与Pn一致,处理间差异显著。胞间CO2浓度(Ci)变化T1、T2、T3处理的趋势与Pn一致,而T4处理高于CK。蒸腾速率(Tr)表现为CK最高,随腐植酸添加量的增加,逐渐降低。
| 表3 腐植酸添加量对番茄幼苗叶片光合参数的影响 Table 3 Effects of humic acid addition on parameters of photosynthetic of tomato seedlings leaves |
 |
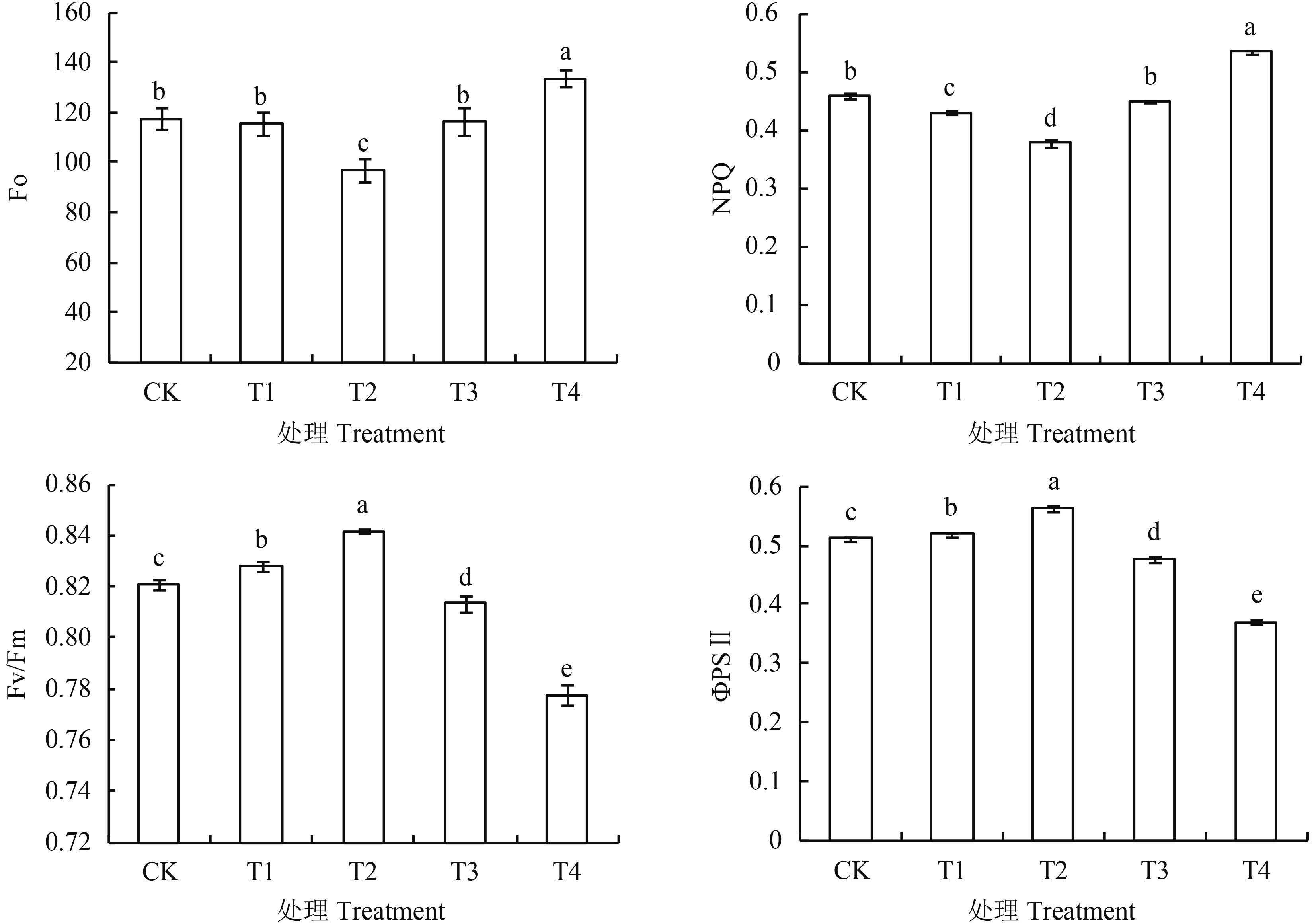
从图 3可以看出,处理番茄幼苗的Fo值,T2处理最小,T4处理最大,均与CK差异显著;其他2个处理与CK差异不显著。NPQ值与Fo值有相似的变化趋势,随基质中腐植酸添加量的增加呈先降低后升高趋势,T2处理显著低于其他处理,较CK低17.6%,T4处理NPQ值最大,较CK高16.6%。各处理Fv/Fm值和ΦPSⅡ值变化趋势相同,大小顺序依次为T2 > T1 > CK > T3 > T4,其中T1、T2处理显著高于CK,分别提高0.9%、2.5% (Fv/Fm)和1.5%、10.1% (ΦPSⅡ);而T3、T4处理Fv/Fm值和ΦPSⅡ值显著低于CK,比CK分别降低0.9%、5.3% (Fv/Fm)和6.6%、27.5% (ΦPSⅡ)。
 |
|
图3
腐植酸添加量对番茄幼苗Fo、NPQ、Fv/Fm和ФPSⅡ的影响
Fig. 3
Effects of humic acid addition on Fo, NPQ, Fv/Fm and ФPSⅡ of tomato seedlings
[注(Note):方柱上不同字母表示处理间在0.05水平差异显著 Different letters above the bars are significantly different among treatments at the 0.05 level.] |
示踪法证明,腐植酸能被植物根系吸收,吸附在细胞壁的腐植酸可提高质膜上的质子泵(H+-ATPase)数量,促进H+向细胞外输出,使细胞壁酸化,多糖水解,细胞壁软化松弛,细胞易于伸长、分裂,从而促进植物生长[21]。植株的生长是反映幼苗同化产物的累积量及其健壮程度最直观的指标,本研究结果表明,基质中适量添加腐植酸可显著提高番茄穴盘苗株高、茎粗,有利于干物质的积累,明显提高壮苗指数;此外,与CK相比,适宜腐植酸添加幼苗的根干重增加幅度(11.5%~20.7%)高于地上部干重(0.2%~4.1%),且有较高的根冠比、根系活力,这表明与地上部相比,光合产物分配更利于根系,腐植酸刺激根系生长,提高根系活力,从而保证地上部的生长,有利于培育壮苗,王汝娟等[22]的研究也表明施用腐植酸钾显著提高了甘薯R/T (某一时期块根鲜重/对应的地上部鲜重),说明腐植酸钾有利于甘薯块根膨大前、中期光合产物向块根运输,促进块根膨大。但基质中腐植酸含量过高也会影响番茄幼苗生长,这与靳志丽等[23]和Fan等[24]在烟草和菊花上的研究结果一致。
硝酸还原酶(NR)是植物体内氮代谢的限速酶,其活性高低影响植物氮吸收,进而影响植物体营养生长;此外其活性可反映植物新陈代谢水平,对叶绿素的合成,光合作用有着重要影响[25-26]。本试验结果表明,随基质中腐植酸添加量的增加,番茄叶片NR活性呈先升高后降低的趋势,说明适量的腐植酸有利于提高硝酸还原酶活性,从而促进植物体内的氮素同化,使番茄穴盘苗生长更健壮。
植物的生长发育离不开光合作用,叶绿素和类胡萝卜素是叶片光合作用的主要色素,是植物吸收、传递和转换光能的基础[27]。有研究表明,腐植酸可显著提高辣椒[28]、小麦[29]、玉米[30]叶片的叶绿素总含量;孙志梅等[31]研究指出,与对照相比,HA系列处理使辣椒叶片叶绿素a的提高幅度在100%左右,而对叶绿素b的影响,提高幅度在60%~80%,腐植酸对叶绿素a的影响大于叶绿素b,赵励军[32]的研究也得到类似结论;Karakurt等[33]研究表明腐植酸降低黄瓜叶片叶绿素b含量,提高甜椒叶片中叶绿素b含量;但几乎所有的研究表明,适量腐植酸可显著提高作物的光合速率。本试验结果表明,适量的腐植酸可显著增加番茄叶片叶绿素和类胡萝卜素的含量,提高光合速率,且适量的腐植酸对叶绿素b的提高幅度大于叶绿素a,表明腐植酸对叶绿素b的影响大于叶绿素a;而腐植酸含量过高,则叶片色素含量下降,光合速率也随之降低(表 2、表 3)。腐植酸有助于提高番茄叶片的叶绿素含量,可能的原因是腐植酸与Mg、Fe、Zn等金属离子形成络合物或螯合物,提高元素的有效性,激活了叶绿素形成过程中的某些酶;此外番茄叶片较高的NR活性(图 2),有利于植物氮代谢,也有助于叶绿素的合成。腐植酸对叶绿素a、b含量的影响,不同的研究结果存在差异,可能与试验中植物种类、培养条件不同有关,亦或是与腐植酸本身的特性(腐植酸来源、腐植酸不同组分等)有关。关于腐植酸影响光合作用主要细胞器-叶绿体的研究还有待进一步深入。腐植酸使番茄光合作用提高可能是基质中适量添加腐植酸使叶片叶绿素含量提高,且对叶绿素b含量的影响大于叶绿素a (表 2),因此更利于LHCⅡ复合体吸收、传递光能,维持类囊体膜结构完整,有助于调节激发能在光系统间的分配[34];其次,番茄叶片较高的类胡萝卜素含量(表 2)可增强光合电子传递量子效率,以及叶黄素循环对光合机构的保护作用,提高了植物的环境适应性[35];第三,腐植酸促进番茄叶片气孔开放,增加了胞间CO2浓度(Ci)(表 3)。本试验结果还表明,腐植酸添加处理降低了番茄叶片的蒸腾速率,且随着基质中腐植酸添加量的增加,降幅增大(表 3),这与前人在玉米[1]、葡萄[36]等作物上的研究结果相似;本研究中,适量腐植酸添加的番茄叶片气孔导度(Gs)显著升高,可见叶片不是通过促进气孔关闭来降低蒸腾作用的,基质中添加腐植酸降低植物蒸腾的相关机理有待进一步研究。
在测定叶片光合作用的过程中,气体交换参数反映了其“表观性”,而叶绿素荧光参数则反映了其“内在性”的特点[37]。Fo、NPQ、Fv/Fm、ФPSⅡ是主要的叶绿素荧光参数,Fo是PSⅡ反应中心(经过充分暗适应以后)处于完全开放状态时的初始荧光产量[38],这部分荧光是天线中的激发能尚未被反应中心捕获之前,由天线叶绿素发出的,它与叶片叶绿素浓度有关;非光化学猝灭(NPQ)反映的是PSⅡ天线色素吸收的光能不能用于光合电子传递而以热的形式耗散掉的光能部分;Fv/Fm是暗适应下PSⅡ反应中心完全开放时的最大光化学效率,反映PSⅡ反应中心最大光能转换效率;ФPSⅡ则是照光下PSⅡ反应中心部分关闭时的实际光化学效率,反映光合电子传递速率[39]。本研究中,基质中腐植酸适量添加时,Fo和NPQ明显降低,Fv/Fm和ФPSⅡ明显增加(图 3),表明叶片光合机构健康,功能良好,因而具有较高的开放程度和激发能捕获效率,荧光和散热减小,进入反应中心进行电荷分离转化的激发能增加(Pn增加),从而促进植物同化力(NADPH、ATP)的形成,影响植物对碳的固定和同化,这种效应以T2处理表现极为明显;腐植酸用量进一步增加时,Fo上升,NPQ高于其他处理,所以导致了ФPSⅡ的降低,叶片吸收的光能不能用于光合电子传递而以热的形式耗散掉的光能多,叶黄素循环耗散变强,从而避免QA的过分还原,也说明PSⅡ的原初光化学反应通过下调光合电子传递来匹配碳代谢对ATP和NADPH的需求减少,这可能是T4处理植株干重较小的原因之一(表 1)。
本试验在供试基质(腐植酸含量11.85%)中添加适量腐植酸(T2添加4.35%),能明显提高番茄幼苗根系活力,叶片NR活性,增加光合色素含量,改善苗期的光合作用,从而使培育的番茄苗表现为株高、茎粗、干重均有较大增加,根冠比和壮苗指数提高,有利于培育壮苗。晋艳等[40]对全国104份商品基质检测得出其腐植酸含量在3.1%~29.7%,并用其中一种基质(腐植酸含量12.8%)添加腐植酸进行烟草漂浮育苗,结果也表明适量添加腐植酸时,烟苗生长较好,但腐植酸含量过高时烟苗生长受到明显抑制,并提出基质中腐植酸含量在20%左右时效果最好,本试验中番茄幼苗生长最好的T2处理基质腐植酸总含量16.2%与其接近。育苗基质成分组成、作物种类、育苗方法或添加腐植酸的来源不同,都可能会导致育苗效果的差异,因此,在大批量育苗选用基质的种类后,应根据作物种类和腐植酸来源事先做好腐植酸添加试验。
目前泥炭(草炭)是应用最多的育苗基质,其中富含腐植酸,但属于不可再生资源。随着科学技术的进步,逐渐可以利用秸秆、木屑等有机废弃物生产生化腐植酸[41],可作为生产使用;或利用蚯蚓粪中存在大量腐殖质的特性,构建种植养殖循环链,生产蚯蚓粪基质[42],以及从污水污泥中提取腐植酸,用于生产腐植酸液肥[43],不仅可以节约泥炭等不可再生资源,而且可以实现腐植酸的可再生循环利用。此外,腐植酸液体肥料因其具有植物生长调节、营养全面、抗旱节水,环境友好等多效[44],必将有利于蔬菜集约化育苗生产的发展,前景广阔。
4 结论番茄穴盘育苗基质中添加适量的腐植酸显著提高番茄幼苗叶片NR活性,增加光合色素含量,提高光能转化效率,改善苗期的光合作用;促进了番茄幼苗根系发育、提高了根冠比和壮苗指数,幼苗株高、茎粗、干重均有较大增加。本试验条件下,在基质中原含有54.5 g/L的基础上,以添加腐植酸量20 g/L的处理表现较佳,促进番茄穴盘苗生长,培育壮苗效果更好。
| [1] | Asli S, Neumann P M. Rhizosphere humic acid interacts with root cell walls to reduce hydraulic conductivity and plant development[J]. Plant and Soil, 2010, 336: 313–322. DOI:10.1007/s11104-010-0483-2 |
| [2] | Schiavon M, Pizzeghello D, Francioso O, et al. High molecular size humic substances enhance phylpropanoid metabolism in maize (Zea mays L.)[J]. Journal of Chemical Ecology, 2010, 36(6): 662–669. DOI:10.1007/s10886-010-9790-6 |
| [3] | Simpson A J, Kingery W L, Hayes M H, et al. Molecular structures and associations of humic substances in the terrestrial environment[J]. Naturwissenschaften, 2002, 89(2): 84–88. DOI:10.1007/s00114-001-0293-8 |
| [4] | Canellas L P, Dantas D J, Aguiar N O, et al. Probing the hormonal activity of fractionated molecular humic components in tomato auxin mutants[J]. Annals of Applied Biology, 2011, 159(2): 202–211. DOI:10.1111/aab.2011.159.issue-2 |
| [5] |
梁太波, 王振林, 史春余, 等. 腐植酸尿素对生姜产量及氮素吸收、同化和品质的影响[J].
植物营养与肥料学报, 2007, 13(5): 903–909.
Liang T B, Wang Z L, Shi C Y, et al. Effects of humic acid urea on yield and nitrogen absorption, assimilation and quality of ginger[J]. Plant Nutrition and Fertilizer Science, 2007, 13(5): 903–909. |
| [6] | Dobbss L B, Canellas L P, Olivares F L, et al. Bioactivity of chemically transformed humic matter from vermicompost on plant root growth[J]. Journal of Agricultural and Food Chemistry, 2010, 58(6): 3681–3688. DOI:10.1021/jf904385c |
| [7] | Tahir M M, Khurshid M, Khan M Z, et al. Lignite-derived humic acid effect on growth of wheat plants in different soils[J]. Pedosphere, 2011, 21(1): 124–131. DOI:10.1016/S1002-0160(10)60087-2 |
| [8] |
王振振, 张超, 史衍玺, 等. 腐植酸缓释钾肥对土壤钾素含量和甘薯吸收利用的影响[J].
植物营养与肥料学报, 2012, 18(1): 249–255.
Wang Z Z, Zhang C, Shi Y X, et al. Effects of Ha-K fertilizer on potassium content of soil and absorption and utilization of potassium in sweet potato[J]. Plant Nutrition and Fertilizer Science, 2012, 18(1): 249–255. |
| [9] | Mora V, Baigorri R, Bacaicoa E, et al. The humic acid-induced changes in the root concentration of nitric oxide, IAA and ethylene do not explain the changes in root architecture caused by humic acid in cucumber[J]. Environmental and Experimental Botany, 2012, 76: 24–32. DOI:10.1016/j.envexpbot.2011.10.001 |
| [10] |
梁太波, 王振林, 王汝娟, 等. 腐植酸钾对生姜根系生长发育及活性氧代谢的影响[J].
应用生态学报, 2007, 18(4): 813–817.
Liang T B, Wang Z L, Wang R J, et al. Effects of potassium humate on ginger root growth and its active oxygen metabolism[J]. Chinese Journal of Applied Ecology, 2007, 18(4): 813–817. |
| [11] | Feller C, Brossard M, Chen Y, et al. Selected pioneering works on humus in soils and sediments during the 20th century:a retrospective look from the international humic substances society view[J]. Physics and Chemistry of the Earth, 2010, 35(15): 903–912. |
| [12] | Chen Y, Clapp C E, Magen H. Mechanisms of plant growth stimulation by humic substances:The role of organo-iron complexes[J]. Soil Science and Plant Nutrition, 2004, 50(7): 1089–1095. DOI:10.1080/00380768.2004.10408579 |
| [13] | Heil C A. Influence of humic, fulvic and hydrophilic acids on the growth, photosynthesis and respiration of the dinoflagellate Prorocentrum minimum (Pavillard) Schiller[J]. Harmful Algae, 2005, 4(3): 603–618. DOI:10.1016/j.hal.2004.08.010 |
| [14] |
郭伟, 王庆祥. 腐植酸浸种对盐碱胁迫下小麦幼苗抗氧化系统的影响[J].
应用生态学报, 2011, 22(10): 2539–2545.
Guo W, Wang Q X. Effects of seed soaking with humic acid on wheat seedlings antioxidant system under salt-alkali stress[J]. Chinese Journal of Applied Ecology, 2011, 22(10): 2539–2545. |
| [15] | Nardi S, Pizzeghello D, Muscolo A, Vianello A. Physiological effects of humic substances on higher plants[J]. Soil Biology and Biochemistry, 2002, 34(11): 1527–1536. DOI:10.1016/S0038-0717(02)00174-8 |
| [16] | Morales-corts M R, Gómez-sánchez M Á, Pérez-sánchez R. Evaluation of green/pruning wastes compost and vermicompost, slumgum compost and their mixes as growing media for horticultural production[J]. Scientia Horticulturae, 2014, 172: 155–160. DOI:10.1016/j.scienta.2014.03.048 |
| [17] | Jayasinghe G Y, Arachchi I L, Yoshihiro T. Evaluation of containerized substrates developed from cattle manure compost and synthetic aggregates for ornamental plant production as a peat alternative[J]. Resources, Conservation and Recycling, 2010, 12(54): 1412–1418. |
| [18] |
游莹卓, 辛国凤, 魏珉, 等. 黄瓜免营养液无土育苗基质组配研究[J].
中国土壤与肥料, 2015(3): 95–99.
You Y Z, Xin G F, Wei M, et al. Study on the composition of soilless media with free nutrient solution for cucumber seedling[J]. Soil and Fertilizer Sciences in China, 2015(3): 95–99. |
| [19] |
赵世杰, 史国安, 董新纯.
植物生理学实验指导
[M]. 北京: 中国农业科学技术出版社, 2002.
Zhao S J, Shi G A, Dong X C. Techniques of plant physiological experiment [M]. Beijing: Chinese Agricultural Science and Technology Press, 2002. |
| [20] |
李合生.
植物生理生化原理和技术
[M]. 北京: 高等教育出版社, 2000.
Li H S. Principles and techniques of plant physiological and biochemical experiment [M]. Beijing: Higher Education Press, 2000. |
| [21] | Hager A, Debus G, Edel H G, et al. Auxin induces exocytosis and the rapid synthesis of a high turnover pool of plasma-membrane H+-ATPase[J]. Planta, 1991, 185(4): 527–537. |
| [22] |
王汝娟, 王振林, 梁太波, 等. 腐植酸钾对食用甘薯品种钾吸收、利用和块根产量的影响[J].
植物营养与肥料学报, 2008, 14(3): 520–526.
Wang R J, Wang Z L, Liang T B, et al. Effects of HA-K fertilizers on the absorption and utilization of potassium and the storage root yield in sweet potato for table use[J]. Plant Nutrition and Fertilizer Science, 2008, 14(3): 520–526. |
| [23] |
靳志丽, 刘国顺, 聂新柏. 腐殖酸对土壤环境和烤烟矿质吸收影响的研究[J].
中国烟草科学, 2002(3): 15–18.
Jin Z L, Liu G S, Nie X B. The study on effect of humic acid on soil environment and absorbing ability to mineral material of flue-cured tobacco[J]. Chinese Tobacco Science, 2002(3): 15–18. |
| [24] | Fan H M, Wang X W, Sun X, et al. Effects of humic acid derived from sediments on growth, photosynthesis and chloroplast ultrastructure in chrysanthemum[J]. Scientia Horticulturae, 2014, 177: 118–123. DOI:10.1016/j.scienta.2014.05.010 |
| [25] |
张生杰, 黄元炯, 任庆成. 氮素对不同品种烤烟叶片衰老、光合特性及产量和品质的影响[J].
应用生态学报, 2010, 21(3): 668–674.
Zhang S J, Huang Y J, Ren Q C. Effects of nitrogen fertilization on leaf senescence, photosynthetic characteristics, yield and quality of different flue-cured tobacco varieties[J]. Chinese Journal of Applied Ecology, 2010, 21(3): 668–674. |
| [26] | Barber M J, Desai S K, Marohnic C C, et al. Synthesis and bacterial expression of a gene encoding the heme domain of assimilatory nitrate reductance[J]. Archives of Biochemistry and Biophysics, 2002, 402(1): 38–50. DOI:10.1016/S0003-9861(02)00035-8 |
| [27] | Ge Y, Wang T, Wang N, et al. Genetic mapping and localization of quantitative trait loci for chlorophyll content in Chinese cabbage (Brassica rapa ssp. pekinensis)[J]. Scientia Horticulturae, 2012, 147(12): 42–48. |
| [28] |
孙志梅, 薛世川, 梁文举, 等. 不同用量腐植酸复合肥在辣椒上的施用效应及其防衰增产机理研究[J].
应用生态学报, 2004, 15(1): 81–84.
Sun Z M, Xue S C, Liang W J, et al. Effects of different application rates of humic acid compound fertilizer on pepper and its mechanism of antisenility and incremental yield[J]. Chinese Journal of Applied Ecology, 2004, 15(1): 81–84. |
| [29] |
梁太波, 王振林, 刘娟, 等. 灌溉和旱作条件下腐植酸复合肥对小麦生理特性及产量的影响[J].
中国生态农业学报, 2009, 17(5): 900–904.
Liang T B, Wang Z L, Liu J, et al. Effect of humate compound fertilizer on physiological characteristics and yield of wheat under irrigated and rain-fed conditions[J]. Chinese Journal of Eco-Agriculture, 2009, 17(5): 900–904. DOI:10.3724/SP.J.1011.2009.00900 |
| [30] | Anjum S A, Wang L, Farooq M, et al. Fulvic acid application improves the maize performance under well-watered and drought conditions[J]. Journal of Agronomy and Crop Science, 2011, 197(6): 409–417. DOI:10.1111/jac.2011.197.issue-6 |
| [31] |
孙志梅, 薛世川, 刘淑萍. 不同组分的腐植酸复合肥在辣椒上的施用效应及其生理机制研究[J].
土壤通报, 2003, 34(5): 440–443.
Sun Z M, Xue S C, Liu S P. Effeets of different compositions of humic acid compound fertilizer on the growth of pepper and physiological mechanism[J]. Chinese Journal of Soil Science, 2003, 34(5): 440–443. |
| [32] |
赵励军.不同来源腐植酸促进植物生长活性及作用机理研究[D].哈尔滨:哈尔滨理工大学硕士学位论文, 2005.
Zhao L J. A research on the humic acid of different sources accelerates the growth activity of plants and its mechanism of action[D]. Harbin: MS Thesis, Harbin University of Science and Technology, 2005. http://cdmd.cnki.com.cn/Article/CDMD-11914-2005150885.htm |
| [33] | Karakurt Y, Unlu H, Unlu H, Padem H. The influence of foliar and soil fertilization of humic acid on yield and quality of pepper[J]. Acta Agriculturae Scandinavica, 2009, 59(3): 233–237. |
| [34] |
曹逼力, 徐坤, 石健, 等. 硅对番茄生长及光合作用与蒸腾作用的影响[J].
植物营养与肥料学报, 2013, 19(2): 354–360.
Cao B L, Xu K, Shi J, et al. Effects of silicon on growth, photosynthesis and transpiration of tomato[J]. Plant Nutrition and Fertilizer Science, 2013, 19(2): 354–360. |
| [35] | Demming-a D B, Adams W W. Photoprotection and other responses of plant to high light stress[J]. Annual Review of Plant Physiology and Plant Molecu, 1992, 43: 599–626. DOI:10.1146/annurev.pp.43.060192.003123 |
| [36] | Morard P, Eyheraguibel B, Morard M, Silvestre J. Direct effects of humic-like substance on growth, water, and mineral nutrition of various species[J]. Journal of Plant Nutrition, 2011, 34(1): 46–59. |
| [37] |
刘明, 孙世贤, 齐华, 等. 水分胁迫对玉米苗期叶绿素荧光参数的影响[J].
玉米科学, 2009, 17(3): 95–98.
Liu M, Sun S X, Qi H, et al. Effects of water stresses on chlorophyll fluorescence parameters in seedlings of different maize hybrids[J]. Journal of Maize Sciences, 2009, 17(3): 95–98. |
| [38] | Qiu Z Y, Wang L H, Zhou Q. Effects of bisphenol a on growth, photosynthesis and chlorophyll fluorescence in above-ground organs of soybean seedlings[J]. Chemosphere, 2013, 90: 1274–1280. DOI:10.1016/j.chemosphere.2012.09.085 |
| [39] | Guidi L, Mori S, Pecchia S, et al. Effects of ozone exposure or fungal pathogen on white lupin leaves as determined by imaging of chlorophyll a fluorescence[J]. Plant Physiology and Biochemistry, 2007, 45: 851–857. DOI:10.1016/j.plaphy.2007.07.001 |
| [40] |
晋艳, 吴玉萍, 段玉琪, 等. 高腐殖酸含量的育苗基质对烟草育苗生长的影响[J].
西南农业学报, 2009, 22(1): 122–125.
Jin Y, Wu Y P, Duan Y Q, et al. Effect of humic acid of media on tobacco seedling-growing in float tray system[J]. Southwest China Journal of Agricultural Sciences, 2009, 22(1): 122–125. |
| [41] |
刘可星, 廖宗文, 谷丽萍, 李胜华. 生化腐植酸:现代肥料新资源和功能化开发的前沿[J].
腐植酸, 2008(4): 10–14.
Liu K X, Liao Z W, Gu L P, Li S H. Bio-humic acid:the important foreland of exploiting new resources and functions of modern fertilizer[J]. Humic Acid, 2008(4): 10–14. |
| [42] |
李扬, 乔玉辉, 莫晓辉, 孙振钧. 蚯蚓粪作为土壤重金属污染修复剂的潜力分析[J].
农业环境科学学报, 2010, 29: 250–255.
Li Y, Qiao Y H, Mo X H, Sun Z J. Analysis for earthworm feces as one of potential repair agents of heavy metal contamination in soil[J]. Journal of Agriculture Environment Science, 2010, 29: 250–255. |
| [43] |
李欢, 金宜英, 聂永丰. 污水污泥中腐殖酸的提取和利用[J].
清华大学学报(自然科学版), 2009, 49(12): 1980–1983.
Li H, Jin Y Y, Nie Y F. Extraction and utilization of humic acid from sewage sludge[J]. Journal of Tsinghua University (Science and Technology Editiom), 2009, 49(12): 1980–1983. |
| [44] |
石朋飞, 玄先路, 侯翠红, 张保林. 腐植酸类肥料的研究现状及展望[J].
河南化工, 2015(7): 7–11.
Shi P F, Xuan X L, Hou C H, Zhang B L. Present situation and prospect of humic acid fertilizers[J]. Henan Chemical Industry, 2015(7): 7–11. |
 2016, Vol. 22
2016, Vol. 22  doi:
doi: 

