文章信息
- 不同雌激素受体状态下化疗周期数对乳腺癌新辅助化疗病理完全缓解率的影响
- Effect of Neoadjuvant Chemotherapy Cycles on Pathologic Complete Response Rate Under Different Estrogen Receptor Status of Breast Cancer
- 肿瘤防治研究, 2017, 44(1): 38-41
- Cancer Research on Prevention and Treatment, 2017, 44(1): 38-41
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2017.01.008
- 收稿日期: 2016-05-09
- 修回日期: 2016-10-09
2. 471000 洛阳,河南科技大学第一附属医院甲状腺乳腺肿瘤外科
2. Department of Thyroid Gland and Breast Tumor Surgery, The First Affiliated Hospital of Henan University of Science and Technology, Luoyang 471000, China
乳腺癌是女性常见的恶性肿瘤之一[1],其中27.8%~36.1%为局部晚期乳腺癌(locally advanced breast cancer, LABC)[2]。新辅助化疗(neoadjuvant chemotherapy, NAC)可降低肿瘤分期,使不可手术的乳腺癌患者获得手术治疗的机会,提高局部晚期乳腺癌保乳率(breast-conserving surgery, BCS)[3-6]。雌激素受体(estrogen receptor, ER)状态是乳腺癌发生和发展的重要决定因素[7-8],本研究分析不同雌激素受体状态下化疗周期长短对新辅助化疗患者病理完全缓解(pathologic complete response, pCR)率的影响。
1 资料与方法 1.1 资料回顾性分析430例2012年4月1日至2016年3月30日于郑州大学附属肿瘤医院首诊为原发性乳腺癌新辅助化疗患者的完整病例资料。入组标准:(1)所有患者在新辅助化疗前均行空心针穿刺活检获得明确病理诊断;(2)临床分期为ⅡB~ⅢC期的局部晚期乳腺癌患者,肿瘤分期参照2010年美国癌症联合委员会(AJCC)标准;(3)行全面检查示内脏器官均无严重器质性病变且无远处转移灶。排除标准:(1)炎性乳腺癌、髓样癌、小管癌、黏液癌、乳腺鳞癌等特殊类型乳腺癌;(2)伴有远处转移及身体不能耐受化疗的患者。
1.2 方法 1.2.1 免疫组织化学法检测ER、孕激素受体(progesterone receptor, PR)、人表皮生长因子受体2(human epidermal growth factor receptor 2, HER2):根据《ER和PR检测指南(ASCO/CAP2012)》用免疫组织化学Envision法对ER、PR进行检测,由高年资病理科医师重新阅片判定,1%作为检测界值,肿瘤细胞核在1%以上的任何强染色时均判为阳性;肿瘤细胞核在1%以下的染色时判为阴性。对于免疫组织化学法HER2初筛为2+的病例再进行FISH检测,HER2的免疫组织化学及FISH的阳性判读标准参考《乳腺癌HER2检测指南(2009版)[9] 》和美国《HER2检测临床实践指南(ASCO/CAP2013)》,即:HER2阳性结果定义为:免疫组织化学检测+++(癌细胞的胞膜30%以上呈全周的强染色)或荧光免疫组织化学检测示HER2基因扩增。根据免疫组织化学及Ki67值(采用14%作为判断Ki67高低的界值),将分子分型分为Luminal A型、Luminal B型、ERBB2+型及Basal-like型[10]。
1.2.2 治疗方法430例患者均行蒽环类联合紫杉类(表柔比星75 mg/m2或吡柔比星45 mg/m2,第1天,21天为1周期,紫杉醇80 mg/m2,第1天,每周1次或多西他赛75 mg/m2,第1天,21天为1周期)化疗方案。HER2阳性根据患者意愿接受靶向治疗(曲妥珠单抗,首次剂量8 mg/kg,之后为6 mg/kg,每3周1次,共12月)。化疗结束后行保乳手术或标准改良根治术。3~4周期新辅助化疗患者术后行3~4周期辅助化疗,淋巴结有转移或保乳术后行放射治疗,ER、PR阳性患者于新辅助化疗结束后行内分泌治疗。
1.3 统计学方法使用SPSS20.0软件进行统计分析,单因素分析使用χ2检验,采用多因素Logistic回归分析不同组间pCR预测因素。P < 0.05为差异有统计学意义。
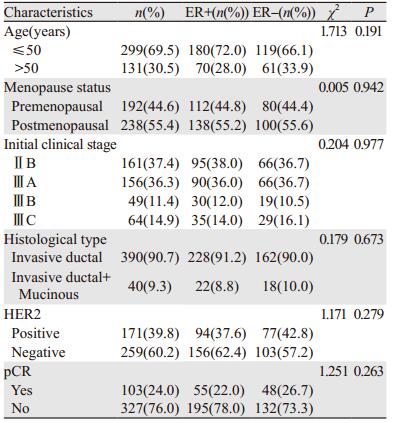
2 结果 2.1 患者临床病理特征年龄19~72岁,中位年龄45岁。430例患者中ER+250例(58.1%),ER-180例(41.9%)。Luminal A型29例(6.7%),Luminal B型223例(51.9%),ERBB2+型101例(23.5%),Basal-like型77例(17.9%)。
189例(44.0%)接受3~4周期新辅助化疗,241例(56.0%)接受6~8周期新辅助化疗。HER2阳性171例,63例(36.8%)接受靶向治疗。化疗结束后4例行保乳手术,余行标准改良根治术。患者临床病理特征见表 1。
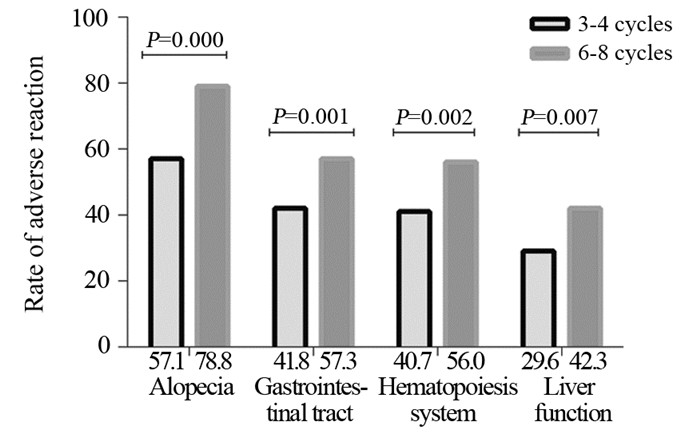
103例(24.0%)患者达pCR(定义为新辅助化疗后术后病理提示乳房无浸润性癌和原位癌残留,ypT0);294例(68.3%)部分缓解,28例(6.5%)疾病稳定;5例(1.2%)疾病进展。ER+患者达pCR者65例(22.0%),ER-患者达pCR48例(26.7%)。接受6~8周期新辅助化疗组pCR率(27.8%)比接受3~4周期组pCR率(19.0%)高,差异有统计学意义(χ2=4.456, P=0.035)。根据ER状态分组时,仅ER+组pCR率增加显著(13.3%~28.3%;χ2=7.924, P=0.005);6~8周期化疗使ER-组pCR率增加(26.2%~27.1%),但增加不显著(χ2=0.018, P=0.893),见表 2。相比3~4周期化疗,6~8周期化疗使患者脱发,胃肠道、造血系统及肝肾功能等不良反应发生率增加,差异有统计学意义,见图 1。

|
|
| 图 1 化疗周期数与乳腺癌患者新辅助化疗不良反应发生率 Figure 1 Relationship between rate of adverse reaction and neoadjuvant chemotherapy cycles of breast cancer patients |
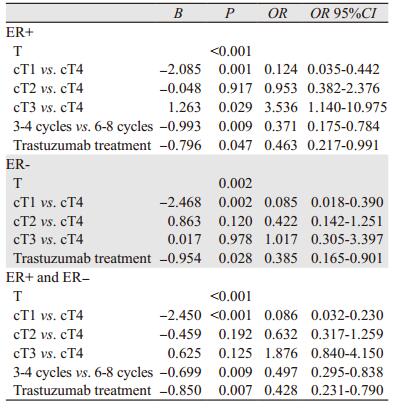
多因素Logistic回归分析不同ER状态下pCR的预测因素。所有患者中,新辅助化疗周期数是pCR的独立预测因素,相比6~8周期新辅助化疗,3~4周期化疗降低了患者的pCR(95%CI: 0.295~0.838, P=0.009)。根据ER状态分层分析,仅在ER+组化疗周期数与pCR差异有统计学意义(95%CI: 0.175~0.784, P=0.009)。此外,原发肿瘤大小(P < 0.05)、靶向治疗(P < 0.05)在ER+组和ER-组间比较差异均有统计学意义,见表 3。
|
新辅助化疗后pCR是乳腺浸润性导管癌独立的生存预后指标[11-12],术后病理证实为pCR的患者可能具有更高的无病生存和总生存[13]。乳腺癌是高度异质性疾病,ER表达状态通过改变基因表达而影响乳腺癌化疗疗效[14-15],是影响乳腺癌生物学行为的重要因素[16-17]。本研究分析不同ER状态下pCR与化疗周期数的相关性,ER状态的分组依据2011年St. Gallen乳腺癌专家共识[18]。
430例患者中pCR占24.0%,与研究[19]一致。不同ER状态新辅助化疗pCR率不同,ER-组中pCR率(26.7%)高于ER+(22.0%)组,与Miglietta等[20]和NSABP B-27研究[21]相符。本研究中6~8周期化疗比3~4周期化疗显著增加pCR率(P=0.035),说明适当延长乳腺癌新辅助化疗周期使pCR率升高,与文献研究一致[22-23]。但仅ER+患者延长化疗周期对以蒽环联合紫杉类化疗方案有益(P=0.005),6~8周期新辅助化疗使ER-组的患者pCR率增加(26.2%~27.1%),但增加不显著(P=0.893),与NSABP B-27研究[21]中8周期化疗相比4周期化疗pCR率增加且ER+组获得更高的pCR率一致。
Logistic回归分析显示化疗周期(P=0.009)、原发肿瘤大小(P < 0.001)及靶向治疗(P=0.007)均是新辅助化疗后pCR的独立预测因素,与文献结果相符[24-25]。ER状态分层分析提示化疗周期(95%CI: 0.175~0.784, P=0.009)仅是ER+组pCR的独立预测因素。ER+患者随着化疗周期延长pCR率可能进一步增加,而ER-患者的pCR率增加不显著,可能由于ER-患者在3~4化疗周期前肿瘤体积缩小较快,后期肿瘤体积缩小减慢或者无缩小,但ER+患者肿瘤体积在整个化疗周期均有缩小[26]。6~8周期化疗相比3~4周期使化疗毒性反应发生率增加,却无明显提高ER-患者的pCR率。因此,对ER-的患者可能并不需要6~8周期化疗。适当延长新辅助化疗周期使ER+患者pCR率升高,但是否会提高患者的无病生存和总生存,有待进一步随访研究。
综上所述,化疗周期是新辅助化疗后ER+患者达pCR的独立预测因素,但延长化疗周期并不能显著提高ER-患者pCR率。ER表达状态是构成新辅助化疗患者不同应答模式的基础,ER状态可能通过指导新辅助化疗周期优化患者的辅助治疗,具有重要的临床意义。
| [1] | Fan L, Strasser-Weippl k, Li JJ, et al. breast cancer in china[J]. Lancet Oncol, 2014, 15 (7) : e279–89. DOI:10.1016/S1470-2045(13)70567-9 |
| [2] | Zheng Y, Wu CX, Wu F, et al. Status and trends of breast cancer mortality in Chinese females[J]. Zhonghua Yu Fang Yi Xue Za Zhi, 2011, 45 (2) : 150–4. [ 郑莹, 吴春晓, 吴凡, 等. 中国女性乳腺癌死亡现况和发展趋势[J]. 中华预防医学杂志, 2011, 45 (2) : 150–4. ] |
| [3] | Mamounas EP. Impact of neoadjuvant chemotherapy on locoregional surgical treatment of breast cancer[J]. Ann Surg Oncol, 2015, 22 (5) : 1425–33. DOI:10.1245/s10434-015-4406-6 |
| [4] | Garg AK, Buchholz TA. Influence of neoadjuvant chemotherapy on radiotherapy for breast cancer[J]. Ann Surg Oncol, 2015, 22 (5) : 1434–40. DOI:10.1245/s10434-015-4402-x |
| [5] | Hayes DF, Schott AF. Neoadjuvant Chemotherapy: What Are the Benefits for the Patient and for the Investigator?[J]. J Natl Cancer Inst Monogr, 2015, 2015 (51) : 36–9. DOI:10.1093/jncimonographs/lgv004 |
| [6] | Golshan M, Cirrincione CT, Sikov WM, et al. Impact of Neoadjuvant Chemotherapy in Stage Ⅱ-Ⅲ Triple Negative Breast Cancer on Eligibility for Breast-conserving Surgery and Breast Conservation Rates: Surgical Results From CALGB 40603 (Alliance)[J]. Ann Surg, 2015, 262 (3) : 434–9. DOI:10.1097/SLA.0000000000001417 |
| [7] | Sánchez-Muñoz A, Plata-Fernández YM, Fernández M, et al. The role of immunohistochemistry in breast cancer patients treated with neoadjuvant chemotherapy: an old tool with an enduring prognostic value[J]. Clin Breast Cancer, 2013, 13 (2) : 146–52. DOI:10.1016/j.clbc.2012.11.006 |
| [8] | Zambetti M, Mansutti M, Gomez P, et al. Pathological complete response rates following different neoadjuvant chemotherapy regimens for operable breast cancer according to ER status, in two parallel, randomized phase Ⅱ trials with an adaptive study design (ECTO Ⅱ)[J]. Breast Cancer Res Treat, 2012, 132 (3) : 843–51. DOI:10.1007/s10549-011-1660-6 |
| [9] | Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours[J]. Nature, 2012, 490 (7418) : 61–70. DOI:10.1038/nature11412 |
| [10] | Jackisch C, Harbeck N, Huober J, et al. 14th St. Gallen International Breast Cancer Conference 2015: Evidence, Controversies, Consensus-Primary Therapy of Early Breast Cancer: Opinions Expressed by German Experts[J]. Breast Care (Basel), 2015, 10 (3) : 211–9. DOI:10.1159/000433590 |
| [11] | Krishnan Y, Al Awadi S, Sreedharan PS, et al. Analysis of neoadjuvant therapies in breast cancer with respect to pathological complete response, disease-free survival and overall survival: 15 years follow-up data from Kuwait[J]. Asia Pac J Clin Oncol, 2016, 12 (1) : e30–7. DOI:10.1111/ajco.2016.12.issue-1 |
| [12] | Guiu S, Arnould L, Bonnetain F, et al. Pathological response and survival after neoadjuvant therapy for breast cancer: a 30-year study[J]. Breast, 2013, 22 (3) : 301–8. DOI:10.1016/j.breast.2012.07.012 |
| [13] | Swisher SK, Vila J, Tucker SL, et al. Locoregional Control According to Breast Cancer Subtype and Response to Neoadjuvant Chemotherapy in Breast Cancer Patients Undergoing Breast-conserving Therapy[J]. Ann Surg Oncol, 2016, 23 (3) : 749–56. DOI:10.1245/s10434-015-4921-5 |
| [14] | Iwamoto T, Bianchini G, Booser D, et al. Gene pathways associated with prognosis and chemotherapy sensitivity in molecular subtypes of breast cancer[J]. J Natl Cancer Inst, 2011, 103 (3) : 264–72. DOI:10.1093/jnci/djq524 |
| [15] | Yang J, AITahan A, Jones DT, et al. Estrogen receptor-alpha directly regulates the hypoxia-inducible factor 1 pathway associated with antiestrogen response in breast cancer[J]. Proc Natl Acad Sci U S A, 2015, 112 (49) : 15172–7. DOI:10.1073/pnas.1422015112 |
| [16] | Das N, Datta N, Chatterjee U, et al. Estrogen receptor alpha transcriptionally activates casein kinase 2 alpha: A pivotal regulator of promyelocytic leukaemia protein (PML) and AKT in oncogenesis[J]. Cell Signal, 2016, 28 (6) : 675–87. DOI:10.1016/j.cellsig.2016.03.007 |
| [17] | Prossnitz ER, Arterburn JB. International Union of Basic and Clinical Pharmacology. XCVII. G Protein-Coupled Estrogen Receptor and Its Pharmacologic Modulators[J]. Pharmacol Rev, 2015, 67 (3) : 505–40. DOI:10.1124/pr.114.009712 |
| [18] | Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011[J]. Ann Oncol, 2011, 22 (8) : 1736–47. DOI:10.1093/annonc/mdr304 |
| [19] | Schipper RJ, Moossdorff M, Nelemans PJ, et al. A model to predict pathologic complete response of axillary lymph nodes to neoadjuvant chemo (immuno) therapy in patients with clinically node-positive breast cancer[J]. Clin Breast Cancer, 2014, 14 (5) : 315–22. DOI:10.1016/j.clbc.2013.12.015 |
| [20] | Miglietta L, Morabito F, Provinciali N, et al. A prognostic model based on combining estrogen receptor expression and Ki-67 value after neoadjuvant chemotherapy predicts clinical outcome in locally advanced breast cancer: extension and analysis of a previously reported cohort of patients[J]. Eur J Surg Oncol, 2013, 39 (10) : 1046–52. DOI:10.1016/j.ejso.2013.06.024 |
| [21] | Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27[J]. J Clin Oncol, 2003, 21 (22) : 4165–74. DOI:10.1200/JCO.2003.12.005 |
| [22] | Han S, Kim J, Lee J, et al. Comparison of 6 cycles versus 4 cycles of neoadjuvant epirubicin plus docetaxel chemotherapy in stages Ⅱ and Ⅲ breast cancer[J]. Eur J Surg Oncol, 2009, 35 (6) : 583–7. DOI:10.1016/j.ejso.2009.01.002 |
| [23] | Iwata H, Sato N, Masuda N, et al. Docetaxel followed by fluorouracil/epirubicin/cyclophosphamide as neoadjuvant chemotherapy for patients with primary breast cancer[J]. Jpn J Clin Oncol, 2011, 41 (7) : 867–75. DOI:10.1093/jjco/hyr081 |
| [24] | Tanaka S, Iwamoto M, Kimura K, et al. Phase II Study of Neoadjuvant Anthracycline-Based Regimens Combined With Nanoparticle Albumin-Bound Paclitaxel and Trastuzumab for Human Epidermal Growth Factor Receptor 2-Positive Operable Breast Cancer[J]. Clin Breast Cancer, 2015, 15 (3) : 191–6. DOI:10.1016/j.clbc.2014.12.003 |
| [25] | Fei F, Messina C, Slaets L, et al. Tumour size is the only predictive factor of distant recurrence after pathological complete response to neoadjuvant chemotherapy in patients with large operable or locally advanced breast cancers: a sub-study of EORTC 10994/BIG 1-00 phase Ⅲ trial[J]. Eur J Cancer, 2015, 51 (3) : 301–9. DOI:10.1016/j.ejca.2014.11.023 |
| [26] | Moon HG, Im SA, Han W, et al. Estrogen receptor status confers a distinct pattern of response to neoadjuvant chemotherapy: implications for optimal durations of therapy: distinct patterns of response according to ER expression[J]. Breast Cancer Res Treat, 2012, 134 (3) : 1133–40. DOI:10.1007/s10549-012-2145-y |
 2017, Vol. 44
2017, Vol. 44