文章信息
- 酸性环境抑制CIK细胞对肝癌HepG2细胞的杀伤活性
- Inhibition Effect of Acid Environment on Cytotoxicity of Cytokine-induced Killer Cells Against Hepatocellular Carcinoma Cells HepG2
- 肿瘤防治研究, 2016, 43(5): 331-334
- Cancer Research on Prevention and Treatment, 2016, 43(5): 331-334
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2016.05.003
- 收稿日期: 2015-07-18
- 修回日期: 2016-01-04
近年来CIK(cytokine-induced killer,CIK)细胞疗法成为新一代抗肿瘤过继细胞免疫治疗的首选方案[1]。为了提高CIK的抗瘤效应,研究者采用了多种方法,包括基因转染CIK细胞[2]、与抗瘤药物联合应用[3]、用特殊的细胞因子刺激[4]以及将其与基因修饰过的DC细胞联合应用[5]等方法,取得了一定进展。但大多数研究关注于CIK本身活性的提高,而忽略了CIK发生作用的肿瘤微环境这个重要因素。有研究发现[6],实体肿瘤细胞有酸性的细胞外pH值,多为pH6.2~6.9,而正常细胞例如CIK细胞都适宜在微偏碱性的条件下(pH7.3~7.4)生存。因此,本研究将在体外探索酸性微环境对CIK细胞抗肝癌细胞HepG2活性的影响,为进一步提高CIK的应用效率提供新的思路。
1 材料与方法 1.1 材料HepG2细胞系购于中国典型培养物保藏中心(武汉大学保藏中心),荧光素酶标记的HepG2细胞(HepG2-luc)稳转株由本实验室构建。DMEM培养液、RPMI 1640培养液及FBS购于美国Hyclone公司。细胞因子IFN-γ、IL-2及CD3抗体购于美国R&D公司。MTT液和Annexin V/PI检测试剂盒购于碧云天公司。小动物活体成像系统(IVIS Spectrum System)由美国Caliper Life Sciences公司生产。
1.2 方法 1.2.1 肝癌HepG2细胞的培养野生型的HepG2细胞及荧光素酶标记的HepG2细胞(HepG2-luc)均用含10%FBS的DMEM完全培养液常规培养。为了后续实验的一致性,将HepG2细胞及HepG2-luc细胞均在pH6.5和pH7.4条件下适应性培养三代后使用。培养液的pH值用乳酸、NaHCO3和Hepes综合调定,用pH计检测pH值。
1.2.2 CIK细胞的获得参照文献[3],用Ficoll密度梯度离心法获得外周血单个核细胞(PBMC)。将PBMC用含10%FBS的RPMI 1640培养液重悬,调整细胞密度为(1~2)×106/ml,培养液中添加 IFN-γ,保证其终浓度为1 000 u/ml。24 h后,培养液中添加IL-2和CD3抗体,浓度分别为500 u/ml和50 ng/ml。之后,每3天用含IL-2的培养液半量换液,保证IL-2的浓度为500 u/ml。
1.2.3 酸性和碱性条件下CIK细胞对HepG2细胞的杀伤活性的检测(1)小鼠活体成像系统(IVIS Spectrum System)检测HepG2-luc细胞荧光强度。 将HepG2-luc细胞按每孔8 000个细胞的密度接种入96孔板中,再将CIK细胞按效靶比0:1、10:1、20:1、40:1及80:1与HepG2-luc细胞混合培养。将混合培养体系培养液pH值分别设为6.5和7.4。在培养0和24 h时,用小动物活体成像系统检测在酸性和碱性环境下、在不同效靶比的CIK的作用下,存活的HepG2-luc细胞的荧光强度。并根据公式CIK cytotoxicity (%) = [(LA0:1-LAx:1)/LA0:1] ×100% ,计算CIK细胞杀伤活性,其中LA表示荧光强度(luminescence activity,LA),LAx:1表示不同效靶比作用下的荧光强度。(2)MTT法检测CIK细胞的杀伤活性。参照前述方法,将CIK细胞和HepG2细胞按10:1,20:1,40:1,80:1效靶比共培养,培养条件分别为pH6.5和pH7.4,效应细胞和靶细胞单独培养作为对照,每种情况设5个复孔。24 h后,参照文献[7],用MTT法检测CIK细胞对HepG2细胞的杀伤活性。计算公式为Cytotoxic activity (%) = [1 − (ODE+T − ODE) / ODT] ×100%,其中OD为酶标仪检测的吸光度值,E表示效应细胞,T表示靶细胞。
1.2.4 酸性和碱性条件下CIK细胞条件培养液对HepG2细胞的杀伤活性检测收集CIK细胞传代后再培养24 h的培养液,作为CIK细胞条件培养液(CIK condition medium,CMCIK)。收集HepG2细胞传代后再培养24 h的培养液,作为对照条件培养液(CMcontrol)。将HepG2细胞按每孔3×105的密度接种入12孔板,培养液分别为CMcontrol中含CMCIK 0、50%及100%。培养液的pH值分别调为6.5和7.4。培养48 h后,收集各孔细胞,离心洗涤,弃上清液后,用PBS重悬,标记Annexin V-FITC和PI,4℃ 避光孵育20 min 后,流式细胞仪检测凋亡和坏死的细胞百分比。实验重复三次。
1.3 统计学方法利用SPSS13.0统计软件检验各组数据间的方差齐性,选用相应的t检验进行统计分析,P<0.05表示差异有统计学意义。
2 结果 2.1 酸性环境对CIK杀伤HepG2细胞活性的抑制作用小动物活体成像系统显示,培养24 h 后,效靶比10:1、20:1、40:1及80:1情况下,pH7.4培养条件下的荧光强度均弱于pH6.5,见图 1A。荧光强度值统计显示,在效靶比20:1、40:1及80:1情况下这种差别更明显,见图 1B。根据荧光强度值及方法中所述公式计算杀伤活性显示,在pH7.4时,效靶比为10:1、20:1、40:1和80:1情况下的HepG2-luc细胞死亡率分别为(19±2.1)%,(38±1.4)%,(60±1.9)% 和(80±3.2)%;而在pH6.5时,相对应的值仅为(4±1.1)%,(18±3.1)%,(30±1.3)% 和(45±4.6)%,见图 1C,表明酸性环境下CIK细胞对HepG2细胞的杀伤活性明显低于碱性环境(P<0.05)。而MTT法与荧光强度分析所得到的结果是一致的,见图 1D。
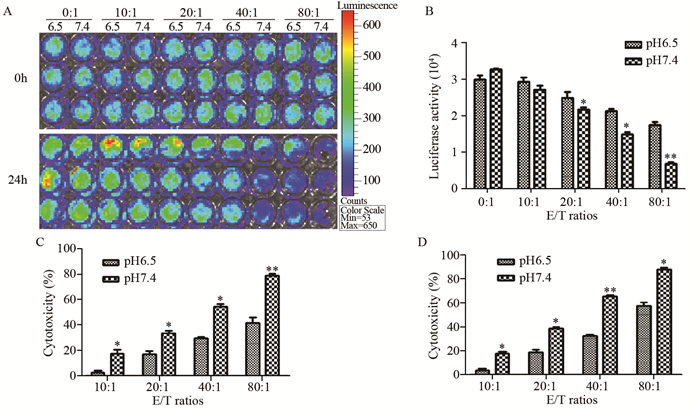
|
| A: the luciferase activity of HepG2-luc cells were measured with IVIS Spectrum System; B: the quantitative analysis of luciferase activity of HepG2-luc cells in pH6.5 medium and pH7.4 medium; C: the cytotoxic activity were calculated based on the luciferase activity; D: the cytotoxic activity was assessed with MTT assay; *: P<0.05, **: P<0.01, compared with pH6.5 condition 图 1 酸性和碱性条件下CIK细胞对HepG2细胞抗瘤活性的影响 Figure 1 Cytotoxicity of CIK cells against HepG2 cells in acid and alkaline condition |
2.2 酸性环境明显影响CIK条件培养液对HepG2细胞的抑制作用
CIK条件培养液作用48 h,HepG2细胞Annexin V-FITC和PI双染后,流式细胞仪检测显示,pH值7.4时,CMCIK为50%和100%条件下,Annexin V阳性和PI阳性细胞总的百分比分别为26%和45%;而在pH6.5时,相对应的值仅为15%和25%,见图 2A。三次实验的统计分析亦显示,pH7.4条件下凋亡坏死细胞比例明显高于pH6.5条件下(P<0.05),见图 2B。
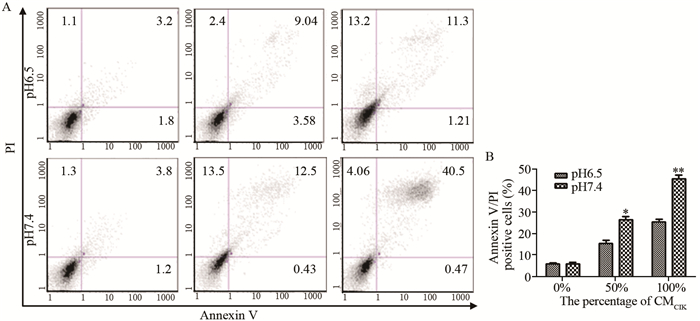
|
| A: the percentage of apoptosis and necrotic HepG2 cells were analyzed by flow cytometry. B: quantitative analysis of the sum of positive Annexin V and PI HepG2 cells; *: P<0.05, **: P<0.01, compared with pH6.5 condition 图 2 酸性和碱性条件下CIK细胞条件培养液对HepG2细胞抗瘤活性的影响 Figure 2 Cytotoxicity of CIK medium against HepG2 cells in acid and alkaline condition |
3 讨论
研究表明,酸性环境可能通过抑制免疫功能而促进肿瘤的生长。Severin等[8]报道,酸性环境抑制人LAK(lymphokine-activated killer,LAK)细胞的细胞毒活性;Loeffler等[9]发现小鼠NK(natural killer,NK)细胞在酸性环境下活性降低;Redegeld等[10]研究表明,CTL(cytotoxic T-lymphocytes,CTL)在酸性环境下溶解肿瘤细胞的能力降低。由于CIK是一群异质细胞,其主要成分是NK细胞及CD8+T淋巴细胞。参照这些报道,我们推测肿瘤酸性微环境有可能抑制CIK的抗瘤效应是比较合理的,近些年来虽然CIK应用于临床非常普遍,却没有人关注到肿瘤的酸性微环境对其的影响。而了解这些内容至关重要,本研究结果说明临床应用CIK进行治疗的过程中对肿瘤微环境的忽略将大大影响CIK的治疗效果。
CIK细胞杀伤肿瘤的机制可以归结如下:(1)CIK细胞通过释放对细胞具有毒性作用的胞质毒性颗粒物,发挥对靶细胞的直接杀伤作用。(2)CIK细胞可以释放大量炎性细胞因子,直接或间接的抑制和杀伤肿瘤细胞。(3)CIK细胞能活化并高表达肿瘤细胞凋亡基因,从而诱导肿瘤细胞凋亡。(4)CIK细胞能促进宿主内T细胞增殖活化。因此,本研究对CIK细胞及其条件培养液在酸性和碱性环境下的抗瘤活性均进行了检测,结果表明酸性环境明显抑制了CIK细胞及其条件培养液的抗瘤活性,与pH7.4相比,分别降低约35%和20%。由此可见,中和或降低实体肿瘤微环境的酸度,与CIK治疗本身同样重要。无论怎样提高CIK的活性、数量或其特异性,如果肿瘤的微环境不能得到适当调整,CIK将无法充分发挥作用。
因此,本研究为临床更高效应用CIK治疗肝癌提供了新的思路。临床应用CIK治疗肝癌,不仅仅要关注CIK本身、患者体质,肿瘤的微环境也应该是关注的重点。后续我们将分别检测CIK细胞和肿瘤细胞在酸性和碱性条件下相关的蛋白和基因的表达水平以及细胞因子分泌的量,以确定产生该现象的分子机制。
| [1] | Rosenberg SA, Restifo NP, Yang JC, et al. Adoptive cell transfer: a clinical path to effective cancer immunotherapy[J]. Nat Rev Cancer, 2008, 8 (4) : 299–308. |
| [2] | Voena C, Chiarle R. Advances in cancer immunology and cancer immunotherapy[J]. Discov Med, 2016, 21 (114) : 125–33. |
| [3] | Huang X, Chen YT, Song HZ, et al. Cisplatin pretreatment enhances anti-tumor activity of cytokine-induced killer cells[J]. World J Gastroenterol, 2011, 17 (25) : 3002–11. |
| [4] | Rettinger E, Meyer V, Kreyenberg H, et al. Cytotoxic capacity of IL-15- stimulated cytokine induced killer cells against human acute myeloid leukemia and rhabdomyosarcoma in humanized preclilical mouse models[J]. Frontiers Oncol, 2012, 2 : 32. |
| [5] | Wang D, Zhang B, Gao H, et al. Clinical research of genetically modified dendritic cells in combination with cytokine-induced killer cell treatment in advanced renal cancer[J]. BMC Cancer, 2014, 14 : 251. |
| [6] | Passaro D, Quang CT, Ghysdael J. Microenvironmental cues for T-cell acute lymphoblastic leukemia development[J]. Immunol Rev, 2016, 271 (1) : 156–72. |
| [7] | Hu S, Li B, Shen X, et al. Induction of antigen-specific cytotoxic T-cell response by dendritic cells generated from ecto-mesenchymal stem cells infected with an adenovirus containing the MAGE-D4a gene[J]. Oncol Lett, 2016, 11 (4) : 2886–92. |
| [8] | Severin T, Müller B, Giese G, et al. pH-dependent LAK cell cytotoxicity[J]. Tumour Biol, 1994, 15 (5) : 304–10. |
| [9] | van Ostaijen-Ten Dam MM, Prins HJ, Boerman GH, et al. Preparation of cytokine-activated NK cells for use in adoptive cell therapy in cancer patients: protocol optimization and therapeutic potential[J]. J Immunother, 2016, 39 (2) : 90–100. |
| [10] | Redegeld F, Filippini A, Sitkovsky M. Comparative studies of the cytotoxic T lymphocyte-mediated cytotoxicity and of extracellular ATP-induced cell lysis. Different requirements in extracellular Mg2+ and pH[J]. J Immunol, 1991, 147 (10) : 3638–45. |
 2016, Vol. 43
2016, Vol. 43


