文章信息
- mFOLFOX6和FOLFIRI方案分别一线和二线治疗晚期胃癌疗效对比观察
- Comparison of Clinical Effect Between mFOLFOX6 and FOLFIRI Regimen as Alternately First-and Second-line Therapy on Advanced Gastric Cancer Patients
- 肿瘤防治研究, 2017, 44(7): 489-492
- Cancer Research on Prevention and Treatment, 2017, 44(7): 489-492
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2017.17.0077
- 收稿日期: 2017-01-22
- 修回日期: 2017-04-27
中国每年胃癌新发病例占世界新发病例的40%以上,相比欧美地区较低的胃癌发病率及日韩地区较高的早期胃癌诊断率,我国超过80%的新发病例一经诊断已达局部进展期或晚期,这部分患者以全身化疗为主,临床研究证实以奥沙利铂或伊立替康为基础的联合化疗方案均可改善进展期和晚期胃癌患者的预后[1],但目前二者一线和二线治疗晚期胃癌优劣对比的临床研究较少,本文通过比较mFOLFOX6和FOLFIRI方案在晚期胃癌患者一线或二线治疗中的疗效和不良反应,探讨二者在晚期胃癌治疗中的价值。
1 资料与方法 1.1 一般资料收集2010年1月至2015年6月在北京大学深圳医院初诊的62例晚期胃癌患者,均经病理学和影像学证实,无合并其他恶性肿瘤及严重脏器疾病,全组男性35例,女性27例。年龄26~69岁,中位年龄45.4岁。胃底贲门癌21例、胃体癌10例、胃窦癌31例。病理类型:高分化腺癌9例、中分化腺癌10例、低分化腺癌32例、印戒细胞癌11例。HER2阳性6例。全部患者均有可评价病灶,可经CT或MRI检测病灶大小,ECOG评分≤2,预计生存期≥3月,治疗期间定期监测患者的肝肾功能、血象、心电图及心脏彩超等,患者均签署知情同意书。
1.2 方法 1.2.1 治疗方法所有患者治疗前均经过影像学检查评价病灶范围,并排除化疗相关禁忌证,HER2阳性患者未联合抗HER2治疗。62例患者分为两组,对照组32例患者,一线采用mFOLFOX6方案,疾病进展后采用FOLFIRI方案;观察组30例患者,一线采用FOLFIRI方案,疾病进展后采用mFOLFOX6方案。mFOLFOX6方案:奥沙利铂85 mg/m2,静脉滴注2 h,d1,亚叶酸钙0.4 g/m2,静脉滴注2 h, dl,5-Fu 0.4 g/m2,静脉推注,dl,5-Fu 1.2 g/m2,持续静脉滴注24 h dl~2, 14 d为1个周期;FOLFIRI方案:伊立替康180 mg/m2,静脉滴注90 min,d1,亚叶酸钙0.4 g/m2,静脉滴注2 h, dl,5-Fu 0.4 g/m2,静脉推注,dl,5-Fu 1.2 g/m2,持续静脉滴注24 h dl~2,14 d为1个周期。每2~3周期评价疗效。药物剂量可根据药物不良反应调整。患者疾病进展后可采用以紫杉类和阿帕替尼等为基础的治疗。有乙肝病史的患者同期口服拉米夫定或替比夫定治疗。治疗期间记录患者的不良反应。
1.2.2 评价指标疗效评价按RECIST标准分为完全缓解(CR)、部分缓解(PR)、疾病稳定(SD)、疾病进展(PD)。客观缓解率(ORR)=CR+PR。疾病进展时间PFS1为一线治疗开始至肿瘤进展的时间或末次随访时间,PFS2为二线治疗开始至肿瘤进展的时间或末次随访时间。总生存期(OS)为治疗开始至死亡的时间或末次随访时间。不良反应按WHO毒性分级标准评价。
1.3 统计学方法采用SPSS 16.0软件分析,临床疗效的比较采用χ2检验,不良反应的比较采用秩和检验,生存分析采用Kaplan-Meier、Log rank检验,P < 0.05为差异有统计学意义。
2 结果 2.1 近期疗效对照组一线平均治疗周期数9.1个,CR 1例,PR 11例,SD 9例,PD 11例,ORR为37.5%,疾病进展后有25例患者采用FOLFIRI方案治疗,平均周期数4.9个,CR 0例,PR 6例,SD 4例,PD 15例,ORR为24.0%,另外7例患者疾病进展后采用紫杉类药物或阿帕替尼等治疗。观察组一线平均周期数10.5个,CR 2例,PR 10例,SD 8例,PD 10例,ORR为40.0%,疾病进展后有25患者采用mFOLFOX6方案治疗,平均周期数4.8个,CR 0例,PR 4例,SD 5例,PD 16例,ORR为16.0%,另外5例患者疾病进展后采用紫杉类药物或阿帕替尼等治疗。经统计学分析,mFOLFOX6和FOLFIRI方案一线和二线治疗晚期胃癌患者ORR差异无统计学意义(P=0.801, P=0.662)。
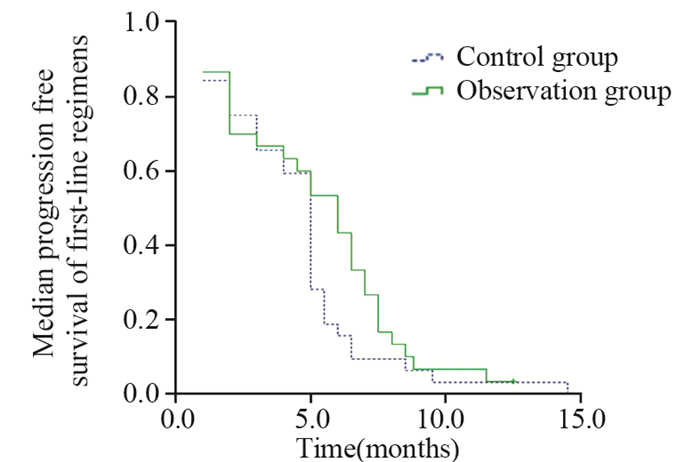
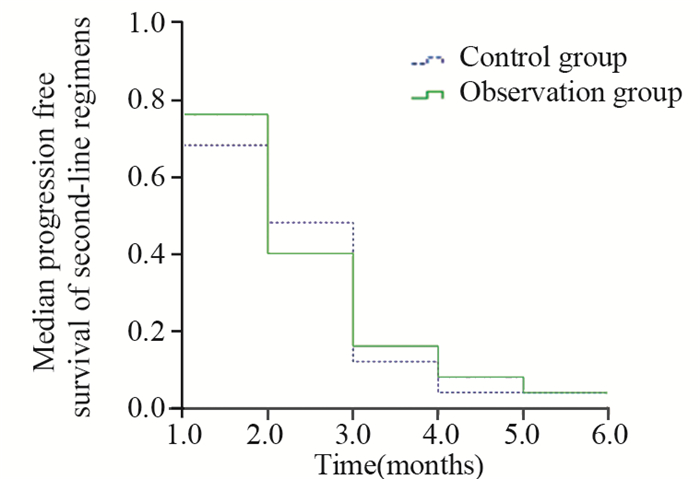
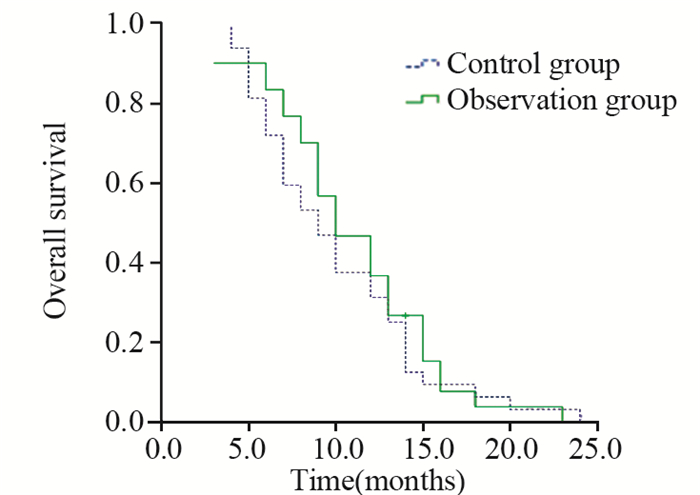
2.2 生存分析末次随访截至2016年6月1日。随访结束时,1例患者健在,中位随访时间11.0月。对照组和观察组患者的PFS1分别为(5.0±0.25)月和(6.0±0.81)月,二组间差异无统计学意义(P=0.178);PFS2分别为(2.0±0.36)月和(2.0±0.27)月,二组间差异无统计学意义(P=0.803)。对照组和观察组患者的OS分别为(10.0±1.06)月和(11.0±1.17)月,二组间差异无统计学意义(P=0.500),见图 1、2、3。
|
| 图 1 62例晚期胃癌患者一线化疗中位无疾病进展生存曲线 Figure 1 Median progression free survival curves of 62 patients with advanced gastric cancer after first–line regimen |
|
| 图 2 50例晚期胃癌患者二线化疗中位无疾病进展生存曲线 Figure 2 Median progression free survival curves of 50 patients with advanced gastric cancer after second-line regimen |
|
| 图 3 62例晚期胃癌患者总生存曲线 Figure 3 Overall survival curves of 62 patients with advanced gastric cancer |
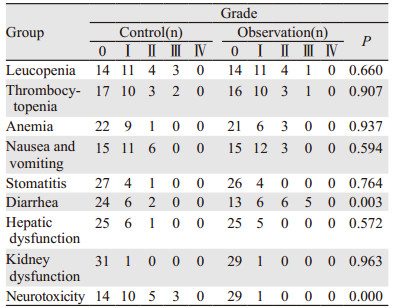
一线和二线治疗过程中,两组不良反应以骨髓抑制、胃肠道反应为主,无治疗相关死亡发生,在一线治疗过程中,其中mFOLFOX6方案周围神经炎发生率比FOLFIRI方案高(56.0% vs. 3.3%, P=0.000),而FOLFIRI方案的腹泻发生率高于mFOLFOX6方案(56.7% vs. 25.0%, P=0.003), 两方案在二线治疗过程中不良反应也是相似的,余相关不良反应两组间差异无统计学意义(P > 0.05),见表 1。
|
胃癌为我国常见恶性肿瘤,初诊时多为晚期,虽然TOGA临床研究证实曲妥珠单抗联合化疗可以明显改善HER2+++和HER2++且FISH阳性的晚期胃癌患者预后,总生存可以延长到16月[2],但多数晚期胃癌患者仍以姑息化疗为主。国内外研究证实以奥沙利铂和伊立替康为基础的联合方案均可以改善晚期胃癌患者的预后,延长患者生存,但目前二者之间的临床研究极少,而本研究主要来比较mFOLFOX6和FOLFIRI方案在晚期胃癌一线和二线治疗的疗效和不良反应,为晚期胃癌患者治疗的选择提供临床依据。
国外Hacibekiroglu等[3]研究采用mFOLFOX6为晚期胃癌一线治疗,ORR达到37.0%,PFS为6.5月,OS为11.4月。Sendur等[4]研究采用FOLFIRI作为晚期胃癌一线治疗,疗效与前者相似,ORR为37.8%,PFS和OS达到5.75和9.72月。本研究发现对照组和观察组的ORR无明显差异,分别为37.5%和40.0%,PFS和OS也无明显差异的,分别为5.0、6.0月和10、11月。国内临床研究结果也相似,如李跃军等[5]和徐智等[6]的研究也证实FOLFOX4和FOLFIRI方案一线治疗进展期或晚期胃癌患者取得的ORR、PFS和OS差别不明显。因此,从近期疗效和预后来看,mFOLFOX6和FOLFIRI一线治疗晚期胃癌患者价值相当。在国外二线治疗晚期胃癌相关临床研究中,Tsuji等[7]研究发现既往经过多种方案治疗后的晚期胃癌患者采用mFOLFOX6方案治疗,ORR达到23.1%,PFS为3月。Kwon等[8]研究发现经过FOLFOX4治疗进展的晚期胃癌患者采用FOLFIRI二线治疗,ORR为17.3%,PFS为5月。本研究发现虽然FOLFIRI作为二线治疗晚期胃癌有效率较mFOLFOX6稍高,但差异无统计学意义,ORR分别为24.0%和16.0%,另外二种方案的PFS差别也不明显。国内临床研究也证实mFOLFOX6和FOLFIRI方案二线治疗晚期胃癌ORR和PFS与本研究相似,如于立江等[9]研究紫杉醇联合FOLFOX方案二线治疗晚期胃癌患者,ORR达到26.7%,PFS为3.7月,而乐薇等[10]研究改良FOLFIRI方案二线治疗晚期胃癌患者,ORR为17.8%,PFS达3月。总的来说,mFOLFOX6和FOLFIRI方案作为二线治疗晚期胃癌患者价值相当,均可改善患者的疗效和预后。
本研究发现两种方案的不良反应均以骨髓抑制和胃肠道反应为主,患者均可耐受,与既往的临床研究[11-12]相似,mFOLFOX6方案以周围神经炎为主,明显高于FOLFIRI方案,目前并无特效药物降低周围神经炎,只能以预防为主,如避免冷刺激等,而FOLFIRI方案以腹泻为主,也是明显高于mFOLFOX6方案,主要表现为延迟性腹泻,使用易蒙停等相关药物对症治疗后均可缓解。因此,两种方案在一线和二线治疗晚期胃癌患者均是安全的。
| [1] | 符涛, 季加孚. 胃癌诊疗的热点和问题[J]. 中国肿瘤临床, 2016, 43(1): 1–5. [ Fu T, Ji JF. The foci and problems in diagnosis and treatment of gastric cancer[J]. Zhongguo Zhong Liu Lin Chuang, 2016, 43(1): 1–5. ] |
| [2] | Sawaki A, Ohashi Y, Omuro Y, et al. Efficacy of trastuzumab in Japanese patients with HER2-positive advanced gastric or gastroesophageal junction cancer: a subgroup analysis of the Trastuzumab for Gastric Cancer(ToGA) study[J]. Gastric Cancer, 2012, 15(3): 313–22. DOI:10.1007/s10120-011-0118-1 |
| [3] | Hacibekiroglu I, Kodaz H, Erdogan B, et al. Comparative analysis of the efficacy and safety of modified FOLFOX-6 and DCF regimens as first-line treatment in advanced gastric cancer[J]. Mol Clin Oncol, 2015, 3(5): 1160–4. |
| [4] | Sendur MA, Ozdemir N, Özatlı T, et al. Comparison the efficacy of second-line modified EOX (epirubicin, oxaliplatin, and capecitabine) and irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) regimens in metastatic gastric cancer patients that progressed on first-line modified docetaxel and cisplatin plus fluorouracil (DCF) regimen[J]. Med Oncol, 2014, 31(9): 153. DOI:10.1007/s12032-014-0153-y |
| [5] | 李跃军, 卓德斌. SOX方案对比FOLFOX4方案治疗进展期胃癌的临床观察[J]. 肿瘤研究与临床, 2014, 26(1): 42–4. [ Li YJ, Zhuo DB. Comparison between the effect of SOX regimen and FOLFOX4 regimen for advanced gastric cancer[J]. Zhong Liu Yan Jiu Yu Lin Chuang, 2014, 26(1): 42–4. ] |
| [6] | 徐智, 陈怡, 陈锦飞. 3种常用联合化疗方案一线治疗晚期胃癌的疗效和安全性比较[J]. 实用老年医学, 2014, 28(12): 1003–6. [ Xu Z, Chen Y, Chen JF. Analysis of efficacy and safety of three commomly used combination chemoregimens in treatment of advanced gastric adenocarcinoma[J]. Shi Yong Lao Nian Yi Xue, 2014, 28(12): 1003–6. ] |
| [7] | Tsuji K, Yasui H, Onozawa Y, et al. Modified FOLFOX-6 therapy for heavily pretreated advanced gastric cancer refractory to fluorouracil, irinotecan, cisplatin and taxanes: a retrospective study[J]. Jpn J Clin Oncol, 2012, 42(8): 686–90. DOI:10.1093/jjco/hys084 |
| [8] | Kwon HJ, Park MI, Park SJ, et al. Efficacy and Safety of FOLFIRI after Failure of FOLFOX-4 in Advanced Gastric Cancer[J]. Korean J Gastroenterol, 2015, 66(1): 10–6. DOI:10.4166/kjg.2015.66.1.10 |
| [9] | 于立江, 潘月琴, 马德, 等. 紫杉醇联合FOLFOX方案在晚期胃癌二线化疗中的应用[J]. 肿瘤基础与临床, 2013, 26(6): 535–6. [ Yu LJ, Pan YQ, Ma D, et al. Paclitaxel combined with FOLFOX regimen in second-line chemotherapy for advanced gastric cancer[J]. Zhong Liu Ji Chu Yu Lin Chuang, 2013, 26(6): 535–6. ] |
| [10] | 乐薇, 项晓军, 张凌, 等. 改良FOLFIRI方案二线治疗老年晚期胃癌患者的临床观察[J]. 实用肿瘤杂志, 2013, 28(2): 200–3. [ Le W, Xiang XJ, Zhang L, et al. Clinical observation of modified FOLFIRI regimen as second-line chemotherapy for elderly patients with advanced gastric cancer[J]. Shi Yong Zhong Liu Za Zhi, 2013, 28(2): 200–3. ] |
| [11] | Xu HB, Huang F, Su R, et al. Capecitabine plus oxaliplatin (XELOX) compared with 5-fluorouracil/leucovorin plus oxaliplatin (FOLFOXs) in advanced gastric cancer: Meta-analysis of randomized controlled trials[J]. Eur J Clin Pharmacol, 2015, 71(5): 589–601. DOI:10.1007/s00228-015-1828-9 |
| [12] | Bajetta E, Floriani I, Di Bartolomeo M, et al. Randomized trial on adjuvant treatment with FOLFIRI followed by docetaxel and cisplatin versus 5-fluorouracil and folinic acid for radically resectedgastric cancer[J]. Ann Oncol, 2014, 25(7): 1373–8. DOI:10.1093/annonc/mdu146 |
 2017, Vol. 44
2017, Vol. 44


