文章信息
- NDRG2上调PTEN表达抑制乳腺癌细胞迁移的实验
- NDRG2 Inhibits Migration of Breast Cancer Cells Through Upregulating PTEN Expression
- 肿瘤防治研究, 2017, 44(1): 5-10
- Cancer Research on Prevention and Treatment, 2017, 44(1): 5-10
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2017.01.002
- 收稿日期: 2016-04-25
- 修回日期: 2016-07-07
乳腺癌是女性常见的恶性肿瘤,严重危及妇女健康及生命。乳腺癌后期的淋巴转移以及肺部、脊椎、肝等组织的转移,是造成患者死亡的首要原因[1]。癌细胞获得高迁移能力是乳腺癌转移的重要条件之一,研究癌细胞迁移调控机制对乳腺癌转移的诊断和治疗具有重要价值。
N-Myc downstream regulated gene 2(NDRG2)是NDRG家族的重要成员,其基因染色体定位于14q11.2,含有16个外显子,15个内含子,表达蛋白含357个氨基酸残基,相对分子质量约41 kDa。NDRG2具有APC样结构域,受原癌基因N-Myc的表达调控。近年研究发现,NDGR2基因除参与组织胚胎发育、细胞分化以及血液免疫之外,还与多种肿瘤的发生、发展具有密切关系[2]。如,NDRG2在中枢神经肿瘤、甲状腺癌、肺癌、和消化系统肿瘤中呈显著低水平的表达,并且与肿瘤的淋巴转移呈负相关[3-8]。NDRG2在肿瘤的发生发展中起到抑癌基因的作用[9]。NDRG2参与调控肿瘤细胞的多种生物学功能,如通过抑制c-Jun磷酸化下调cyclin D1或通过磷酸化p38抑制肿瘤细胞增殖[10];过表达NDRG2可促进由p53介导的肝癌细胞凋亡[11];NDRG2还可通过促进葡萄糖转运蛋白GLUT1降解阻断肿瘤细胞能量代谢抑制肿瘤生长[12]。但NDRG2是否参与调控乳腺癌细胞迁移尚不清楚。
本研究应用乳腺癌细胞MCF-7和具有高转移能力的亚克隆LM-MCF-7细胞系(获得国家发明专利,ZL.03131026415) [13]作为细胞模型,并以高转移MDA-MB-231细胞作为验证,研究NDRG2对乳腺癌细胞迁移的调控作用。
1 材料与方法 1.1 细胞及试剂人乳腺癌细胞系LM-MCF-7由南开大学叶丽虹教授惠赠。MCF-7、MDA-MB-231细胞购自中国科学院典型培养物保藏委员会细胞库;RPMI1640培养液、胎牛血清、脂质体Lipofectamine 2000购自美国Invitrogen公司;兔抗人NDRG2多克隆抗体、鼠抗人β-actin单克隆抗体购自美国Sigma-aldrich公司;兔抗人PTEN(Phosphatase and Tensin Homolog)多克隆抗体购自美国Santa Cruz公司;小量总RNA提取试剂盒购自德国Qiagen公司;QuantScript RT试剂盒购自北京天根生化科技有限公司。靶向NDRG2和PTEN的小RNA干扰片段由广州锐博生物科技有限公司合成,NDRG2 siRNA:正义5'-ACAUCCUGGCGAGAUAUGCUCUUAA-3',反义5'-UUAAGAGCAUAUCUCGCCAGGAUGU-3';对照siRNA序列:正义5'-UUCUCCGAACGUGUCACGUTT-3',反义5'-ACGUGACACGUUCGGAGAATT-3'。PTEN siRNA序列:正义5'-AACCCACCACAGCUAGAACTT-3';反义5'-AAGUUC UAGCUGUGGUGGGTT-3',对照siRNA序列正义5'-UUCUCCGAACGUGUCACGUTT-3';反义5'-ACGUGACACGUUCGGAGAATT-3';其余试剂为国产分析纯。
1.2 方法 1.2.1 细胞培养MCF-7、LM-MCF-7和MDA-MB-231细胞采用含10%胎牛血清和100 u/ml青霉素,100 μg/ml硫酸链霉素的RPMI 1640培养液,37℃、5% CO2条件下培养。
1.2.2 RT-PCR检测NDRG2和PTEN表达以及pCMV-NDRG2和pCMV-PTEN表达载体构建用Qiagen公司小量总RNA提取试剂盒提取MCF-7和LM-MCF-7细胞总RNA,利用天根公司QuantScript RT试剂盒合成cDNA。检测NDRG2和PTEN mRNA表达水平的PCR引物为:NDRG2扩增引物:正义5'-ATGGCGGAGCTGCAGGAGGTGC-3'和反义5'-TGAGGAACGAGGTCTGGGTGGG-3';PTEN扩增引物:正义5'-AGTTCCCTCAGCCGTTACCT-3'和反义5'-ATTTGACGGC-TCCTCTACTG-3';内参GAPDH扩增引物:正义5'-GCCTTCCGTGTCCCCACTGC-3'和反义5'-CAATGCCAGCCCCAGCGTCA-3'。PCR反应条件为:94℃ 30 s,52℃ 40 s, 72℃ 1 min, 72℃延伸7 min,共30个循环。
根据NDRG2(GENBANK:NM_201535.1)mRNA CDS序列设计引物:上游加入EcoRⅠ酶切位点,下游加入SalⅠ酶切位点。上、下游引物序列分别为:5'-GAATTCATGGCGGAGCTGCAGGAGGTG-3',5'-GTCGACTCAACAGGAG-ACCTCCATGGT-3'。以反转录的cDNA为模板。用上述引物PCR扩增,获得目的基因片段(PCR反应参数:94℃变性5 min;94℃变性30 s,55℃退火30 s,72℃延伸90 s,共20个循环,72℃延伸7 min)。
根据PTEN(GENBANK:NM_000314.6)mRNA CDS序列设计引物:上游加入BamHⅠ酶切位点,下游加入HindⅢ酶切位点。上、下游引物序列分别为:5’-GGATCCATGGCAGCCATCATCAAAGAGATCG-3’和5’-AAGCTTT-CAGACTTTTGTAATTTGTGTATGCTG-3’。PCR反应条件如下:94℃预变性5 min,94℃ 1 min,52℃ 1 min,72℃ 3 min,20个循环,72℃延伸10 min。
扩增产物经EcoRⅠ/SalⅠ、BamHⅠ/HindⅢ双酶切纯化后,与pCMV重组,转化DH5α菌。提取转化菌质粒,经双酶切和DNA测序分析,对NDRG2和PTEN序列进行鉴定,得到重组真核表达载体pCMV-NDRG2和pCMV-PTEN。
1.2.3 细胞转染将培养至覆盖率达80%的MCF-7、LM-MCF-7和MDA-MB-231细胞用于转染实验。将8 μg质粒(或80 nmol/L siRNA)和24 μl Lipofectamine 2000分别加入到500 μl的OMEM培养液中,室温静置5 min,然后将两者混匀并于37℃静置15 min。随后将质粒(或siRNA)-Lipofectamine 2000-OMEM混合物加入细胞中,37℃孵育6 h,随后换为无抗生素的完全培养液,继续培养72 h后进行免疫印迹分析或Transwell细胞迁移实验。
1.2.4 Transwell细胞迁移实验收获转染后的细胞,以PBS洗2次,用无血清培养液重悬。取0.2 ml细胞悬液(2×105个每毫升)加入上室,将含10%胎牛血清的完全培养液600 μl加入24孔板下室,每组设3个复孔。在37℃、5%CO2条件下培养24 h。取出上室,擦去膜上面未穿过膜的细胞,无水乙醇固定10 min,随后用0.1%结晶紫染色20 min。在显微镜下随机选取10个视野计数,以穿膜细胞的数目代表乳腺癌细胞的迁移能力。
1.2.5 免疫印迹分析收获正常培养的细胞和转染后的细胞,用预冷的PBS洗细胞2次,随后加入细胞裂解液(10 mmol/L Tris-HCl; pH8.0, 0.1 mmol/L EDTA; 150 mmol/L NaCl; 1% NP-40; 1 mmol/LPMSF; 1% SDS;蛋白酶抑制剂)冰上放置20 min,4℃,12 000 g离心20 min,收集上清定量分析,取30 μg总蛋白进行12% SDS-PAGE电泳,随后电转印至PVDF膜上,5%脱脂奶粉封闭2 h,加入相应一抗,室温孵育2 h,TBST洗膜后加入相应二抗室温孵育1 h,TBST洗膜后,应用增强型ECL显色试剂盒于暗室曝光显影,实验均重复3次。
1.3 统计学方法对本组研究的数据采用SPSS13.0统计软件进行分析。实验数据采用(x±s)表示,组间比较采用t检验;P≤0.05为差异有统计学意义。
2 结果 2.1 NDRG2和PTEN表达同乳腺癌细胞迁移能力负相关为探明NDRG2和PTEN表达与乳腺癌细胞迁移的关系,检测了低转移能力的MCF-7细胞和其具有高转移能力的LM-MCF-7亚克隆细胞[13]以及MDA-MB-231细胞中NDRG2和PTEN mRNA和蛋白表达差异。RT-PCR结果显示MCF-7细胞中NDRG2 mRNA水平显著高于LM-MCF-7和MDA-MB-231细胞(P=0.0078和P=0.017),并且PTEN mRNA水平高于LM-MCF-7和MDA-MB-231细胞(P=0.033和P=0.006),见图 1A;蛋白免疫印迹结果显示低转移的MCF-7细胞中NDRG2的蛋白表达水平显著高于LM-MCF-7和MDA-MB-231细胞(P=0.022和P=0.039),且PTEN表达水平也显著高于LM-MCF-7和MDA-MB-231细胞(P=0.041和P=0.037),见图 1B。结果说明NDRG2和PTEN的表达同乳腺癌细胞迁移能力呈负相关。
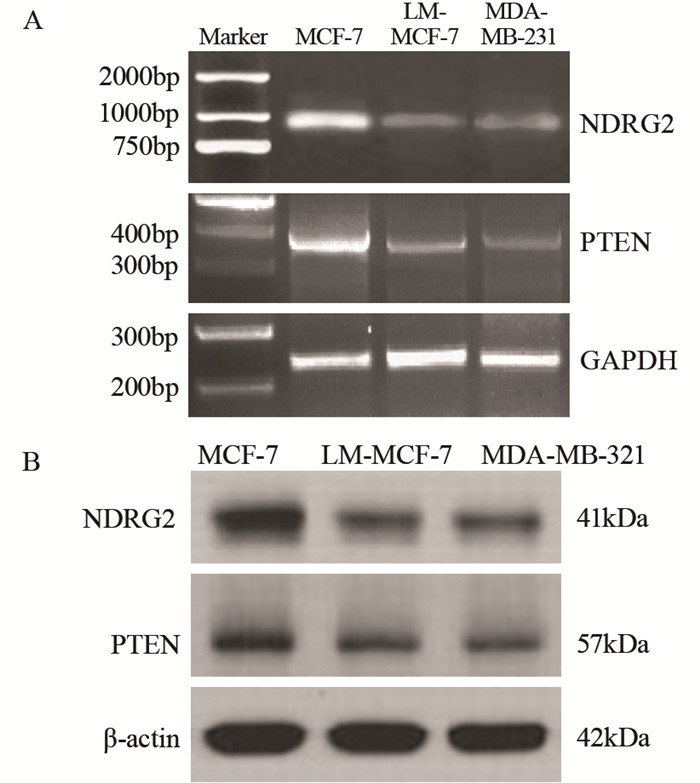
|
| 图 1 RT-PCR (A)和Western blot (B)法检测MCF-7、LM-MCF-7和MDA-MB-231细胞中NDRG2和PTEN的mRNA和蛋白表达水平 Figure 1 mRNA and protein levels of NDRG2 and PTEN in MCF-7, LM-MCF-7 and MDA-MB-231 cells examined via RT-PCR (A) and Western blot (B) |
利用pCMV-tag2B载体分别构建了NDRG2和PTEN的真核表达载体。提取MCF-7细胞总RNA,并反转录cDNA。利用NDRG2和PTEN引物PCR克隆NDRG2和PTEN编码基因,并通过EcoRⅠ/SalⅠ和BamHⅠ/HindⅢ酶切位点克隆至pCMV-tag2B载体上,构建pCMV-NDRG2,pCMV-PTEN表达载体。插入序列测序结果正确。pCMV-NDRG2和pCMV-PTEN双酶切鉴定结果见图 2。
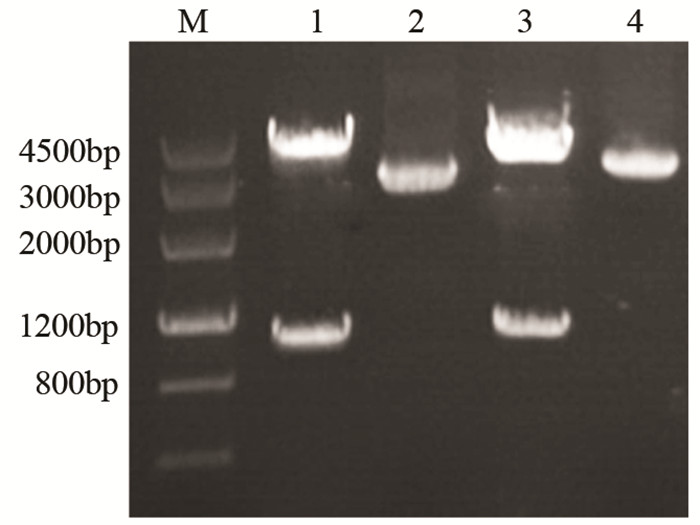
|
| 图 2 双酶切鉴定pCMV-NDRG2和pCMV-PTEN质粒 Figure 2 pCMV-NDRG2 and pCMV-PTEN plasmid digested by EcoRⅠ/SalⅠand BamHⅠ/HindⅢ respectively |
为明确NDRG2和PTEN在乳腺癌细胞中的调控关系,利用转染的方法改变NDRG2的表达水平,并通过免疫印迹检测PTEN蛋白和mRNA水平表达改变。MCF-7细胞中NDRG2表达水平相对较高,通过转染NDRG2 siRNA,沉默NDRG2表达。RT-PCR和Western blot检测结果显示,降低NDRG2的表达可下调PTEN mRNA水平和蛋白表达,见图 3A;LM-MCF-7和MDA-MB231细胞中NDRG2表达水平较MCF-7细胞低,通过转染细胞pCMV-NDRG2质粒,过表达NDRG2。RT-PCR和Western blot检测PTEN表达,结果显示提高NDRG2表达可上调PTEN mRNA和蛋白表达水平,见图 3B~3C。结果说明PTEN为NDRG2的下游调控因子,参与调控乳腺癌细胞迁移。
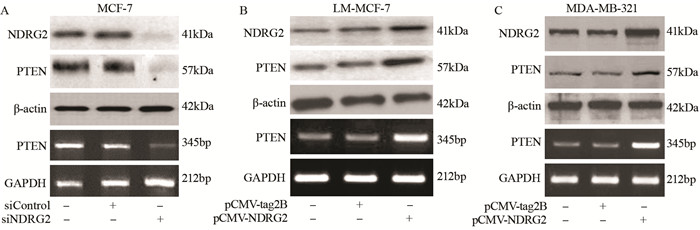
|
| 图 3 Western blot和RT-PCR法检测干扰NDRG2表达的MCF-7细胞(A)和过表达NDRG2的LM-MCF-7细胞(B)和MDA-MB-231细胞(C)中的PTEN mRNA和蛋白表达水平 Figure 3 mRNA and protein expression levels of PTEN detected by RT-PCR and Western bot after transfecting MCF-7 cells with NDRG2 siRNA (A), or transfecting LM-MCF-7(B) and MDA-MB-231(C) cells with pCMV-NDRG2 |
为明确NDRG2/PTEN通路在调控乳腺癌细胞迁移中的作用,转染MCF-7细胞NDRG2 siRNA,沉默NDRG2表达,Transwell实验结果显示,MCF-7细胞迁移能力增强至(354.62±38.02)%,与对照组1比较差异具有统计学意义(P=0.0031);共转染MCF-7细胞NDRG2 siRNA和pCMV-PTEN,细胞迁移能力下降至(139.51±49.13)%,同对照组1相比差异无有统计学意义(P=0.73),与实验组3比较差异有统计学意义(P=0.0079),见图 4A。转染LM-MCF-7细胞pCMV-NDRG2,上调NDRG2表达水平,细胞迁移能力下降至(13.02±4.31)%,与对照组1相比差异有统计学意义(P=0.0032);共转染LM-MCF-7细胞PTEN siRNA和pCMV-NDRG2,Transwell实验显示LM-MCF-7细胞迁移能力恢复至未转染的(78.33±10.07)%,与对照组1和实验组3相比差异有统计学意义(P=0.048和P=0.0082),见图 4B。随后我们在MDA-MB-231中进一步验证了以上结果,发现在MDA-MB-231细胞中过表达NDRG2,细胞迁移能力下降至(26.33±5.29)%,同对照组1相比差异有统计学意义(P=0.0077);过表达NDRG2的同时沉默PTEN的表达,MDA-MB-231细胞迁移能力恢复至未转染的(42.79±19.62)%,同对照组1和对照组3相比差异均具有统计学意义(P=0.018和P=0.042),见图 4C。以上结果说明,PTEN作为NDRG2下游重要的调控因子,参与NDRG2抑制乳腺癌细胞迁移。PTEN/NDRG2信号途径的沉默是乳腺癌细胞获得高迁移能力的重要分子机制。
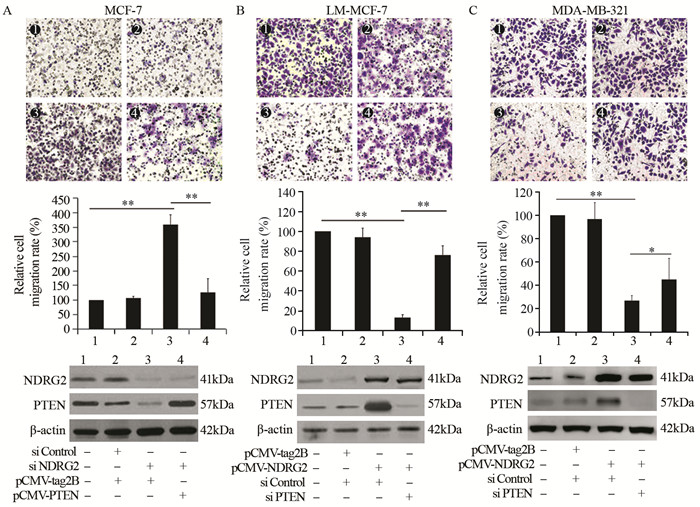
|
| A: the migration ability of MCF-7 cells was examined by Transwell migration assay; 1: untransfected MCF-7 cells; 2: MCF-7 cells co-transfected with Control siRNA/pCMV-tag2B; 3: MCF-7 cells co-transfected with NDRG2 siRNA/pCMV-tag2B (**: P=0.0031, compared with group 1); 4: MCF-7 cells co-transfected with NDRG2 siRNA/pCMV-PTEN (**: P=0.0079, compared with group 3); B: the migration ability of LM-MCF-7 cells was examined by Transwell migration assay; 1: untransfected LM-MCF-7 cells; 2: LM-MCF-7 cells co-transfected with Control siRNA/pCMV-tag2B; 3: LM-MCF-7 cells co-transfected with Control siRNA/pCMV-NDRG2 (**: P=0.0032, compared with group 1); 4: LM-MCF-7 cells co-transfected with PTEN siRNA/pCMV-NDRG2 (**: P=0.0082, compared with group 3); C: the migration ability of MDA-MB-231 cells was examined by Transwell migration assay; 1: untransfected MDA-MB-231 cells; 2: MDA-MB-231 cells co-transfected with Control siRNA/pCMV-tag2B; 3: MDA-MB-231 cells co-transfected with Control siRNA/pCMV-NDRG2 (**: P=0.0077, compared with group 1); 4: MDA-MB-231 cells co-transfected with PTEN siRNA/pCMV-NDRG2 (*: P=0.042, compared with group 3) 图 4 NDRG2通过调控PTEN表达抑制乳腺癌细胞迁移 Figure 4 NDRG2 inhibited cell migration via regulating PTEN expression |
乳腺癌细胞获得高迁移能力是乳腺癌转移的必备条件。因此深入研究乳腺癌细胞迁移的分子调控机制对肿瘤治疗具有重要价值。本研究利用具有不同转移能力的乳腺癌细胞MCF-7、LM-MCF-7和MDA-MB-231,对NDRG2调控乳腺癌细胞迁移的作用及机制进行了研究。结果发现肿瘤细胞中NDRG2的mRNA水平及蛋白表达水平与细胞迁移能力呈负相关,提高NDRG2的表达可抑制乳腺癌细胞的迁移。Ma等在研究乳腺癌的转移和浸润中发现NDRG2可抑制血管内皮生长因子VEGF以及低氧诱导因子HIF-1α的表达,进而抑制乳腺肿瘤的增殖和血管生成,抑制肿瘤转移[14]。肿瘤细胞中CD24的表达与NDRG2关系紧密,NDRG2能够通过下调CD24的表达,降低肿瘤细胞中由整合素介导的细胞黏附能力,进而抑制肿瘤细胞的迁移和侵袭能力[15-17]。基质金属蛋白酶(MMPs)能降解细胞外基质中的基质成分,破坏肿瘤细胞侵袭的组织学屏障,在肿瘤侵袭转移中起关键性作用。研究发现NDRG2与MMP家族中的MMP-2和MMP-9关系密切。NDRG2可通过诱导骨形成蛋白4抑制MMP-9活性,以及抑制ERK1/2信号通路活性,抑制癌细胞转移[18-19]。NDRG2还可通过NF-κB信号途径调控MMP-9和MMP-2,抑制肿瘤细胞的迁移和侵袭行为[20]。上述研究结果说明NDRG2作为重要的蛋白因子,可通过多种途径参与抑制肿瘤细胞迁移和侵袭。
PTEN是具有脂质磷酸酶和蛋白磷酸酶双重活性的抑癌基因,与人类多种肿瘤的发生发展密切相关。它的突变或表达沉默会使肿瘤细胞获得较强的抗凋亡和迁移能[21]。本研究发现PTEN表达受NDRG2的表达调控,在乳腺癌细胞中NDRG2可通过上调PTEN的表达抑制细胞迁移。PTEN作为抑癌基因,参与调控细胞内多种信号转导通路,包括负调控肿瘤细胞生长,诱导肿瘤细胞凋亡,抑制肿瘤细胞侵袭及转移[22]。PTEN可通过抑制FAK的磷酸化功能,阻断FAK/P130信号,进而影响肌动蛋白骨架重构以及黏着斑复合体的形成,抑制肿瘤细胞迁移[23];还可通过阻断FAK抑制Ras-MAPK通路,从而抑制肿瘤细胞的转移和黏附[24]。PTEN还能通过PI3K/Akt信号途径调控MMP蛋白活性,抑制肿瘤细胞的浸润、转移[25]。肿瘤血管新生是肿瘤细胞侵袭及远处转移的基础。PTEN与VEGF及其受体相互作用,参与调控血管新生,并抑制了多种肿瘤的侵袭和转移[26]。
本研究发现NDRG2/PTEN信号途径沉默是乳腺癌获得高迁移能力的重要分子机制,对阐明乳腺癌后期转移的机制以及对乳腺癌的诊断及治疗具有重要参考价值。
| [1] | Jin X, Mu P. Targeting Breast Cancer Metastasis[J]. Breast Cancer (Auckl), 2015, 9 (Suppl 1) : 23–34. |
| [2] | Hu W, Fan C, Jiang P, et al. Emerging role of N-myc downstream-regulated gene 2 (NDRG2) in cancer[J]. Oncotarget, 2016, 7 (1) : 209–23. |
| [3] | Lorentzen A, Lewinsky RH, Bornholdt J, et al. Expression profile of the N-myc Downstream Regulated Gene 2 (NDRG2) in human cancers with focus on breast cancer[J]. BMC Cancer, 2011, 11 : 14. DOI:10.1186/1471-2407-11-14 |
| [4] | Mordalska A, Latek J, Ferenc T, et al. Evaluation of NDRG2 gene expression in primary papillary thyroid carcinoma and in metastases of this neoplasm to regional lymph nodes[J]. Thyroid Res, 2010, 3 (1) : 6. DOI:10.1186/1756-6614-3-6 |
| [5] | Park MY, Choi SC, Lee HS, et al. A quantitative analysis of N-myc downstream regulated gene 2 (NDRG 2) in human tissues and cell lysates by reverse-phase protein microarray[J]. Clin Chim Acta, 2008, 387 (1-2) : 84–9. DOI:10.1016/j.cca.2007.09.010 |
| [6] | Piepoli A, Cotugno R, Merla G, et al. Promoter methylation correlates with reduced NDRG2 expression in advanced colon tumour[J]. BMC Med Genomics, 2009, 2 : 11. DOI:10.1186/1755-8794-2-11 |
| [7] | Shi H, Li N, Li S, et al. Expression of NDRG2 in esophageal squamous cell carcinoma[J]. Cancer Sci, 2010, 101 (5) : 1292–9. DOI:10.1111/cas.2010.101.issue-5 |
| [8] | Skiriute D, Tamasauskas S, Asmoniene V, et al. Tumor grade-related NDRG2 gene expression in primary and recurrent intracranial meningiomas[J]. J Neurooncol, 2011, 102 (1) : 89–94. DOI:10.1007/s11060-010-0291-9 |
| [9] | Golestan A MSc, Mojtahedi Z PhD, Ghalamfarsa G PhD, et al. The Effects of NDRG2 Overexpression on Cell Proliferation and Invasiveness of SW48 Colorectal Cancer Cell Line[J]. Iran J Med Sci, 2015, 40 (5) : 430–9. |
| [10] | Kim YJ, Yoon SY, Kim JT, et al. NDRG2 suppresses cell proliferation through down-regulation of AP-1 activity in human colon carcinoma cells[J]. Int J Cancer, 2009, 124 (1) : 7–15. DOI:10.1002/ijc.v124:1 |
| [11] | Cao W, Zhang JL, Feng DY, et al. The effect of adenovirus-conjugated NDRG2 on p53-mediated apoptosis of hepatocarcinoma cells through attenuation of nucleotide excision repair capacity[J]. Biomaterials, 2014, 35 (3) : 993–1003. DOI:10.1016/j.biomaterials.2013.09.096 |
| [12] | Ma J, Liu W, Guo H, et al. N-myc downstream-regulated gene 2 expression is associated with glucose transport and correlated with prognosis in breast carcinoma[J]. Breast Cancer Res, 2014, 16 (2) : R27. |
| [13] | Zhou X, Liu Y, You J, et al. Myosin light-chain kinase contributes to the proliferation and migration of breast cancer cells through cross-talk with activated ERK1/2[J]. Cancer Lett, 2008, 270 (2) : 312–27. DOI:10.1016/j.canlet.2008.05.028 |
| [14] | Ma J, Liu W, Yan X, et al. Inhibition of endothelial cell proliferation and tumor angiogenesis by up-regulating NDRG2 expression in breast cancer cells[J]. PLoS One, 2012, 7 (2) : e32368. DOI:10.1371/journal.pone.0032368 |
| [15] | Zheng J, Liu Q, Li Y, et al. NDRG2 expression regulates CD24 and metastatic potential of breast cancer cells[J]. Asian Pac J Cancer Prev, 2010, 11 (6) : 1817–21. |
| [16] | Baumann P, Thiele W, Cremers N, et al. CD24 interacts with and promotes the activity of c-src within lipid rafts in breast cancer cells, thereby increasing integrin-dependent adhesion[J]. Cell Mol Life Sci, 2012, 69 (3) : 435–48. DOI:10.1007/s00018-011-0756-9 |
| [17] | Zheng J, Li Y, Yang J, et al. NDRG2 inhibits hepatocellular carcinoma adhesion, migration and invasion by regulating CD24 expression[J]. BMC Cancer, 2011, 11: 251 : 1–9. |
| [18] | Guo Y, Ma J, Wu L, et al. Hyperthermia-induced NDRG2 upregulation inhibits the invasion of human hepatocellular carcinoma via suppressing ERK1/2 signaling pathway[J]. PLoS One, 2013, 8 (4) : e61079. DOI:10.1371/journal.pone.0061079 |
| [19] | Shon SK, Kim A, Kim JY, et al. Bone morphogenetic protein-4 induced by NDRG2 expression inhibits MMP-9 activity in breast cancer cells[J]. Biochem Biophys Res Commun, 2009, 385 (2) : 198–203. DOI:10.1016/j.bbrc.2009.05.038 |
| [20] | Kim A, Kim MJ, Yang Y, et al. Suppression of NF-kappaB activity by NDRG2 expression attenuates the invasive potential of highly malignant tumor cells[J]. Carcinogenesis, 2009, 30 (6) : 927–36. DOI:10.1093/carcin/bgp072 |
| [21] | Wang Y, Dai B. PTEN genomic deletion defines favorable prognostic biomarkers in localized prostate cancer: a systematic review and meta-analysis[J]. Int J Clin Exp Med, 2015, 8 (4) : 5430–7. |
| [22] | Xu W, Yang Z, Zhou SF, et al. Posttranslational regulation of phosphatase and tensin homolog (PTEN) and its functional impact on cancer behaviors[J]. Drug Des Devel Ther, 2014, 8 : 1745–51. |
| [23] | Zhang LL, Liu J, Lei S, et al. PTEN inhibits the invasion and metastasis of gastric cancer via downregulation of FAK expression[J]. Cell Signal, 2014, 26 (5) : 1011–20. DOI:10.1016/j.cellsig.2014.01.025 |
| [24] | Ebbesen SH, Scaltriti M, Bialucha CU, et al. Pten loss promotes MAPK pathway dependency in HER2/neu breast carcinomas[J]. Proc Natl Acad Sci U S A, 2016, 113 (11) : 3030–5. DOI:10.1073/pnas.1523693113 |
| [25] | Tian T, Nan KJ, Guo H, et al. PTEN inhibits the migration and invasion of HepG2 cells by coordinately decreasing MMP expression via the PI3K/Akt pathway[J]. Oncol Rep, 2010, 23 (6) : 1593–600. |
| [26] | Zhu L, Loo WT, Louis WC. PTEN and VEGF: possible predictors for sentinel lymph node micro-metastasis in breast cancer[J]. Biomed Pharmacother, 2007, 61 (9) : 558–61. DOI:10.1016/j.biopha.2007.08.015 |
 2017, Vol. 44
2017, Vol. 44
