文章信息
- 嗜蛋白阿克曼菌通过保护肠道屏障功能抑制小鼠非酒精性脂肪性肝炎相关肝癌
- Akkermansia Muciniphila Suppresses Non-alcoholic Steatohepatitis-associated Liver Cancer in Mice by Recovering Intestinal Barrier Function
- 肿瘤防治研究, 2023, 50(5): 463-469
- Cancer Research on Prevention and Treatment, 2023, 50(5): 463-469
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2023.22.1259
- 收稿日期: 2022-10-25
- 修回日期: 2023-03-01
2. 510000 广州,南方医科大学南方医院消化内科
2. Department of Gastroenterology, Nanfang Hospital of Southern Medical University, Guangzhou 510000, China
随着生活水平的快速提高,非酒精性脂肪肝(non-alcoholic fatty liver disease, NAFLD)发病率高达25%,逐渐成为世界上最常见的慢性肝病[1-2]。NAFLD包括单纯非酒精性脂肪肝、非酒精性脂肪性肝炎(NASH)和肝硬化[3-4]。当非酒精性脂肪肝发展为NASH时,继续发展为肝细胞癌(hepatocellular carcinoma, HCC)的风险急剧增加[5]。并且NASH可以在没有肝硬化的情况下直接发展为HCC[3],是NAFLD患者不良预后的独立预测因子[6]。此外,合并NASH的病毒性肝炎患者更容易发生HCC[7]。因此,目前急需找到有效抑制NAFLD进展为NASH并预防其进展为HCC的治疗策略。有研究表明二代益生菌嗜蛋白阿克曼菌(A. muciniphila)能抑制NAFLD[8],并且通过改善肠道屏障功能抑制酒精性脂肪肝病。NAFLD相关HCC患者肠道中A. muciniphila的丰度在明显降低,并且与炎性反应标志物呈负相关,推测A. muciniphila的降低可能是促进NASH进展为HCC的发病机制[9-10]。在此基础上本研究探究补充A. muciniphila菌是否可以抑制NAFLD进展为HCC及其作用机制。
1 材料与方法 1.1 实验动物和材料8周龄SPF级C57BL/6J雌雄小鼠各8只,体重(20±2)g,购自广州百沐赛业生物科技有限公司,动物生产许可证号:SYXK(粤)2020-0242。小鼠丙氨酸转氨酶(ALT/GPT)ELISA试剂盒(CSB-E16539m)和小鼠天冬氨酸转氨酶(AST)ELISA试剂盒(CSB-E12649m)购自华美生物工程有限公司。60 kcal%高脂饲料(high fat diet, HFD)(Research Diets, D12492)购自杭州昕诚生物技术有限公司。佐脲链菌素(Sigma-Aldrich, MO, USA)购自上海昊阳生物技术有限公司。DMEM、青霉素-链霉素液(索莱宝)和胰蛋白酶(碧云天)购自广州硕恒生物技术有限公司。异硫氰酸荧光素标记的葡聚糖(FITC-Dextran,FD4)购自西安凯西生物科技有限公司。
1.2 方法 1.2.1 分组造模方法:出生4天的小鼠每只背部皮下注射链脲佐菌素(STZ)200 μg,4周龄时选取雄性小鼠给予HFD,此模型小鼠简称STAM小鼠(STZ+HFD mice)[11]。1雄2雌配笼生育小鼠,选取所有的雄性小鼠,按随机数字表法分为三大组:普通饲料组(对照组)、STAM组和STAM+A. muciniphila组。每组共14只鼠,其中6只小鼠从4周龄开始每天给予AKK菌灌喂,直到8周龄(STAM小鼠进展为NASH);另外8只小鼠从8周龄开始每天给予AKK菌灌喂,直到20周龄(STAM小鼠进展为HCC)。STAM+A. muciniphila组每只灌胃A. muciniphila 1.0×109 CFU,2次/天,普通饲料组(对照组)和STAM模型组给予等体积的0.9%氯化钠溶液灌胃,自由饮用高压灭菌水。
1.2.2 小鼠处死及标本采集于4、8、16、20周龄时取血以备血清生化指标检测,脱臼处死小鼠,取肝组织,拍摄大体照片,对肿瘤数目和最大肿瘤直径计数。取部分肝组织用于RT-PCR检测,部分肝组织用于HE染色。取小鼠远端回肠组织用于免疫荧光和RT-PCR检测,取结肠组织用于阿利新蓝染色。
1.2.3 RT-PCR检测采用RT-PCR法检测肝组织肿瘤坏死因子(TNF)-α、单核细胞趋化蛋白-1(MCP-1)、IL-1β、IL-6 mRNA表达水平。取大约100 mg肝组织,加入1 ml TRIzol,研磨,12 000 r/min、4℃离心5 min,提取总RNA,反转录成cDNA,ABI prism 7900HT荧光定量PCR仪进行PCR扩增,以2-ΔΔCt表示mRNA的相对表达水平。引物序列:TNF-α:上游引物:3′-AAGGGAGAGTGGTCAGG-5′,下游引物:3′-TCTGTGAGGAAGGCT-5′;MCP-1:上游引物:3′-TCCCAATGAGTAGGCTG-5′,下游引物:3′-TCTGGACCCATTC-5′;IL-6:上游引物:3′-ACAACGATGATGCACTTGCAGA-5′,下游引物3′-TGGTACTCCAGAAGACCAGAGG-5′;IL-1β:上游引物:3′-GCAACTGTTCCTGAACTCAACT-5′,下游引物:3′-ATCTTTTGGGGTCCGTCAACT-5′。
1.2.4 肝组织病理学观察分离肝脏,观察肝脏大体,固定、脱水、石蜡包埋,HE染色后于光学显微镜下观察小鼠肝脏病理改变,参照美国国立卫生研究院NAFLD临床研究网病理工作组指南,进行NAFLD活动度积分(NAS)评分[9]。制作冰冻切片,油红O染色后观察肝脏脂滴含量。
1.2.5 A. muciniphila的培养A. muciniphila(ATCC-BAA-835)购自美国ATCC,在37℃严格厌氧条件下用脑心浸出液琼脂培养,从培养皿中收集细菌,悬浮于含甘油的厌氧无菌磷酸盐缓冲液中,-80℃保存,使用前用磷酸盐缓冲液稀释A. muciniphila进行小鼠灌胃。
1.2.6 肠屏障通透性检测实验前,用磷酸盐缓冲液(PBS)稀释异硫氰酸荧光素标记的葡聚糖(FITC-Dextran, FD4)溶液并进行避光冷藏,FD4的平均分子量为10 000,实验浓度为25 mg/ml。STAM小鼠20周龄时,取材前1 h打开腹腔,结扎8 cm左右中段小肠,形成封闭肠袢,在肠袢内注射0.5 ml的FD4溶液,关闭腹腔。0.5 h后用肝素抗凝管从门静脉收集0.3~0.4 ml血液,避光,4℃下500×g离心5 min后,根据标准曲线计算血浆中FITC浓度。
1.3 统计学方法所有结果均以均数±标准差表示,两组间的差异采用Graphpad Prism 5的非配对t检验进行分析,P < 0.05为差异有统计学意义。
2 结果 2.1 STAM模型的成功建立首先我们构建了NASH-HCC小鼠模型(简称STAM小鼠)。从大体上看,所有STAM小鼠肝脏在8周时呈淡黄色,16周时表面呈颗粒状,20周时表面形成大的肿瘤结节,见图 1A。STAM小鼠血糖水平明显升高,血清丙氨酸转氨酶(ALT)水平升高,肝脏组织做HE和O油红染色后,在显微镜下对肝组织进行NASH活性评分(NAS),STAM小鼠的NAS明显增高,见图 1B~D。显微镜下可见,STAM(STZ-HFD)小鼠8周的时候非酒精性脂肪肝以脂质沉积为主要特点,16周以后肝脏组织出现大量纤维化,20周时肝脏内以肝组织结构混乱和炎症细胞浸润为主要特征,见图 1E。
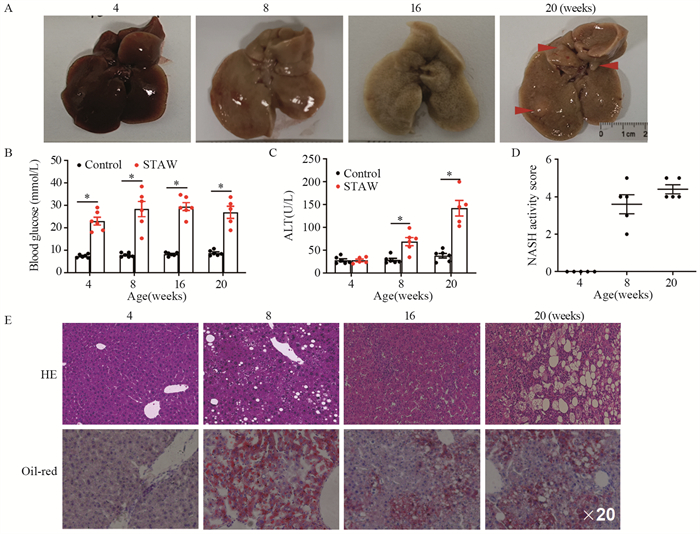
|
| A: macroscopic image of the liver surface; the serum levels of blood glucose (B) and ALT (C) were detected by ELISA; D: STAM mice progressed with increased NASH activity scores (NAS) from 8 weeks of age; E: paraffin-embedded and frozen mouse liver sections were stained with HE and oil-red to determine liver histology. ALT: alanine aminotransferase. *: P < 0.05, compared with the control group. 图 1 NASH相关HCC动物模型的构建 Figure 1 Establishment of STAM model of NASH-associated HCC |
为了检测补充A. muciniphila是否可以减少肝脏炎性反应,在STAM模型小鼠开始高脂饮食同时给予口服A. muciniphila,每天两次,见图 2A。HE染色结果显示,STAM小鼠8周龄时肝脏中空泡细胞、脂滴融合、炎症细胞浸润和球囊样结构明显增加,肝脏结构严重紊乱,而补充A. muciniphila可显著减少肝脏组织的结构异常。与STAM组相比,虽然STAM+A. muciniphila组小叶炎性反应评分差异无统计学意义,但脂肪变性评分、气球化和NAS活性评分明显减低(均P < 0.05),并且STAM+A. muciniphila组的血浆ALT水平也明显降低。补充A. muciniphila可以降低STAM小鼠肝脏组织的炎症因子MCP-1、IL-1β、IL-6和TNF-α的水平,见图 2~3。
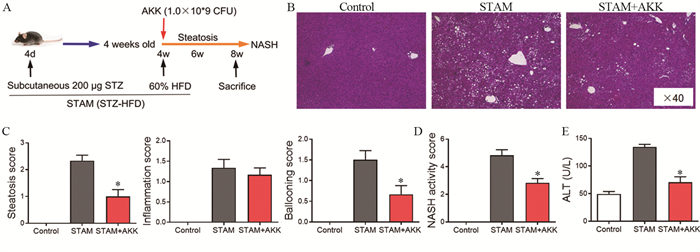
|
| AKK: A.muciniphila. A: experimental design and protocol; B: histological images of liver tissues by HE staining. Scale bars=50 µm; C: steatosis, inflammation score, and ballooning score were evaluated in the livers of STAM mice at 8 weeks of age; D: the total NASH score was calculated by combining the sum of steatosis score, inflammation score, and ballooning score (n=6 in each group); E: the serum ALT levels were detected by ELISA; *: P < 0.05, compared with the STAM group. 图 2 补充A. muciniphila可改善8周龄STAM小鼠的非酒精性脂肪性肝炎 Figure 2 A. muciniphila supplementation ameliorated non-alcoholic steatohepatitis in 8-week-old STAM mice |
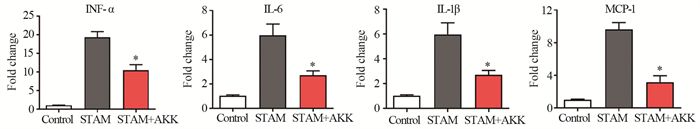
|
| *: P < 0.05, compared with the STAM group. 图 3 A. muciniphila减少8周龄STAM小鼠肝脏的炎性因子水平 Figure 3 A. muciniphila reduced levels of inflammatory factors in the livers of 8-week-old STAM mice |
为了探究长期补充A. muciniphila对NASH进展的影响,我们延长了口服灌胃A. muciniphila的时间,当STAM小鼠患有NASH时(8周龄)开始给予A. muciniphila灌胃直到小鼠20周龄时发生HCC。每组8只小鼠,STAM组中,6只发生了类似HCC的多发性肝肿瘤,而STAM+A. muciniphila组只有4只发生肝肿瘤,各组间HCC发生率(75% vs. 50%)差异无统计学意义(P=0.3017)。补充了A. muciniphila的STAM小鼠肝表面肿瘤结节平均数仅有1.13±0.52,而STAM组同窝小鼠有4.25±1.21个肿瘤结节(P=0.0319),见图 4A~D。STAM+A. muciniphila组的肝肿瘤大小明显较小,肝脏结构严重紊乱得到缓解,肝内脂肪变性、炎性反应和坏死减少,STAM+A. muciniphila组的血浆IL-6水平显著降低,见图 4E。

|
| A: macroscopic image of the liver surface (arrowheads: tumor); B: histological images of liver tissues by HE staining. Scale bars=200 µm; C: the total number of tumor nodules on the liver surface; D: the maximum diameter of the tumor nodules; E: the serum levels of IL-6 of the STAM mice (20 weeks of age) were measured by ELISA. *: P < 0.05, compared with the STAM group. 图 4 A. muciniphila抑制STAM小鼠肝癌发生 Figure 4 A. muciniphila administration blunted hepatocarcinogenesis in STAM mice |
与对照组小鼠相比,高脂饮食破坏了肠黏液层,STAM小鼠肠黏膜厚度明显减小,而A. muciniphila灌胃能减少高脂饮食引起的黏液层破坏,这与A. muciniphila灌胃能增加小鼠肠道产生黏液的杯状细胞有关,见图 5A~B。为了进一步探究A. muciniphila对肠道屏障功能的影响,我们在体内将小鼠肠道黏膜暴露于FD4,并检测血清中从肠道渗漏进来的FD4。与STAM组相比,STAM+A. muciniphila组的小鼠血清中的FD4明显升高,见图 5C。与恢复的肠道屏障功能一致,我们发现A. muciniphila能降低STAM小鼠血浆内毒素水平,见图 5D。
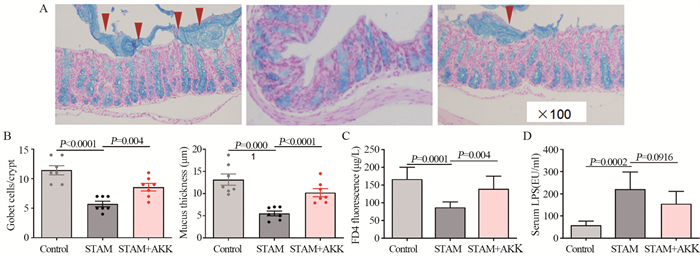
|
| A, B: quantification of mucus thickness after 10 weeks of A. muciniphila supplementation in STAM mice. Quantification of colonic goblet cells identified by alcian blue staining; C: serum FD4 level by the in vivo gut permeability assay; D: serum lipopolysaccharide concentrations in STAM mice. 图 5 A. muciniphila恢复STAM肠道屏障功能 Figure 5 A. muciniphila administration restored the intestinal barrier function |
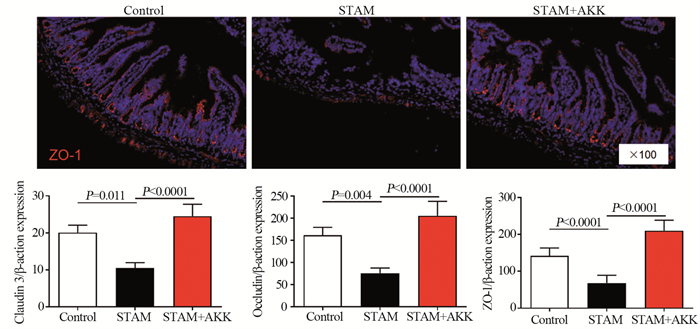
进一步评估A. muciniphila对回肠紧密连接蛋白表达的影响,结果显示,A. muciniphila灌胃能够增加回肠上皮紧密连接蛋白ZO-蛋白表达和上皮紧密连接蛋白ZO-1、Claudin-3和Occludin的mRNA表达水平,见图 6。
|
| 图 6 A. muciniphila增加STAM小鼠肠道紧密连接蛋白的表达 Figure 6 A. muciniphila administration increased the expression of tight junction proteins ZO-1, Claudin-3, and Occludin in ileal epithelial cells of STAM mice |
肠道微生物群通过肠-肝轴决定NAFLD和NAFLD相关HCC的发生发展[12-14]。因此,肠道菌群逐渐成为NAFLD的一个新的治疗靶点和预防NAFLD相关HCC的靶点[14]。补充益生菌可以改善NAFLD[15-16],但益生菌对NAFLD向HCC进展的作用仍不清楚。已有研究表明,益生菌可以抑制转移的和化学药物诱导的HCC生长[17-18]。NAFLD相关HCC患者肠道中A. muciniphila明显减少,并与炎性反应标志物呈负相关[9-10]。在动物模型中,我们发现补充A. muciniphila可以抑制NAFLD向HCC的进展。
越来越多的研究发现益生菌的抗肿瘤能力,然而,目前研究主要关注益生菌对肠道相关的肿瘤的作用。由于肝脏通过门静脉循环不断暴露于肠道源性因子中,因此益生菌对肝癌的影响也引起了研究者的关注。本研究探究了二代益生菌A. muciniphila在NASH-HCC微环境中的抗炎和抗肿瘤能力,发现A. muciniphila能抑制NASH并且能预防NASH进展为HCC,并且证实了A. muciniphila能通过增加肠道屏障功能减少NASH相关的肝癌发生。
A. muciniphila是一种很有发展前景的二代益生菌,它能够调节全身代谢和免疫稳定性[8]。最近多项研究表明A. muciniphila还具有抗肿瘤活性[19]。A. muciniphila菌可以维持患者对PD-1相关的肿瘤免疫治疗的敏感度[20-21]。更重要的是,在体外A. muciniphila可以直接抑制癌细胞活性[22],在体内可以增强机体的抗肿瘤免疫反应[23]。在肠道中,A. muciniphila通过调节细胞毒性T淋巴细胞来对抗结肠炎相关的结直肠癌[24]。肠道微生物群能调节全身免疫系统,因此补充益生菌A. muciniphila也能作用于肝脏免疫系统,我们的研究发现给与NASH相关HCC小鼠灌胃A. muciniphila能减少肝癌的发生。
A. muciniphila对肥胖和糖尿病等代谢相关疾病的改善已经得到研究证实[25-26],A. muciniphila可减少肥胖小鼠的脂肪量、胰岛素抵抗和血脂异常,改善肥胖小鼠的肥胖,维持葡萄糖稳态。肥胖和糖尿病不仅是导致非酒精性脂肪肝病的两大主要诱因,还是患者发生非酒精性脂肪肝相关肝癌的两个独立危险因素[27]。最近的研究初步表明其还具有抗非酒精性脂肪肝病的作用[15-16],本研究进一步证明了补充A. muciniphila可以减轻非酒精性脂肪肝相关肝癌。
肠道屏障功能损伤是NAFLD的发病机制[28],研究表明A. muciniphila发挥抗酒精性脂肪肝作用的机制与肠上皮黏液层产生的增加和屏障功能的恢复有关[29],因此本研究探究了A. muciniphila是否通过改善肠道屏障抑制NASH-HCC。有报道发现NAFLD和NAFLD-HCC中肠道通透性增加,肠道细菌过度生长和血清内毒素升高[30]。探究其中的因果关系发现,肠道菌群失调通过破坏肠道屏障,引起肠道通透性增加,肠源的因子特别是内毒素渗漏到体循环中,在肝脏中发挥促炎作用来驱动NAFLD的发生和癌变[31-32],因此,屏障功能损伤是NAFLD和相关肝癌的发病机制。本研究结果表明,补充A. muciniphila具有保护肠道屏障功能,能改善肠道通透性的作用,这与A. muciniphila能够维持肠道内环境稳态、维持NAFLD小鼠肠道菌群平衡和多样性、增加肠道物种丰富度有关[17, 33]。本研究探究了口服补充A. muciniphila对NASH发生并进展为HCC的抑制作用,并证实了其发挥作用的机制是促进肠道屏障功能恢复。
综上,除了能抑制NASH外,A. muciniphila可以通过改善肠道屏障功能抑制NASH向HCC的进展。A. muciniphila可能成为预防NASH发生发展为相关HCC的一种有前景的补充药物。
利益冲突声明:
所有作者均声明不存在利益冲突。
作者贡献:
张武剑:实验实施、论文撰写
李 桃:课题构思、实验设计与指导、数据处理及论文修改
| [1] |
Loomba R, Sanyal AJ. The global NAFLD epidemic[J]. Nat Rev Gastroenterol Hepatol, 2013, 10(11): 686-690. DOI:10.1038/nrgastro.2013.171 |
| [2] |
Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes[J]. Hepatology, 2016, 64(1): 73-84. DOI:10.1002/hep.28431 |
| [3] |
Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention[J]. Nat Rev Gastroenterol Hepatol, 2018, 15(1): 11-20. DOI:10.1038/nrgastro.2017.109 |
| [4] |
Anstee QM, Reeves HL, Kotsiliti E, et al. From NASH to HCC: Current concepts and future challenges[J]. Nat Rev Gastroenterol Hepatol, 2019, 16(7): 411-428. DOI:10.1038/s41575-019-0145-7 |
| [5] |
Vernon G, Baranova A, Younossi ZM. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults[J]. Aliment Pharmacol Ther, 2011, 34(3): 274-285. DOI:10.1111/j.1365-2036.2011.04724.x |
| [6] |
Younossi ZM, Marchesini G, Pinto-Cortez H, et al. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Implications for liver transplantation[J]. Transplantation, 2019, 103(1): 22-27. DOI:10.1097/TP.0000000000002484 |
| [7] |
Pfister D, Núñez NG, Pinyol R, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC[J]. Nature, 2021, 592(7854): 450-456. DOI:10.1038/s41586-021-03362-0 |
| [8] |
Zhang T, Li Q, Cheng L, et al. Akkermansia muciniphila is a promising probiotic[J]. Microb Biotechnol, 2019, 12(6): 1109-1125. DOI:10.1111/1751-7915.13410 |
| [9] |
Ponziani FR, Bhoori S, Castelli C, et al. Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease[J]. Hepatology, 2019, 69(1): 107-120. DOI:10.1002/hep.30036 |
| [10] |
Schneider KM, Mohs A, Gui W, et al. Imbalanced gut microbiota fuels hepatocellular carcinoma development by shaping the hepatic inflammatory microenvironment[J]. Nat Commun, 2022, 13(1): 3964. DOI:10.1038/s41467-022-31312-5 |
| [11] |
Fujii M, Shibazaki Y, Wakamatsu K, et al. A murine model for non-alcoholic steatohepatitis showing evidence of association between diabetes and hepatocellular carcinoma[J]. Med Mol Morphol, 2013, 46(3): 141-152. DOI:10.1007/s00795-013-0016-1 |
| [12] |
Gerbes A, Zoulim F, Tilg H, et al. Gut roundtable meeting paper: Selected recent advances in hepatocellular carcinoma[J]. Gut, 2018, 67(2): 380-388. DOI:10.1136/gutjnl-2017-315068 |
| [13] |
Jia W, Rajani C. The influence of gut microbial metabolism on the development and progression of non-alcoholic fatty liver disease[J]. Adv Exp Med Biol, 2018, 1061: 95-110. |
| [14] |
Borrelli A, Bonelli P, Tuccillo FM, et al. Role of gut microbiota and oxidative stress in the progression of non-alcoholic fatty liver disease to hepatocarcinoma: Current and innovative therapeutic approaches[J]. Redox Biol, 2018, 15: 467-479. DOI:10.1016/j.redox.2018.01.009 |
| [15] |
Kim S, Lee Y, Kim Y, et al. Akkermansia muciniphila Prevents Fatty Liver Disease, Decreases Serum Triglycerides, and Maintains Gut Homeostasis[J]. Appl Environ Microbiol, 2020, 86(7): e03004-e03019. |
| [16] |
Rao Y, Kuang Z, Li C, et al. Gut Akkermansia muciniphila ameliorates metabolic dysfunction-associated fatty liver disease by regulating the metabolism of L-aspartate via gut-liver axis[J]. Gut Microbes, 2021, 13(1): 1-19. |
| [17] |
Li J, Sung CYJ, Lee N, et al. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice[J]. Proc Natl Acad Sci U S A, 2016, 113(9): E1306-E1315. |
| [18] |
Zhang HL, Yu LX, Yang W, et al. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats[J]. J Hepatol, 2012, 57(4): 803-812. DOI:10.1016/j.jhep.2012.06.011 |
| [19] |
Kaźmierczak-Siedlecka K, Skonieczna-Żydecka K, Hupp T, et al. Next-generation probiotics-do they open new therapeutic strategies for cancer patients?[J]. Gut Microbes, 2022, 14(1): 2035659. DOI:10.1080/19490976.2022.2035659 |
| [20] |
Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients[J]. Science, 2018, 359(6371): 104-108. DOI:10.1126/science.aao3290 |
| [21] |
Zheng Y, Wang T, Tu X, et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma[J]. J Immunother Cancer, 2019, 7(1): 193. DOI:10.1186/s40425-019-0650-9 |
| [22] |
Meng X, Zhang J, Wu H, et al. Akkermansia muciniphila aspartic protease Amuc_1434* inhibits human colorectal cancer LS174T cell viability via TRAIL-mediated apoptosis pathway[J]. Int J Mol Sci, 2020, 21(9): 3385. DOI:10.3390/ijms21093385 |
| [23] |
Shi L, Sheng J, Chen G, et al. Combining IL-2-based immunotherapy with commensal probiotics produces enhanced antitumor immune response and tumor clearance[J]. J Immunother Cancer, 2020, 8(2): e000973. DOI:10.1136/jitc-2020-000973 |
| [24] |
Wang L, Tang L, Feng Y, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8+T cells in mice[J]. Gut, 2020, 69(11): 1988-1997. DOI:10.1136/gutjnl-2019-320105 |
| [25] |
Shin NR, Lee JC, Lee HY, et al. An increase in the akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice[J]. Gut, 2014, 63(5): 727-735. DOI:10.1136/gutjnl-2012-303839 |
| [26] |
Yoon HS, Cho CH, Yun MS, et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice[J]. Nat Microbiol, 2021, 6(5): 563-573. DOI:10.1038/s41564-021-00880-5 |
| [27] |
Pennisi G, Celsa C, Giammanco A, et al. The Burden of Hepatocellular Carcinoma in Non-Alcoholic Fatty Liver Disease: Screening Issue and Future Perspectives[J]. Int J Mol Sci, 2019, 20(22): 5613. DOI:10.3390/ijms20225613 |
| [28] |
Nicoletti A, Ponziani FR, Biolato M, et al. Intestinal permeability in the pathogenesis of liver damage: From non-alcoholic fatty liver disease to liver transplantation[J]. World J Gastroenterol, 2019, 25(33): 4814-4834. DOI:10.3748/wjg.v25.i33.4814 |
| [29] |
Grander C, Adolph TE, Wieser V, et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease[J]. Gut, 2018, 67(5): 891-901. DOI:10.1136/gutjnl-2016-313432 |
| [30] |
Schwabe RF, Greten TF. Gut microbiome in HCC-Mechanisms, diagnosis and therapy[J]. J Hepatol, 2020, 72(2): 230-238. DOI:10.1016/j.jhep.2019.08.016 |
| [31] |
Miele L, Valenza V, La Torre G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease[J]. Hepatology, 2009, 49(6): 1877-1887. DOI:10.1002/hep.22848 |
| [32] |
Said I, Ahad H, Said A. Gut microbiome in non-alcoholic fatty liver disease associated hepatocellular carcinoma: Current knowledge and potential for therapeutics[J]. World J Gastrointest Oncol, 2022, 14(5): 947-958. DOI:10.4251/wjgo.v14.i5.947 |
| [33] |
Morrison MC, Gart E, van Duyvenvoorde W, et al. Heat-Inactivated Akkermansia muciniphila Improves Gut Permeability but Does Not Prevent Development of Non-Alcoholic Steatohepatitis in Diet-Induced Obese Ldlr-/-. Leiden Mice[J]. Int J Mol Sci, 2022, 23(4): 2325. |
 2023, Vol. 50
2023, Vol. 50


