文章信息
- 组蛋白去乙酰化酶抑制剂SAHA抑制乳腺癌细胞MDA-MB-435增殖及促凋亡的作用机制
- Effect of SAHA on Proliferation and Apoptosis of Breast Cancer Cells MDA-MB-435 and Related Mechanism
- 肿瘤防治研究, 2016, 43(12): 1023-1029
- Cancer Research on Prevention and Treatment, 2016, 43(12): 1023-1029
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2016.12.003
- 收稿日期: 2016-04-20
- 修回日期: 2016-05-23
2. 510006 广州,中山大学药学院微生物与生化药学实验室
2. Department of Microbial and Biochemical Pharmacy, School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou 510006, China
三阴性乳腺癌(TNBC)是一种缺乏雌激素受体、孕激素受体和人表皮生长因子Ⅱ型受体的一类乳腺癌亚型,占乳腺癌患者总数的15%~20%,较非三阴性乳腺癌愈后和存活率更差,目前为止TNBC仍无有效的靶向药物[1-3]。组蛋白乙酰化作用在肿瘤进程中广泛分布,组蛋白去乙酰化酶抑制剂(HDACi)在抑制多种肿瘤的增殖、分化、凋亡、自噬、免疫调节中发挥着显著的作用,逐渐被临床认可[4-7]。辛二酰基酰替苯胺异羟肟酸(suberoylanilide hydroxamic acid, SAHA)是首个被美国FDA批准临床使用的HDACi,作为一种有前景的抗肿瘤药物正在被进行广泛且深入的机制研究[8]。本实验以TNBC细胞株MDA-MB-435作为研究对象,探讨SAHA对TNBC的可能作用及其相关分子机制。
1 材料与方法 1.1 细胞株人乳腺癌MDA-MB-435细胞购自ATCC公司(美国马里兰州)。
1.2 主要试剂DMEM培养液(Hyclone公司,南美);胎牛血清(WISENT公司,南美);异羟肟酸(Selleck公司,美国);JC-1(线粒体膜电位ΔΨm荧光探针)、RAC1(ROS阻滞剂)、碘化丙啶(PI)、噻唑蓝(MTT)、cyclinA1(Cat# ab53699)和cyclinB(Cat# ab32053) (Abcam公司,英国);bax(Cat#AB026)、bcl-2(Cat#AB112)和GAPDH(Cat#AG019)抗体(碧云天生物技术公司,中国);磷酸化P38抗体p-P38(Cat#4511S)、P38(Cat#8690S)、磷酸化ERK抗体p-ERK(Cat#75165)、磷酸化JNK抗体p-JNK(Cat#4688T)、JNK(Cat#9258S)、磷酸化P53抗体p-P53(Cat#9285T)和P53(Cat#9282T)(CST公司,美国);反转录试剂盒(PrimeScriptTM RT Reagent Kit Cat#RR037A Lot#AK5302)和SYBR荧光染料(Premix Ex TaqTM Ⅱ Cat#RR820A Lot#AK6702)(TaKaRa公司,日本);BAY11-7082(S2913)、SB203580(S1076)、SP600125(S1460)、LY294002(S1105)和PD98059(S1177) (Selleck公司,美国)。
1.3 细胞培养MDA-MB-435细胞贴壁生长于含10%无支原体无胎牛血清的DMEM培养液中,在37℃、5%CO2饱和湿度下培养,每隔1至2天换液或传代,取对数生长期细胞进行实验。
1.4 MTT法检测细胞增殖胰酶消化对数期细胞,终止后离心收集,制成5×104/ml细胞悬液,每孔各取100 μl加入96孔板。条件培养至细胞贴壁后加入相应浓度SAHA培养液继续培养24 h,再每孔加入20 μl的MTT溶液,4 h后吸去培养液,每孔加入150 μl DMSO,混匀15 min后用490 nm波长测量吸光度值(OD)。实验重复三次。
1.5 流式细胞术检测细胞周期胰酶消化加药处理细胞,2 000 r/min离心5 min后加入75%乙醇固定过夜。次日用PBS清洗细胞两次,加入0.1 μl/ml PI避光染色15 min后用流式细胞仪检测细胞周期。
1.6 流式细胞术检测细胞凋亡细胞处理方法同1.3。加药培养24 h后用PBS洗涤细胞一次,加入1 μl/ml JC-1工作液常温孵育20 min。吸弃上清液,PBS洗涤后用荧光显微镜拍照,胰酶消化后再用PBS洗涤一次,流式细胞仪检测。
1.7 流式细胞术检测活性氧(reactive oxygen species, ROS)水平细胞处理方法同1.3,加药培养24 h后用胰酶消化细胞,PBS洗涤后加入0.8 μl/ml DCFH-DA避光染色30 min,PBS再次洗涤后用流式细胞仪检测ROS;第二次处理细胞前加入15 μmol/ml RAC1,再进行如上操作。
1.8 Western blot检测蛋白表达细胞加药处理24 h后冰上裂解提取蛋白,离心定量变性后应用10%和12%的SDS-PAGE分离胶进行电泳(80~120V),电转(200 mA 1.5 h)操作后5%BSA封闭薄膜,根据相应蛋白大小裁剪条带,装入含有相应抗体的管中,4℃摇床上孵育过夜。次日用PBST(1×PBS加入1:1000吐温-20)清洗两次,每次10 min,用相应的二抗孵育2 h,再用PBST清洗三次,每次10 min。取出条带放置曝光盒中,加入蛋白底物荧光发光液,盖上胶片30 min后依次用显影液和定影液洗涤。
1.9 荧光定量PCR检测基因表达加药处理细胞24 h后,用TRIzol液裂解细胞提取RNA,应用TaKaRa反转录试剂盒反转录成cDNA后,用含有荧光标记的引物进行RT-PCR操作。引物按照Primer premier5软件设计,上海捷瑞生物工程公司合成。引物序列如下,TP53-F:5’-GTTGGTCGGTGGGTTGGTAGTTT-3’;TP53-R:5’-GGTGTGGGATGGGGTGAGATTT-3’。GAPDH-F:5’-CGGAGTCAACGGATTTGGTCGTAT-3’; GAPDH-R:5’-AGCCTTCTCCATGGTGGTGAAGAC-3’。应用TaKaRa 10 μl体系,加入SYBR作为荧光染料。PCR反应程序:预扩增94℃ 2 min;92℃ 20 s,56℃ 30 s,72℃ 45 s,共30 个循环;末次延伸72℃ 7 min。
1.10 统计学方法所有试验结果均由三次重复实验得出,应用SPSS17.0分析软件,配对t检验进行数据分析,P<0.05为差异有统计学意义。荧光定量PCR计算目的基因在MDA-MB-435细胞系中的相对表达量,每个样品的PCR反应均重复3次,取平均值。对照组ΔΔCt=对照组测定基因Ct值-(对照组平均Ct值-内参平均Ct值),其他组Ct值=测定基因Ct值-(对照组平均Ct值-内参平均Ct值)。
2 结果 2.1 SAHA对MDA-MB-435细胞的增殖抑制作用0.1~9 μmol/L浓度的SAHA处理细胞24、48 h后的细胞增殖情况,见图 1。结果表明SAHA可显著抑制MDA-MB-435细胞的增殖,且随着药物浓度增大及时间延长,抑制效应越显著。不同浓度SAHA处理细胞组与阴性对照组相比,差异均具有统计学意义(P<0.05)。SAHA对MDA-MB-435细胞24及48 h的IC50分别为5.9 μmol/L和4.1 μmol/L。
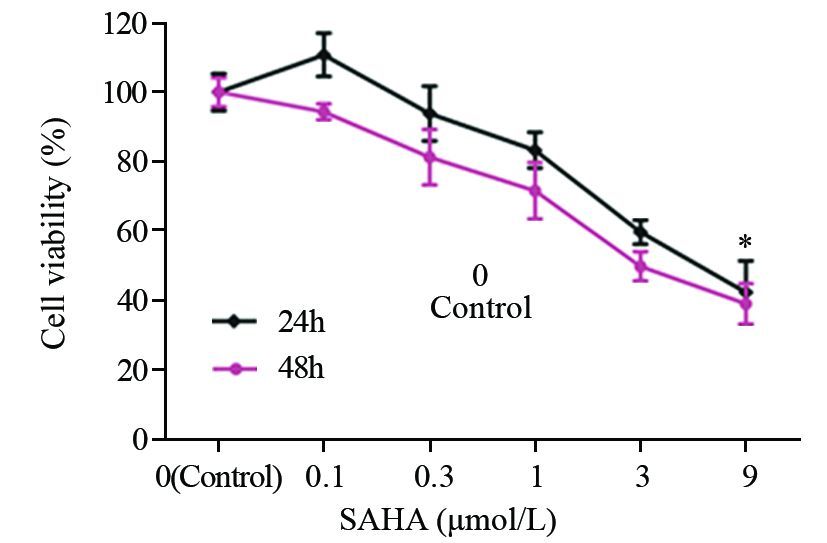
|
| MDA-MB-435 cells were treated with increasing concentrations of SAHA(0.1-9μmol/L) for 24h, and then the cell viability was assessed by MTT method. With the increasing dose of SAHA, the proliferation of MDA-MB-435 was obviously inhibited. The quantitation results of data represented the average of three independent experiments; *: P<0.05, compared with control gorup 图 1 SAHA对MDA-MB-435细胞增殖的影响 Figure 1 Effects of SAHA on proliferation of MDA-MB-435 cells |
利用流式细胞仪观察1~5 μmol/LSAHA处理细胞24 h后,MDA-MB-435细胞G2/M期及S期的比例降低,G0/G1及亚G1期细胞的比例增高,见图 2。
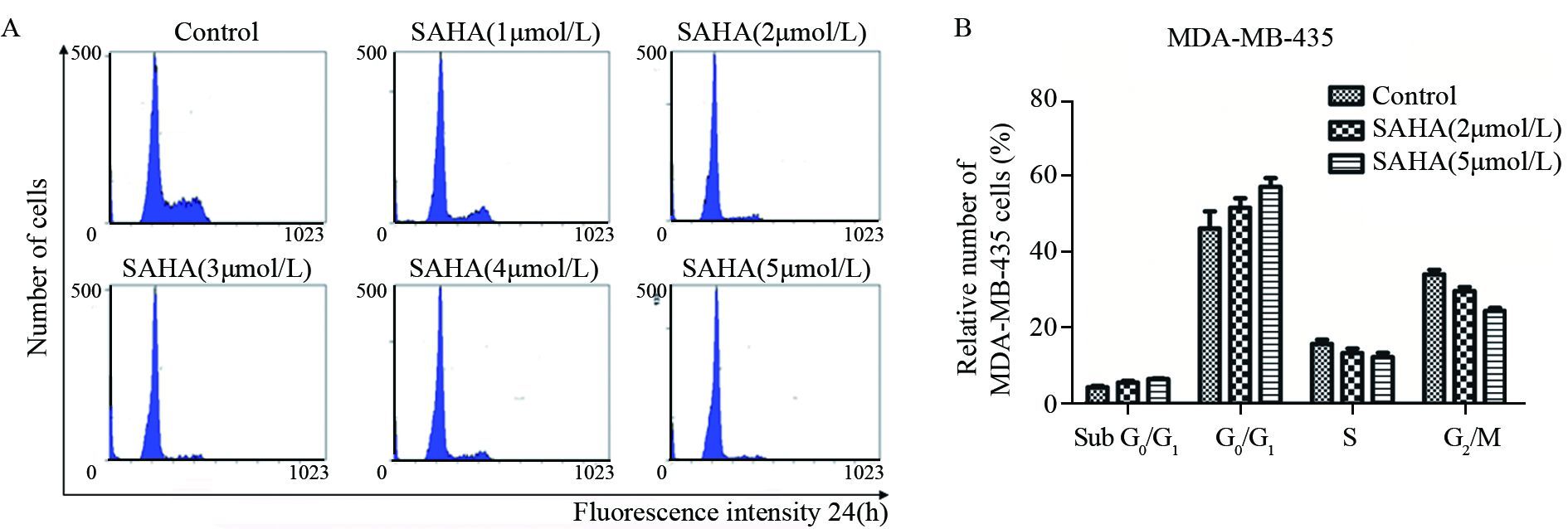
|
| MDA-MB-435 cells were synchronized at the G2/M transition by a double TdR block, and then treated with different doses of SAHA(1-5μmol/L) for 24h. The cell cycles were analyzed by FCM; A: flow cytometry graph of MDA-MD-435 cells with different doses of SAHA; B: the cell cycle cycle ratio of MDA-MB-435 cells with different doses of SAHA 图 2 SAHA对MDA-MB-435细胞周期的影响 Figure 2 Effects of SAHA on cell cycle of MDA-MB-435 cells |
荧光显微镜下可见,SAHA处理后的细胞JC-1由红色向绿色转变增多,表明膜电位下降。流式细胞仪分析结果表明SAHA可导致MDA-MB-435细胞膜电位剂量依赖型下调,见图 3。说明细胞发生了凋亡。
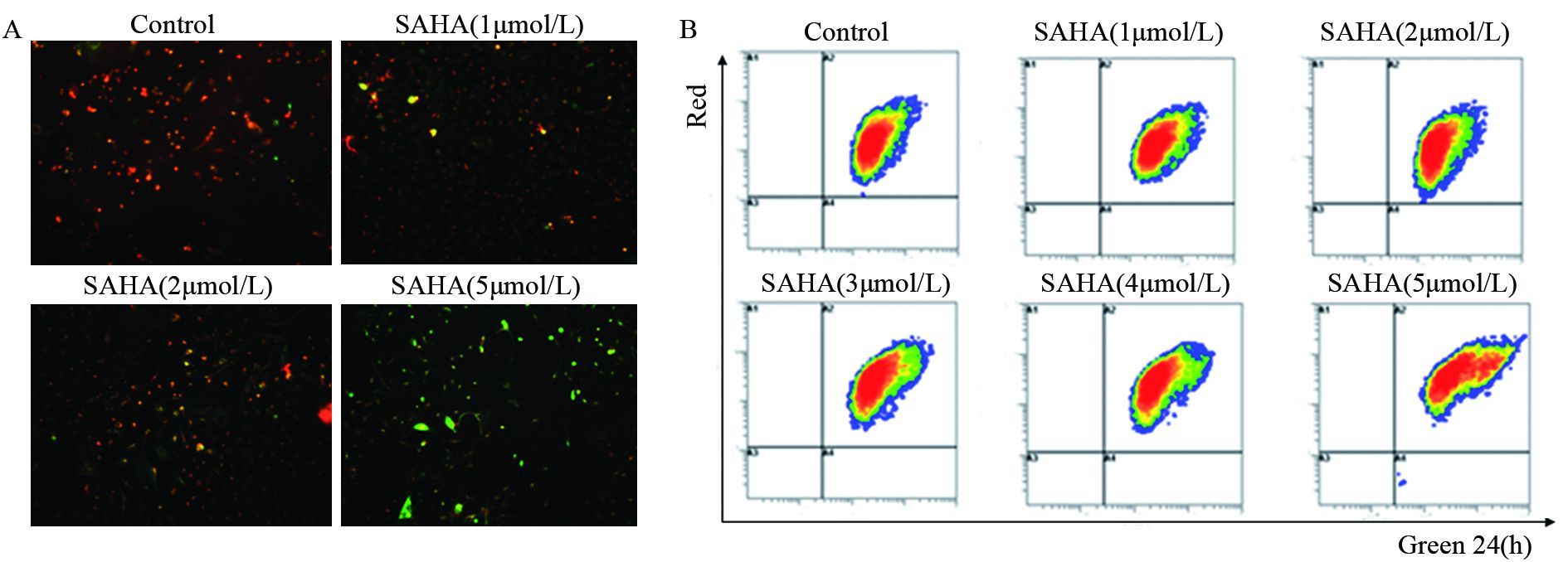
|
| MDA-MB-435 cells were treated with different doses of SAHA(1-5 μmol/L) as the indicated concentrations for 24h, and then JC-1, the mitochondria-specific dye, was added to measure the membrane polarity (ΔΨm) and cell apoptosis. Apoptotic cells mainly showed as green fluorescence, while healthy cells showed as red fluorescence; A: MDA-MB-435 cells under fluorescence microscope with different doses of SAHA; B: flow cytometry scatter plot of MDA-MB-436 cells with different doses of SAHA 图 3 JC-1检测SAHA对MDA-MB-435线粒体膜电位的影响 Figure 3 Effect of SAHA on mitochondrial membrane potentials in MDA-MB-435 cells detected by JC-1 |
流式细胞仪检测结果表明SAHA可剂量依赖性上调MDA-MB-435细胞内的ROS水平。细胞加入15 μmol/L Nac1后再加入SAHA则不会引起ROS的上调,表明SAHA可以上调MDA-MB-435细胞的活性氧水平,且这种上调可被Nac1阻断,见图 4。
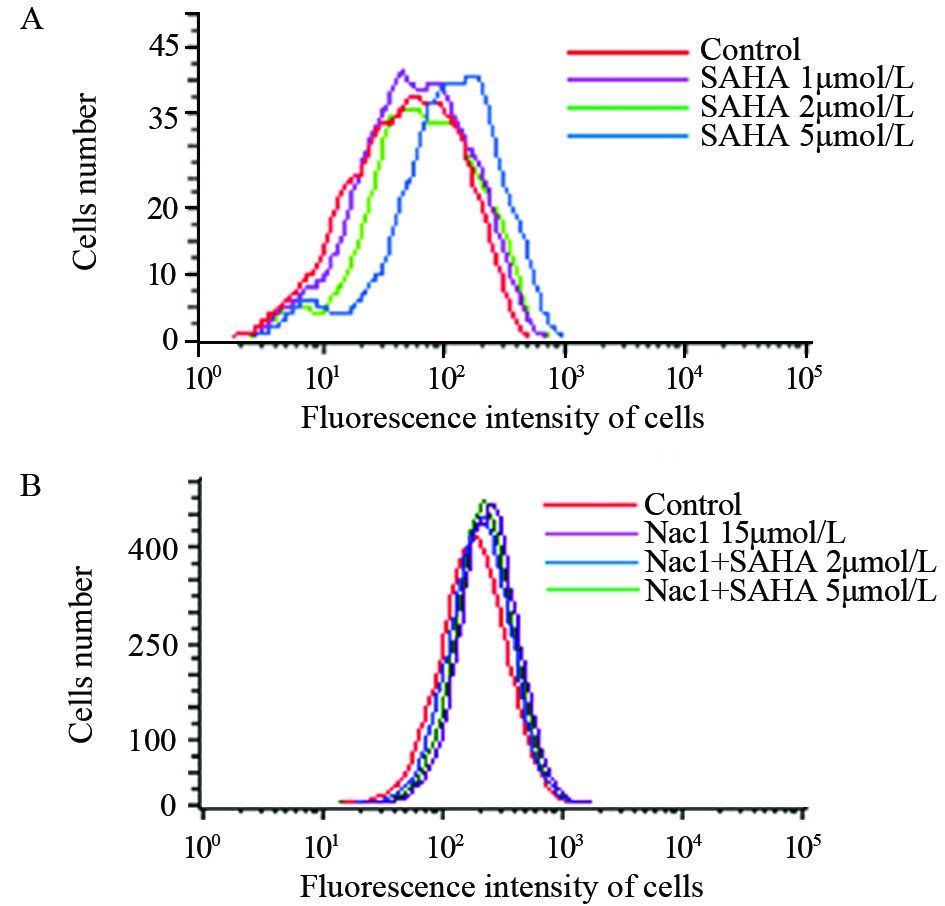
|
| A: MDA-MB-435 cells were treated with different doses of SAHA(1, 2, 5μmol/L) for 24h, then added 0.8μl/ml DCFH-DA with 30mins in the dark, then ROS were tested by flow cytometry; B: MDA-MB-435 cells were treated with 15μmol/L Nac1 and SAHA for 24h, then ROS were tested by flow cytometry as above 图 4 SAHA对MDA-MB-435细胞ROS水平的影响 Figure 4 Effects of SAHA on ROS generation of MDA-MB-435 cells |
不同浓度(1~5 μmol/L)SAHA处理24 h后可抑制周期蛋白cyclin A及cyclin B的表达,并下调Bcl-2的表达,促进Bax的蛋白表达。表明SAHA可通过诱导线粒体途径的凋亡抑制MDA-MB-435细胞增殖,见图 5。

|
| MDA-MB-435 cells were treated with different doses of SAHA(1-5μmol/L) as the indicated concentrations for 24h, then Bcl-2, Bax, cyclinA, cyclin B, and GAPDH protein expression levels were analyzed by Western blot. Data were presented as means±s of three independent experiments 图 5 SAHA对凋亡蛋白表达的影响 Figure 5 Effects of SAHA on apoptosis protein expression |
SAHA处理MDA-MB-435细胞24 h后可显著增加P38及JNK的磷酸化,表明SAHA可能通过活化P38及JNK来抑制细胞增殖,见图 6A。进一步选择五种常见通路抑制剂SB203580(P38-MAPK通路抑制剂)、LY294002(PI3K通路抑制剂)、PD98059(ERK1/2通路抑制剂)、BAY117082(NF-κB通路抑制剂)和SP600125(JNK1/2/3通路抑制剂)进行验证,见图 6B。结果显示加入抑制剂后并不能明显改变细胞增殖情况,证明可能与这些通路无关。
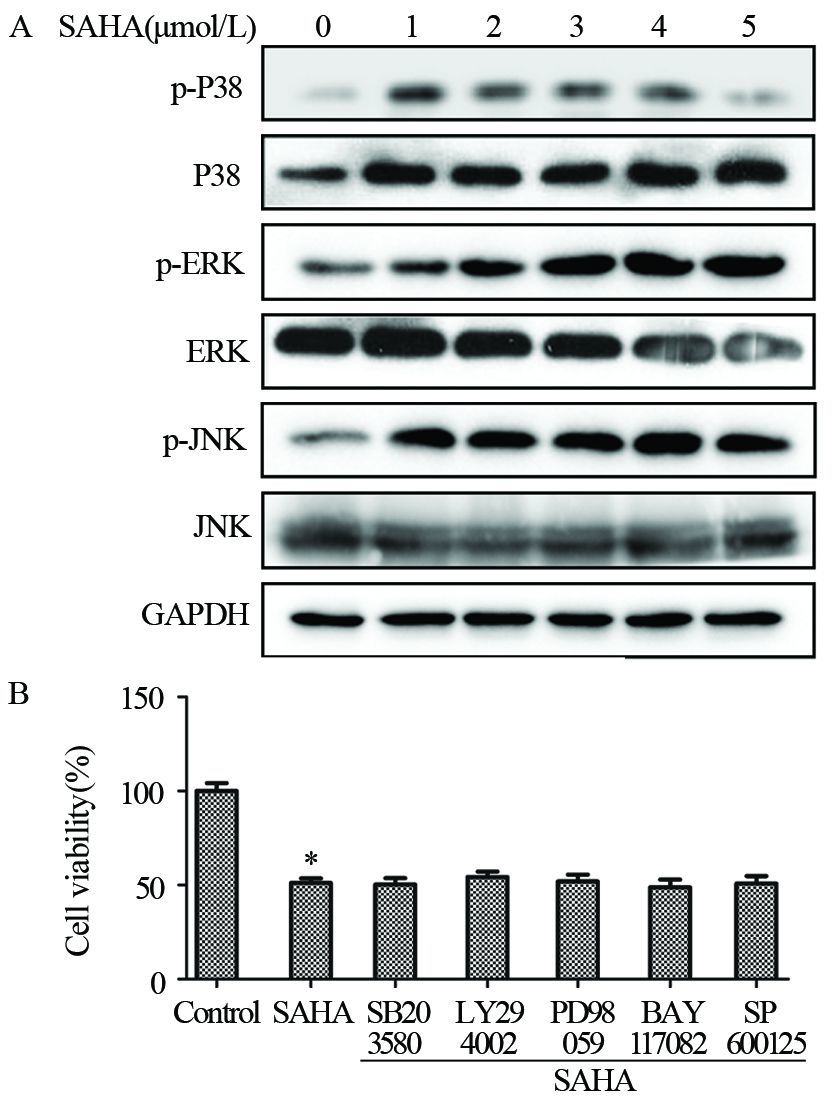
|
| A: MDA-MB-435 cells were treated with different doses of SAHA(1-5μmol/L) as the indicated concentrations for 24h, and then p-P38, P38, p-ERK, ERK, p-JNK, JNK and GAPDH protein expression levels were analyzed by Western blot; Data were presented as x±s of three independent experiments. B: MDA-MB-435 cells were pretreated with 10 μmol/L ERK1/2 inhibitor PD98059(PD), P38 MAPK inhibitor SB203580 (SB), PI3K inhibitor LY294002 (LY), NF-κB inhibitor BAY 117028 (BAY), and JNK1/2/3 pathway inhibitor SP600125 for 90 min, and then exposed to 2mol/L SAHA for further 24h. MTT assays showed the slight change in the cells; *: P<0.05, compared with control group 图 6 SAHA对相关MAPK蛋白的影响 Figure 6 Effects of SAHA on MAPK protein expression |
Western blot结果表明SAHA可抑制P53的总蛋白及其磷酸化水平的表达,荧光定量PCR结果发现SAHA可抑制P53的转录。表明SAHA可能通过抑制P53从而抑制MDA-MB-435细胞增殖,见图 7A~7B。选择五种常见通路抑制剂SB203580(P38 MAPK通路抑制剂)、LY294002(PI3K通路抑制剂)、PD98059(ERK1/2通路抑制剂)、BAY117082(NF-κB通路抑制剂)和SP600125(JNK1/2/3通路抑制剂)验证抑制通路后SAHA是否会逆转MDA-MB-435中P53的下调情况,结果呈现阴性,见图 7C。
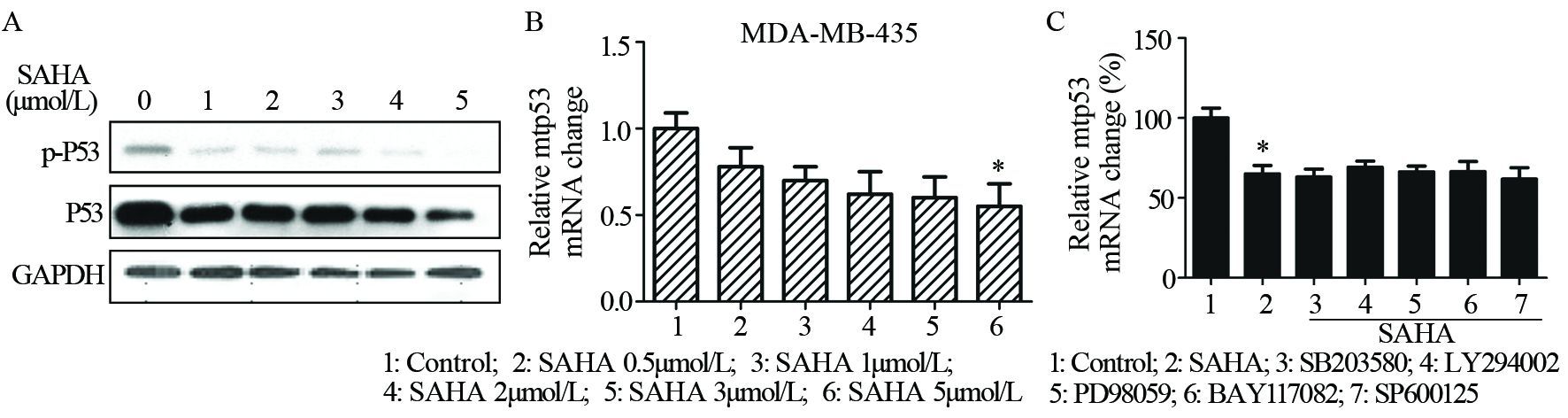
|
| A: MDA-MB-435 cells were treated with different doses of SAHA(1-5μmol/L) for 24h, and then p-P53, P53 and GAPDH protein expression levels were analyzed by Western blot; B: MDA-MB-435 cells were treated with different doses of SAHA(1-5μmol/L) for 24h, and then the mRNA levels of P53 were measured by RT-PCR. Data represented the average of three independent experiments. *: P<0.05, compared with control group; C: MDA-MB-435 cells were pretreated with 10μmol/L ERK1/2 inhibitor PD98059(PD), p38 MAPK inhibitor SB203580 (SB), PI3K inhibitor LY294002 (LY), NF-κB inhibitor BAY 11-7028 (BAY), and JNK1/2/3 pathway inhibitor SP600125 for 90min, and then exposed to 2μmol/L SAHA for further 24h. P53 mRNA level was measured by RT-PCR and the result was negative.Data were presented as x±s of three independent experiments 图 7 SAHA对MDA-MB-435细胞变异P53 mRNA的表达影响 Figure 7 Effects of SAHA on P53 mRNA expression in MDA-MB-435 cells |
目前有关TNBC的治疗研究进展涉及多个方面:(1)放疗疗法和乳房切除术仍是极有争议的治疗方法。(2)靶向DNA修复复合体,如修复P53和细胞增殖复合体。(3)新辅助化学疗法作用于TNBC细胞上有更高的反应性。(4)用蒽环霉素和紫杉醇进行特殊辅助疗法。(5)应用诸如聚腺苷二磷酸核糖聚合酶-1、mTOR以及去乙酰化酶抑制剂等。由于不易接受内分泌疗法(三苯氯胺和芳香酶抑制剂)以及缺乏靶向药物(曲妥珠单抗和拉帕替尼)且受多种临床因素的制约,直至目前为止治疗TNBC仍无有效的靶向药物[4]。
目前科研界对于HDACi的研究已较为深入,主要集中在HDACi抑制肿瘤细胞克隆形成、阻滞细胞周期和抑制生长速率、促进细胞分化或自噬以及激活免疫系统反应方面。但肿瘤细胞种类繁多,HDACi也分为Ⅰ、Ⅱ、Ⅲ、Ⅳ型,不同类型HDACi药物作用于不同类型肿瘤细胞的机制也不尽相同,尚缺乏此方面的具体研究[10]。TNBC作为存活率较低的癌症已被作为热门的研究对象,且TNBC细胞属于P53高度变异类型的细胞,对于HDACi是否会下调TNBC细胞变异型P53的研究同样缺乏[11]。
本研究的前期实验证实了SAHA也能对部分TNBC细胞(如MDA-MB-231和BT549)起到同样抑制增殖、阻滞细胞周期及促进凋亡的作用[12],而SAHA药物对于MDA-MB-435增殖凋亡机制等研究目前仍比较缺乏。本实验验证了SAHA对于MDA-MB-435的增殖抑制和G2/M周期阻滞作用、及可以上调G1及Sub-G1期细胞比例,并从凋亡蛋白的表达、线粒体膜电位的变化、ROS的变化、突变型P53的改变和通路机制的初步探讨五方面验证了促凋亡作用,发现MDA-MB-435细胞与MDA-MB-231及BT549细胞[12]一样,可在SAHA作用下发生细胞周期阻滞,细胞增殖受抑、细胞凋亡被促,突变型P53下调及部分MAPKs通路蛋白受到影响。但两者促凋亡及抑制增殖机制未必相同,比如MDA-MB-231在SAHA作用下的促ROS生成能力较弱,且ERK、P38、JNK等蛋白变化情况不全然一致,推测SAHA作用细胞的机制也许不同。
通过本实验,可以得知2 μmol/L浓度以上的SAHA作用于MDA-MB-435细胞时能抑制细胞增殖,轻微阻滞细胞周期。Bcl-2的浓度相关性下调,Bax的浓度相关性上调,JC-1实验中绿光随SAHA浓度的加大而趋势性增加,ROS的浓度相关性上调都是SAHA诱导MDA-MB-435细胞早期凋亡的力证[13]。JC-1用于检测细胞的线粒体膜电位的变化,如果细胞发生早期凋亡,线粒体膜电位会随之发生改变,导致红色荧光减少而绿光荧光增加,此数据可以作为SAHA诱导MDA-MB-435细胞产生早期凋亡的佐证;在众多肿瘤细胞中已证实,部分HDACi可以增加ROS的生成并上调Caspase以促进细胞凋亡[14]。加入SAHA24 h后,ROS显著增加(P<0.05)。且ROS对于凋亡的作用已被证实与Bcl-2的减少和Bax的增加有关[15-16],可以作为下一步的研究对象。关于MDA-MB-435细胞MAPKs通路相关蛋白的变化,SAHA确实能在磷酸化和蛋白表达方面起作用;ROS的上升常常会激活JNK通路,实验也显示磷酸化的JNK被显著激活并且有一定程度的ERK协同性失活[17]。SAHA激活磷酸化的ERK1/2,降低ERK的表达从而影响细胞增殖。同时,SAHA能显著激活MDA-MB-435细胞的P38-MAPK信号通路,在一定剂量范围内起到正调控的作用,同时增加P38的磷酸化蛋白和蛋白的表达水平,HDACi的抗肿瘤活性也常常与P38通路的激活相关,而该现象同样受制于细胞的种类,结合前期结果及本实验可以证实在TNBC中HDACi与P38通路是正相关的[18]。另外加入SAHA后,MDA-MB-435细胞中突变型P53的表达不论是mRNA水平和蛋白水平(包括磷酸化水平)均显著下降。P53作为一种常见的抑癌基因对肿瘤的发生发展起抑制作用,当P53突变后抑制作用随即消失,甚至转变为促肿瘤作用。在乳腺癌中,P53多为突变型P53,P53的下调证明细胞中的突变型P53有所下调。HDACi会乙酰化P53,此现象可作为一个靶点进行深入的机制分析,究竟这种下降是不是ROS独立型的也有待进一步验证。虽然在三阴性乳腺癌中P53多为突变型,但本文中仍沿用P53的说法。通过通路抑制剂实验,证明SAHA对MDA-MB-435的作用可能不是通过PI3K通路、P38 MAPK通路、ERK1/2通路、NF-κB和JNK通路起作用。修复TNBC细胞内变异型P53已成为一个肿瘤治疗的热点,突变型P53的下降对肿瘤生长有利或有害,是直接作用还是间接作用都有待进一步研究[19-20]。在Champion ChIP Transcription Factor Search Portal软件公司的网站上预测P53启动子结合位点显示HOXA5、YY1、LUN-1、NF-κB等转录因子可上调P53的转录水平,P53的下调是否由转录因子的乙酰化导致或是否是转录因子与P53共结合能力的减弱导致的都需要进一步的验证。TNBC及HDACi作为两个研究的热点,各类HDACi对于MDA-MB-435细胞的作用仍不明晰,下一步实验将继续探讨SAHA对MDA-MB-435的作用究竟是哪个通路或蛋白发挥了重要作用。
本研究进一步证实了SAHA确实可以起到抑制MDA-MB-435细胞增殖、促进其凋亡并促使P53下调的作用,并且排除了部分的通路影响,证实SAHA可以作为治疗TNBC的潜在药物。
| [1] | Bose S. Triple-negative Breast Carcinoma: Morphologic and Molecular Subtypes[J]. Adv Anat Pathol, 2015, 22 (5) : 306–13. DOI:10.1097/PAP.0000000000000084 |
| [2] | Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence[J]. Clin Cancer Res, 2007, 13 (15) : 4429–34. DOI:10.1158/1078-0432.CCR-06-3045 |
| [3] | Wahba H A, El-Hadaad HA. Current approaches in treatment of triple-negative breast cancer[J]. Cancer Biol Med, 2015, 12 (2) : 106. |
| [4] | Barbetti V, Gozzini A, Cheloni G, et al. Time-and residue-specific differences in histone acetylation induced by VPA and SAHA in AML1/ETO-positive leukemia cells[J]. Epigenetics, 2013, 8 (2) : 210–9. DOI:10.4161/epi.23538 |
| [5] | Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer[J]. Nat Rev Cancer, 2006, 6 (1) : 38–51. DOI:10.1038/nrc1779 |
| [6] | Drummond DC, Noble CO, Kirpotin DB, et al. Clinical development of histone deacetylase inhibitors as anticancer agents[J]. Annu Rev Pharmacol Toxicol, 2005, 45 : 495–528. DOI:10.1146/annurev.pharmtox.45.120403.095825 |
| [7] | Slingerland M, Guchelaar H J, Gelderblom H. Histone deacetylase inhibitors: an overview of the clinical studies in solid tumors[J]. Anti-Cancer Drug, 2014, 25 (2) : 140–9. DOI:10.1097/CAD.0000000000000040 |
| [8] | Dickinson M, Johnstone RW, Prince HM. Histone deacetylase inhibitors: potential targets responsible for their anti-cancer effect[J]. Invest New Drug, 2010, 28 (1) : 3–20. |
| [9] | Huang L, Pardee AB. Suberoylanilide hydroxamic acid as a potential therapeutic agent for human breast cancer treatment[J]. Mol Med, 2000, 6 (10) : 849. |
| [10] | Marks PA, Xu WS. Histone deacetylase inhibitors: Potential in cancer therapy[J]. J Cell Biochem, 2009, 107 (4) : 600–8. DOI:10.1002/jcb.v107:4 |
| [11] | Li D, Marchenko ND, Moll UM. SAHA shows preferential cytotoxicity in mutant p53 cancer cells by destabilizing mutant p53 through inhibition of the HDAC6-Hsp90 chaperone axis[J]. Cell Death Differ, 2011, 18 (12) : 1904–13. DOI:10.1038/cdd.2011.71 |
| [12] | Wang ZT, Chen ZJ, Jiang GM, et al. Histone deacetylase inhibitors suppress mutant p53 transcription via HDAC8/YY1 signals in triple negative breast cancer cells[J]. Cell Signal, 2016, 28 (5) : 506–15. DOI:10.1016/j.cellsig.2016.02.006 |
| [13] | Lee JS, Jeong SH, Soung YH, et al. SAHA treatment overcomes the anti-apoptotic effects of Bcl-2 and is associated with the formation of mature PML nuclear bodies in human leukemic U937 cells[J]. Chem-Biol Interact, 2009, 181 (1) : 61–70. DOI:10.1016/j.cbi.2009.02.007 |
| [14] | Kim BM, Chung HW. Hypoxia/reoxygenation induces apoptosis through a ROS-mediated caspase-8/Bid/Bax pathway in human lymphocytes[J]. Biochem Bioph Res Co, 2007, 363 (3) : 745–50. DOI:10.1016/j.bbrc.2007.09.024 |
| [15] | Arbab IA, Looi CY, Abdul AB, et al. Dentatin induces apoptosis in prostate cancer cells via Bcl-2, Bcl-xL, Survivin downregulation, caspase-9,-3/7 activation, and NF-κB inhibition[J]. Evid Based Complement Alternat Med, 2012, 2012 : 856029. |
| [16] | Hagenbuchner J, Ausserlechner MJ. Mitochondria and FOXO3: breath or die[J]. Front Physiol, 2013 : 4. |
| [17] | Yu C, Subler M, Rahmani M, et al. Induction of apoptosis in BCR/ABL+ cells by histone deacetylase inhibitors involves reciprocal effects on the RAF/MEK/ERK and JNK pathways[J]. Cancer Biol Ther, 2003, 2 (5) : 544–51. DOI:10.4161/cbt.2.5.454 |
| [18] | Portanova P, Russo T, Pellerito O, et al. The role of oxidative stress in apoptosis induced by the histone deacetylase inhibitor suberoylanilide hydroxamic acid in human colon adenocarcinoma HT-29 cells[J]. Int J Oncol, 2008, 33 (2) : 325–31. |
| [19] | Dai C, Gu W. p53 post-translational modification: deregulated in tumorigenesis[J]. Trends Mol Med, 2010, 16 (11) : 528–36. DOI:10.1016/j.molmed.2010.09.002 |
| [20] | Hui L, Zheng Y, Yan Y, et al. Mutant p53 in MDA-MB-231 breast cancer cells is stabilized by elevated phospholipase D activity and contributes to survival signals generated by phospholipase D[J]. Oncogene, 2006, 25 (55) : 7305–10. DOI:10.1038/sj.onc.1209735 |
 2016, Vol. 43
2016, Vol. 43


