文章信息
- ROCK2调控骨肉瘤细胞的侵袭及迁移
- ROCK2 Regulates Invasion and Migration of Osteosarcoma Cells
- 肿瘤防治研究, 2022, 49(2): 95-100
- Cancer Research on Prevention and Treatment, 2022, 49(2): 95-100
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2022.21.0562
- 收稿日期: 2021-05-17
- 修回日期: 2021-08-22
骨肉瘤是起源于间叶组织的恶性肿瘤,好发于股骨远端及胫骨近端,主要发生于青少年,具有恶性程度高、易发生肺部转移等特点[1]。骨肉瘤预后较差,其术后5年生存率为60%[2]。骨肉瘤严重影响青少年儿童的健康,尽管当前骨肉瘤的临床诊断技术及外科治疗技术取得了很大进展,但骨肉瘤患者的整体预后依旧较差,因此,亟需寻找有效的分子靶点。肿瘤的恶性转移是导致患者治疗失败的主要原因,肿瘤远处转移的发生是一个多因素多步骤的结果[3]。Rho相关卷曲螺旋形成蛋白激酶2(ROCK2)在肿瘤的发生及恶性进展中扮演着重要角色,是Rho信号通路的关键蛋白,目前研究表明其可以调节细胞的收缩、黏附、迁移、增殖和凋亡[4-5]。研究还证实其在肝癌[6]、结直肠癌[7]及乳腺癌[8]中异常高表达,且证实了转移性肿瘤组织中ROCK2的表达高于其原位癌中的表达[9],然而,其具体调控机制有待进一步研究。基于此,本研究旨在分析ROCK2在骨肉瘤组织中的表达,并进一步分析其对骨肉瘤侵袭及迁移的影响和机制,为骨肉瘤的分子靶向治疗提供更多的理论依据。
1 材料与方法 1.1 主要试剂人骨肉瘤细胞U2-OS及Saos-2购自中科院上海细胞库,兔抗人单克隆ROCK2、E-钙黏蛋白(E-cadherin)、N-钙黏蛋白(N-cadherin)、波形蛋白(Vimentin)及鼠抗人GAPDH(内参照)单克隆抗体购自英国Abcam公司。辣根过氧化物酶(horseradish peroxidase,HRP)标记的山羊抗鼠IgG和山羊抗兔IgG均购自英国Abcam公司,ROCK2及GAPDH引物由广州锐博公司合成,空转质粒(shNC)、ROCK2下调质粒(shROCK2)均由上海吉玛基因公司合成,其序列如下:F:5'-CACCGCAGCTGGAATCTAACAATAGCGAACTATTGTTAGATTCCAGCTGC-3', R:5'-AAAAGCAGCTGGAATCTAACAATAGTTCGCTATTGTTAGATTCCAGCTGC-3'。
1.2 免疫组织化学检测经南昌市第一医院医学伦理委员会批准,并经患者或其家属知情同意,收集25例外科手术后切取的骨肉瘤组织及其相应的癌旁组织标本。所有患者均未接受放化疗,标本经4%中性多聚甲醛固定,脱水后石蜡进行固定,切成4 μm的薄片,5%BSA封闭30 min,滴加ROCK2抗体(1:200稀释)4℃孵育过夜,二抗室温孵育2 h,显色,根据细胞染色(棕色、黄色、蓝色)深浅及比例进行评分。
1.3 实时荧光定量PCR将收集好的骨肉瘤组织经过研磨后采用TRIzol(Invitrogen,美国)法提取组织RNA及总RNA。按照TAKARA(日本)试剂说明,将其转录合成cDNA,实时荧光定量PCR扩增。将阈值定义为循环数Ct,依据标准曲线计算目的基因ROCK2及GAPDH的mRNA量,每个样品ROCK2与GAPDH基因浓度比值,即为校正后的相对含量,最终结果以2-ΔΔCt表示,实验重复3次。
1.4 Western blot检测将收集好的骨肉瘤组织通过组织抽提试剂盒(北京碧云天公司)提取组织蛋白,RIPA(北京碧云天公司)裂解法提取骨肉瘤细胞蛋白,通过BCA(北京碧云天公司)法测定其蛋白浓度,并加入蛋白质Buffer将其稀释,经沸水煮10 min使其发生变性,每个上样孔加入约30 μg蛋白,SDS-PAGE凝胶电泳,将蛋白转移至PVDF膜上,置于含ROCK2(1:1 000稀释)一抗中孵育过夜(4℃),相应种属二抗室温孵育1.5 h,将其置于凝胶成像系统中检测。
1.5 划痕实验按照空转质粒(shNC)、ROCK2下调质粒(shROCK2)、ROCK2过表达质粒(HA-ROCK2)进行分组,采用0.25%的胰酶消化后并计数,以每孔50×104个细胞接种于六孔板中,待细胞铺匀后,转染携带有shROCK2的质粒及空转质粒于骨肉瘤细胞中,待细胞铺满整个六孔板,使用200 μm枪头进行垂直划痕处理,PBS清洗细胞2次,按照既定的时间进行拍照观察并分析愈合率。
1.6 Transwell细胞侵袭实验按照shNC、shROCK2、HA-ROCK2进行分组,骨肉瘤经胰酶消化后并计数,以每孔1×104个细胞接种于Transwell板中,预冷处理实验器械,每孔加入50 μl稀释基质胶,37℃孵育30 min,待其凝固后,上室加入200 μl含1×104个细胞的无血清培养基,下室加入500 μl含10%血清的培养基,培养箱孵育48 h后,用棉签轻轻擦拭未穿透的细胞,甲醇固定20 min,1%结晶紫染色30 min,室温晾干后,电子显微镜下拍照计数。
1.7 动物实验雄性裸鼠来源于湖南斯莱克景达动物实验有限公司(BALB/c-nu/nu, 6周);按照shNC及shROCK2进行分组,每组6只。构建转染shNC及shROCK2质粒的骨肉瘤细胞,PBS清洗2次,经胰酶消化后收集细胞沉淀并将细胞稀释成每毫升1×107个备用。尾静脉接种100 μl shNC及shROCK2组细胞,无菌环境下继续喂养30天后处死,取出双肺,HE染色。
1.8 统计学方法所有实验数据均通过软件SPSS22.0进行统计分析。以平均数±标准差(x±s)表示计量数据,实验数据间的两两比较采用t检验或者Welch校正t检验方法,多组实验均数之间的比较采用单因素方差分析方法,P < 0.05为差异有统计学意义,每个独立实验均重复3次以上。
2 结果 2.1 ROCK2蛋白在骨肉瘤组织中的表达情况qRT-PCR结果表明骨肉瘤组织中ROCK2 mRNA表达水平约为相应非癌组织的2.5倍(P < 0.01),见图 1A;25例骨肉瘤组织标本中,21例蛋白表达水平显著提升,4例表达水平无明显差异(P < 0.01),见图 1B~C,;免疫组织化学结果表明ROCK2在骨肉瘤组织中表达升高(P < 0.05),见图 1D~E。
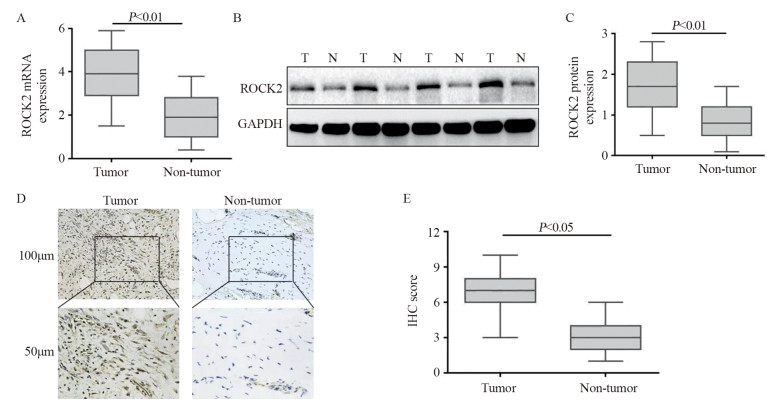
|
| A: the results of qRT-PCR showed that the expression level of ROCK2 mRNA in osteosarcoma tissues was higher than that in non-tumor tissues (n=31); B, C: the results of Western blot assay showed that the expression level of ROCK2 in osteosarcoma tissue was significantly higher than that in non-tumor tissues (n=25); T: Tumor, N: Non-tumor; D, E: immunohistochemical results also proved that ROCK2 was highly expressed in osteosarcoma tissues. 图 1 ROCK2蛋白在骨肉瘤组织中的表达 Figure 1 Expression of ROCK2 protein in osteosarcoma tissues |
下调骨肉瘤细胞U2-OS中ROCK2的表达后,qRT-PCR及Western blot证实其蛋白表达下调效果显著(P < 0.01),见图 2A。划痕愈合及Transwell结果表明其迁移及侵袭能力明显被抑制(P < 0.05; P < 0.01),见图 2C、E;相反,过表达ROCK2后(P < 0.01),其蛋白表达上调,见图 2B,骨肉瘤细胞的侵袭及迁移能力显著提升(P < 0.05; P < 0.01),见图 2D、F。
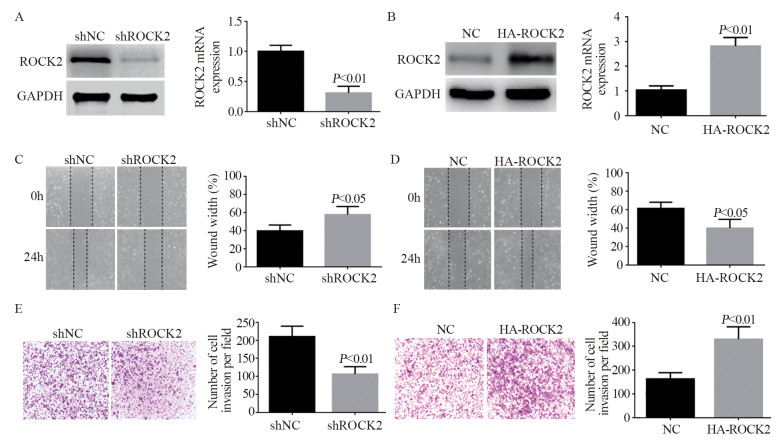
|
| A: Western blot and qRT-PCR confirmed that ROCK2 was successfully down-regulated after the shROCK2 plasmid was transfected into osteosarcoma cells; B: Western blot and qRT-PCR results showed that ROCK2 was successfully up-regulated; C: after the expression of ROCK2 was down-regulated in osteosarcoma cells, the wound-healing experiment showed that its migration ability was significantly weakened (P < 0.05); D: after the expression of ROCK2 was up-regulated in osteosarcoma cells, wound-healing assays showed that its migration ability was enhanced; E: Transwell assays also confirmed that after down-regulating ROCK2, the invasion ability of osteosarcoma cells was significantly weakened; F: Transwell assays also confirmed that the invasion ability of osteosarcoma cells was enhanced after upregulating ROCK2. 图 2 ROCK2促进骨肉瘤细胞侵袭及迁移 Figure 2 ROCK2 promoted invasion and migration of osteosarcoma cells |
下调骨肉瘤细胞中ROCK2的表达后,Western blot结果显示E-cadherin表达下降,而N-cadherin及Vimentin蛋白明显增加(P < 0.05),见图 3A。免疫荧光同样也证实此结果,见图 3B。上调后则与其相反,见图 3C。进一步采用TGF-β诱导骨肉瘤细胞上皮间质转化(epithelial-mesenchymal transition, EMT)的发生,下调ROCK2的表达,Western blot结果表明TGF-β能够减弱下调ROCK2对E-cadherin蛋白的抑制作用,能够减弱下调ROCK2对N-cadherin及Vimentin蛋白的促进作用,见图 3D。划痕愈合及Transwell实验表明TGF-β能够减弱下调ROCK2对骨肉瘤细胞侵袭及迁移的抑制作用(P < 0.05),见图 3E~F。
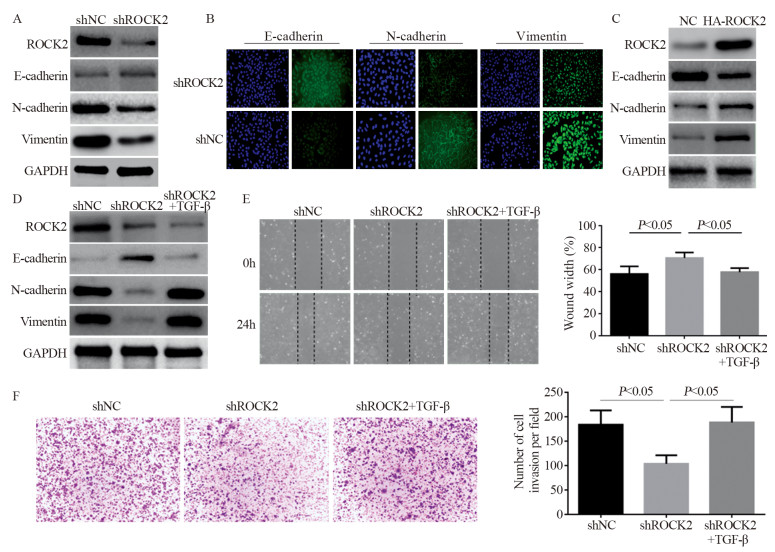
|
| A: after down-regulating the expression of ROCK2 in osteosarcoma cells, Western blot was used to detect the expression of EMT-related proteins; B: immunofluorescence was used to detect E-cadherin, N-cadherin and Vimentin protein expression in osteosarcoma cells; C: after up-regulating the expression of ROCK2 in osteosarcoma cells, Western blot was used to detect the expression of EMT-related proteins; D: EMT inducer was added to osteosarcoma cells with down-regulated ROCK2 expression, and Western blot was used to detect the expression of EMT-related proteins; E, F: Transwell and wound-healing assays showed that the induction of EMT in osteosarcoma cells could attenuate the inhibitory effect of down-regulation of ROCK2 on the invasion and migration of osteosarcoma cells. 图 3 ROCK2诱导骨肉瘤细胞EMT的发生 Figure 3 ROCK2 induced EMT in osteosarcoma cells |
裸鼠尾静脉转移实验表明,下调骨肉瘤细胞中ROCK2的表达后,其转移的发生率明显降低,肺转移发生率为0,而相应的对照组有5例发生肺转移(n=6)。
3 讨论骨肉瘤是骨组织来源最为常见的恶性肿瘤,约占骨恶性肿瘤的三分之一,早期即易发生肺部转移,通常患者预后很差[10-11]。以往的骨肉瘤治疗局限于临床手术切除根治治疗。据报道,患者5年的预后存活率仅为20%[2, 12]。目前骨肉瘤的治疗主要是新辅助化疗和临床手术两种方式,患者的5年预后存活率也仅仅保持在50%左右,较其他肿瘤而言存活率低[13-14]。研究证实肿瘤进展是一个多因素多步骤的复杂生物学过程,在此过程中总会伴随着基因及蛋白质水平的不断变化,最终诱导细胞的生物学表象改变,肿瘤恶性生长及向远处发生转移是当前临床治疗的难点,也是患者死亡的主要原因,因此,探究其恶性生长及远处转移的原因及机制,对临床肿瘤的指导治疗尤为重要。
Rho家族相关基因担负着蛋白分子开关的作用,在细胞信号转导方面也起着关键作用。Rho激酶(Rho kinase, ROCK)是Rho亚家族下游的关键靶点效应蛋白分子之一[15-16]。研究证实在肝癌、肾癌、乳腺癌等恶性肿瘤中观察到ROCK2表达升高, 然而在不同肿瘤中其机制却不相同:ROCK2介导GSK-3β/β-catenin通路抑制CEBPD表达,进而促进肝癌细胞增殖[6];ROCK2通过介导β-catenin/TCF4通路来抑制SCARA5表达,从而促进肾癌细胞增殖[7];ROCK2基因的Thr431Asn多态性可能是乳腺癌转移的危险因素[8]。ROCK2参与了许多细胞生物学活动,主要包括细胞形态发生、运动性、细胞分裂、增殖、迁移和细胞黏附。ROCK2是与Rho/GTP酶两者间相互作用而激活,并通过一些分子信号途径来调节肌动蛋白细胞骨架,进而影响细胞的侵袭迁移。研究也证实ROCK2在平滑肌和神经再生以及神经元发育中起着重要作用[17]。此外,多数研究表明,ROCK2活化通过直接影响肿瘤细胞的运动以及对癌症相关成纤维细胞的间接影响来增加细胞外基质并促进肿瘤细胞的生长,从而在增加肿瘤细胞的侵袭和转移中发挥直接作用[18]。在此方面,很多Rho相关通路抑制剂可减少肿瘤的生长、迁移和进展,例如乳腺癌、卵巢癌、肺癌等。然而ROCK2与骨肉瘤的研究报道不多,因此探讨ROCK2在骨肉瘤中的表达及其影响机制尤为重要。
上皮间质转化是上皮细胞表型向间质细胞表型转变的过程,可导致肿瘤上皮细胞的极性和细胞间连接的逐渐丧失,细胞骨架重构,在肿瘤发生及演变中发挥着重要的作用[19]。EMT的发生涉及多种通路的参与,如转化生长因子-β(TGF-β/Smad)通路、Wnt/β-catenin通路、Notch通路等[20]。Ding等发现TGF-β可通过介导Slug信号通路连接、进而抑制miR-203启动子的表达,促进上皮间质转换从而增强乳腺癌细胞的转移特性[21]。有多种转录因子在EMT过程中发挥重要作用,如E-cadherin作为上皮表型的标志物,是一种跨膜细胞黏附蛋白,调节细胞间黏附和维持上皮组织的结构和功能完整性,其缺失或者降低被认为是发生EMT的重要标志,其他如Snail、Twist、ZEB等E-cadherin转录抑制的相关因子在EMT过程中表达增多,从而抑制E-cadherin的表达[22]。此外,N-cadherin、Vimentin等反映细胞的间质成分变化的蛋白表达升高[23]。研究表明,EMT在多种肿瘤的转移过程中扮演着重要角色。Akalay等[24]发现,乳腺癌细胞呈现EMT表型改变,抑制Snail、逆转EMT可抑制其侵袭与迁移能力。近年研究表明EMT在骨肉瘤转移中发挥重要作用。Yuan等[25]证实EMT可以诱导骨肉瘤肺转移的发生。由此可见EMT在骨肉瘤的转移过程中扮演着重要角色。本研究首先通过提取骨肉瘤患者中的mRNA及蛋白质,发现癌组织的ROCK2表达量明显升高,免疫组织化学检测也证实了同样的结果。Transwell和细胞划痕实验发现在骨肉瘤细胞中ROCK2促进细胞迁移能力,而通过RNAi干扰技术下调骨肉瘤细胞中ROCK2的表达,其侵袭迁移能力减弱,提示ROCK2在骨肉瘤发展中有促进作用。Western blot检测发现,ROCK2过表达的骨肉瘤细胞中E-cadherin蛋白减少,N-cadherin、Vimentin蛋白增加;ROCK2敲低的骨肉瘤细胞结果则相反,免疫荧光实验也得到了同样的结果。本实验还发现,TGF-β可以减弱敲低ROCK2对骨肉瘤细胞迁移侵袭的抑制作用。以上结果证实了ROCK2是通过诱导骨肉瘤EMT的发生进而促进其侵袭及迁移的发生。动物实验也发现降低ROCK2表达能明显降低骨肉瘤细胞的肺转移率。
综上,本研究揭示了ROCK2在骨肉瘤中的表达及其对骨肉瘤细胞侵袭及迁移的影响,为骨肉瘤的靶向治疗提供更多的理论基础,提示ROCK2有可能成为骨肉瘤的潜在治疗靶点。
作者贡献:
单文豪:文献查阅与论文撰写
易伟江:细胞实验
张司军:动物实验
江伟:数据统计
夏剑:实验整体设计及论文指导
| [1] |
Bozorgi A, Sabouri L. Osteosarcoma, personalized medicine, and tissue engineering; an overview of overlapping fields of research[J]. Cancer Treat Res Commun, 2021, 27: 100324. DOI:10.1016/j.ctarc.2021.100324 |
| [2] |
Faisham WI, Mat Saad AZ, Alsaigh LN, et al. Prognostic factors and survival rate of osteosarcoma: A single-institution study[J]. Asia Pac J Clin Oncol, 2017, 13(2): e104-e110. DOI:10.1111/ajco.12346 |
| [3] |
Su B, Yan X, Li Y, et al. Effects of Inonotus obliquus Polysaccharides on Proliferation, Invasion, Migration, and Apoptosis of Osteosarcoma Cells[J]. Anal Cell Pathol (Amst), 2020, 2020: 4282036. |
| [4] |
Li M, Ke J, Wang Q, et al. Upregulation of ROCK2 in gastric cancer cell promotes tumor cell proliferation, metastasis and invasion[J]. Clin Exp Med, 2017, 17(4): 519-529. DOI:10.1007/s10238-016-0444-z |
| [5] |
Zucchini C, Manara MC, Cristalli C, et al. ROCK2 deprivation leads to the inhibition of tumor growth and metastatic potential in osteosarcoma cells through the modulation of YAP activity[J]. J Exp Clin Cancer Res, 2019, 38(1): 503. DOI:10.1186/s13046-019-1506-3 |
| [6] |
Li M, Zhou W, Yuan R, et al. ROCK2 promotes HCC proliferation by CEBPD inhibition through phospho-GSK3beta/beta-catenin signaling[J]. FEBS Lett, 2015, 589(9): 1018-1025. DOI:10.1016/j.febslet.2015.03.004 |
| [7] |
Xu Z, Hong Z, Ma M, et al. Rock2 promotes RCC proliferation by decreasing SCARA5 expression through beta-catenin/TCF4 signaling[J]. Biochem Biophys Res Commun, 2016, 480(4): 586-593. DOI:10.1016/j.bbrc.2016.10.097 |
| [8] |
Kalender ME, Demiryurek S, Oztuzcu S, et al. Association between the Thr431Asn polymorphism of the ROCK2 gene and risk of developing metastases of breast cancer[J]. Oncol Res, 2010, 18(11-12): 583-591. |
| [9] |
Libanje F, Raingeaud J, Luan R, et al. ROCK2 inhibition triggers the collective invasion of colorectal adenocarcinomas[J]. EMBO J, 2019, 38(14): e99299. |
| [10] |
Ayerza MA, Farfalli GL, Aponte-Tinao L, et al. Does increased rate of limb-sparing surgery affect survival in osteosarcoma?[J]. Clin Orthop Relat Res, 2010, 468(11): 2854-2859. DOI:10.1007/s11999-010-1423-4 |
| [11] |
Papakonstantinou E, Stamatopoulos A, I Athanasiadis D, et al. Limb-salvage surgery offers better five-year survival rate than amputation in patients with limb osteosarcoma treated with neoadjuvant chemotherapy. A systematic review and meta-analysis[J]. J Bone Oncol, 2020, 25: 100319. DOI:10.1016/j.jbo.2020.100319 |
| [12] |
Pruksakorn D, Phanphaisarn A, Arpornchayanon O, et al. Survival rate and prognostic factors of conventional osteosarcoma in Northern Thailand: A series from Chiang Mai University Hospital[J]. Cancer Epidemiol, 2015, 39(6): 956-963. DOI:10.1016/j.canep.2015.10.016 |
| [13] |
Bacci G, Forni C, Ferrari S, et al. Neoadjuvant chemotherapy for osteosarcoma of the extremity: intensification of preoperative treatment does not increase the rate of good histologic response to the primary tumor or improve the final outcome[J]. J Pediatr Hematol Oncol, 2003, 25(11): 845-853. DOI:10.1097/00043426-200311000-00006 |
| [14] |
Restrepo DJ, Huayllani MT, Boczar D, et al. Factors that Influence Chemotherapy Treatment Rate in Patients With Upper Limb Osteosarcoma[J]. Anticancer Res, 2019, 39(10): 5611-5615. DOI:10.21873/anticanres.13756 |
| [15] |
Fromigue O, Hay E, Modrowski D, et al. RhoA GTPase inactivation by statins induces osteosarcoma cell apoptosis by inhibiting p42/p44-MAPKs-Bcl-2 signaling independently of BMP-2 and cell differentiation[J]. Cell Death Differ, 2006, 13(11): 1845-1856. DOI:10.1038/sj.cdd.4401873 |
| [16] |
Xia Y, Cai X, Fan J, et al. RhoA/ROCK pathway inhibition by fasudil suppresses the vasculogenic mimicry of U2OS osteosarcoma cells in vitro[J]. Anticancer Drugs, 2017, 28(5): 514-521. DOI:10.1097/CAD.0000000000000490 |
| [17] |
Shimizu T, Fukumoto Y, Tanaka S, et al. Crucial role of ROCK2 in vascular smooth muscle cells for hypoxia-induced pulmonary hypertension in mice[J]. Arterioscler Thromb Vasc Biol, 2013, 33(12): 2780-2791. DOI:10.1161/ATVBAHA.113.301357 |
| [18] |
Shimizu T, Narang N, Chen P, et al. Fibroblast deletion of ROCK2 attenuates cardiac hypertrophy, fibrosis, and diastolic dysfunction[J]. JCI Insight, 2017, 2(13): e93187. DOI:10.1172/jci.insight.93187 |
| [19] |
潘琦璐, 马礼兵. 上皮-间质转化在恶性肿瘤发病和侵袭转移中的作用研究进展[J]. 实用医学杂志, 2020, 36(17): 2443-2447. [Pan QL, Ma LB. Research progress based on the role of epithelial-to-mesenchymal transition in the pathogenesis and invasion of malignant tumors[J]. Shi Yong Yi Yue Za Zhi, 2020, 36(17): 2443-2447.] |
| [20] |
Akalay I, Janji B, Hasmim M, et al. Epithelial-to-mesenchymal transition and autophagy induction in breastcarcinoma promote escape fromT-cell-mediated lysis[J]. Cancer Res, 2013, 73(8): 2418-2427. DOI:10.1158/0008-5472.CAN-12-2432 |
| [21] |
Ding X, Park SI, McCauley LK, et al. Signaling between transforming growth factor β (TGF-β) and transcription factor SNAI2 represses expression of microRNA miR-203 to promote epithelial-mesenchymal transition and tumor metastasis[J]. J Biol Chem, 2013, 288(15): 10241-10253. DOI:10.1074/jbc.M112.443655 |
| [22] |
Nieto MA, Huang RY, Jackson RA, et al. EMT: 2016[J]. Cell, 2016, 166(1): 21-45. DOI:10.1016/j.cell.2016.06.028 |
| [23] |
Loh CY, Chai JY, Tang TF, et al. The E-cadherin and N-cadherin switch in epithelial-to-mesenchymal transition: Signaling, therapeutic implications, and challenges[J]. Cells, 2019, 8(10): 1118-1150. DOI:10.3390/cells8101118 |
| [24] |
Akalay I, Janji B, Hasmim M, et al. Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T cell-mediated lysis[J]. Cancer Res, 2013, 73(8): 2418-2427. DOI:10.1158/0008-5472.CAN-12-2432 |
| [25] |
Yuan X, Piao L, Wang L, et al. Erythrocyte membrane protein band 4.1-like 3 inhibits osteosarcoma cell invasion through regulation of Snai1-induced epithelial-to-mesenchymal transition[J]. Aging (Albany NY), 2020, 13(2): 1947-1961. |
 2022, Vol. 49
2022, Vol. 49


