文章信息
- TET1蛋白对人乳腺癌MDA-MB-231细胞株增殖和侵袭能力的影响及其相关机制
- Effects of TET1 on Proliferation and Invasion of Human Breast Cancer MDA-MB-231 Cells and Related Mechanism
- 肿瘤防治研究, 2017, 44(7): 447-453
- Cancer Research on Prevention and Treatment, 2017, 44(7): 447-453
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2017.17.0040
- 收稿日期: 2017-01-11
- 修回日期: 2017-02-23
TET(ten-eleven translocation)家族包括3个成员:TET1、TET2和TET3,此家族通过介导DNA去甲基化的表观遗传调控重新激活沉默基因,在胚胎发育、机体造血、造血系统疾病及肿瘤的发生发展中均起重要作用[1-4]。TET2基因突变多见于骨髓恶性肿瘤,TET3与肿瘤的关系至今研究尚少[3-6]。目前研究提示,TET1参与肿瘤的增殖及侵袭转移过程[6-8],但其具体作用及相关机制尚未完全清楚。为此,本研究应用慢病毒载体构建稳定转染的TET1过表达细胞株,通过体外实验研究TET1对乳腺癌细胞增殖、迁移及侵袭的影响,并初步探究其相关机制,旨在为临床肿瘤诊治寻找新的靶点。
1 材料与方法 1.1 材料人乳腺癌MDA-MB-231和人胚肾HEK 293T慢病毒包装细胞均购自中国科学院上海细胞库;大肠杆菌菌株DH5α购自北京安赞诺公司。RPMI 1640培养液和胎牛血清(FBS)购自美国Gibco公司;TrizolTMReagent核酸分离试剂购自日本Takara公司;FastQuant RT Kit(with gDNase)(KR106)和SuperReal PreMix Plus(SYBR Green)(FP205)均购自北京TIANGEN公司;TET1鼠抗人单克隆抗体(GT1462)购自美国GeneTex公司;GAPDH兔抗人多克隆抗体(AP0063)、兔抗E-cadherin、N-cadherin、Vimentin、β-catenin一抗均购自美国Bioworld公司;羊抗兔荧光二抗、羊抗鼠荧光二抗均购自美国ROCKLAND公司;免疫荧光二抗购自美国KPL公司;Matrigel基质胶购自美国BD公司;Transwell小室购自美国Costar公司;CCK-8细胞增殖-毒性检测试剂盒购自上海贝博公司。
1.2 方法 1.2.1 质粒的构建及制备委托广州复能基因公司构建EX-E2856-Lv201表达载体及EX-NEG-Lv201表达载体。两质粒均具备嘌呤霉素耐药基因,可用嘌呤霉素筛选;将连接产物转化DH5α大肠杆菌,挑单克隆菌种扩大化培养;抽提质粒,测定所提质粒浓度,并进行双向测序验证。
1.2.2 慢病毒感染及筛选在进行转染前,提前48 h将293T包装细胞铺于10 cm细胞培养皿中,按照病毒包装试剂盒说明进行,转染293T细胞72 h后,收集培养液上清液,即慢病毒液;将其感染靶细胞(MDA-MB-231),同时加入终浓度为8 μg/ml的聚凝胺,并做感染无关序列的对照。以1 μg/ml终浓度的嘌呤霉素进行加压筛选,命名为MDA-MB-231-TET1细胞及阴性对照MDA-MB-231-NC细胞;未经感染的细胞命名为空白对照MDA-MB-231细胞。
1.2.3 qRT-PCR检测TET1的表达按照说明书使用TRIzol提取各组细胞总RNA,并将其反转录成cDNA,实时荧光定量PCR仪检测TET1的过表达情况。GAPDH为内参,上游引物为:5' -CAAGGCTGAGAACGGGAA-3' ,下游引物为:5' -GCATCGCCCCACTTGATTTT-3' ;TET1上游引物为:5' -CCCGAATCAAGCGGAAGAATA-3' ,下游引物为:5' -TACTTCAGGTTGCACGGT-3' 。反应条件为:95℃ 15 min,95℃ 10 s,63℃ 32 s,42个循环。采用2-ΔΔCT法分析所得qRT-PCR数据,并进行统计学分析。
1.2.4 Western blot检测TET1的表达收集各组细胞,采用RIPA强效裂解液提取细胞总蛋白,BCA蛋白定量检测试剂盒进行蛋白定量,处理后的蛋白进行SDS-PAGE、转膜;加入相应一抗,4℃孵育过夜,洗膜,加入相应荧光二抗,室温避光孵育1 h,洗膜,在Odyssey红外显像系统中成像进行分析,目的基因相对表达量用目的基因灰度值与GAPDH灰度值的比值表示,并进行统计学分析。
1.2.5 细胞增殖实验(CCK-8法)取对数生长期的各组细胞,以每孔1×103个细胞加入96孔板内,每组设6复孔;设最初加入CCK-8的时间为0 h,分别在细胞培养的0、24、48和72 h,以10微升/孔加入CCK-8试剂,于37℃、5%CO2的细胞培养箱中继续培养;1~4 h后取出96孔板,酶标仪检测各孔450 nm处的OD值;实验至少重复3次,并对数据进行统计学分析。
1.2.6 细胞周期检测取对数生长期的各组细胞,常规消化、离心后制备成2×106个细胞悬液,加入70%无水乙醇4℃放置过夜,次日用PBS清洗细胞2次,加核糖核酸酶A(10 mg/ml)2.5 μl及PI(1 mg/ml)25 μl,50 μm尼龙网过滤,上机检测。
1.2.7 划痕实验取对数生长期的各组细胞,以每孔5×105个细胞接种于6孔板,培养24 h后细胞融合度达80%~90%时,用200 μl枪头垂直行“十”字划痕,加入无血清RMPI 1640培养液,倒置光学显微镜下随机取6个固定点观察、照相,此时记为0 h;继续培养24 h后取出6孔板,在同一观察点处观察划痕愈合情况,并于倒置显微镜下拍照。计算划痕愈合率(%)=(0 h划痕宽度-24 h划痕宽度)/0 h划痕宽度×100%。
1.2.8 细胞侵袭能力的测定取对数生长期的各组细胞,制备成无血清细胞悬液,浓度为5×105个/毫升,向涂布基质胶的上室加入200 μl细胞悬液;下室加入600 μl含有5%FBS的培养液,培养20~24 h;取出小室,擦去膜上未迁移的细胞,10%多聚甲醛固定细胞,结晶紫染色,洗去染料,于倒置显微镜下观察(200倍),随机选取膜上、下、左、右、中各2个视野拍照,计数,计算平均值。
1.2.9 免疫荧光染色检测取对数生长期的各组细胞,常规消化、离心后铺入24孔板中,待细胞融合度达50%~60%时,进行染色,PBS洗3次,每次5 min,应用4%多聚甲醛固定细胞20 min,室温;弃4%多聚甲醛,PBS洗3次,每次5 min;0.5% Triton-X-100透膜10 min(若为膜蛋白,此步可省),PBS漂洗细胞,3次,每次5 min;10%BSA室温封闭1 h;弃10% BSA,一抗200 μl,4℃孵育过夜;加入二抗200 μl,37℃避光孵育1.5~2 h;PBS漂洗3次,每次5 min,5 μg/ml DAPI染色10 min,室温;PBS漂洗3次,每次5 min,于荧光显微镜下观察、拍照。
1.3 统计学方法采用SPSS13.0软件进行数据分析,组间差异分析采用t检验或方差分析,P < 0.05为差异有统计学意义。
2 结果 2.1 稳定过表达TET1的MDA-MB-231细胞系的建立将收集的病毒液感染靶细胞MDA-MB-231,感染72 h后,经嘌呤霉素筛选,感染效率明显提高,MDA-MB-231-NC组和MDA-MB-231-TET1组的95%以上细胞表达绿色荧光蛋白(eGFP),见图 1。
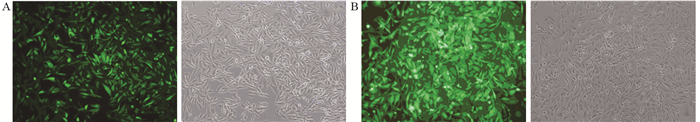
|
| The figures were taken at the same location under fluorescent field and bright field; A: the eGFP-negative control was stably transfected into MDA-MB-231 cells (×100); B: the TET1 plasmid was stably transfected into MDA-MB-231 cells (×100) 图 1 稳定表达eGFP及TET1 MDA-MB-231细胞系的建立 Figure 1 Establishment of MDA-MB-231 cell lines with stable expression of eGFP and TET1 |
与阴性对照组和空白对照组细胞比较,MDA-MB-231-TET1细胞TET1 mRNA(P=0.003)及蛋白(P=0.03)表达水平明显升高,差异均具有统计学意义,见图 2。

|
| 1: MDA-MB-231; 2: MDA-MB-231-NC; 3: MDA-MB-231-TET1; A: qPCR analysis was performed to detect TET1 mRNA levels after transfection and it showed that cell strain of stable overexpression of TET1 was effectively established; B: the expression of TET1 protein in different cell lines were detected by Western blot. C: the histograms were made by Prism 6 according to figure B; n=3 图 2 慢病毒感染前后MDA-MB-231细胞中TET1 mRNA及蛋白的表达 Figure 2 Expression of TET1 mRNA and protein in MDA-MB-231 cells before and after lentiviral transfection tested by qPCR and Western blot |
与阴性对照组和空白对照组细胞相比,MDA-MB-231-TET1细胞增殖速度显著降低,差异有统计学意义(P < 0.001),见图 3。
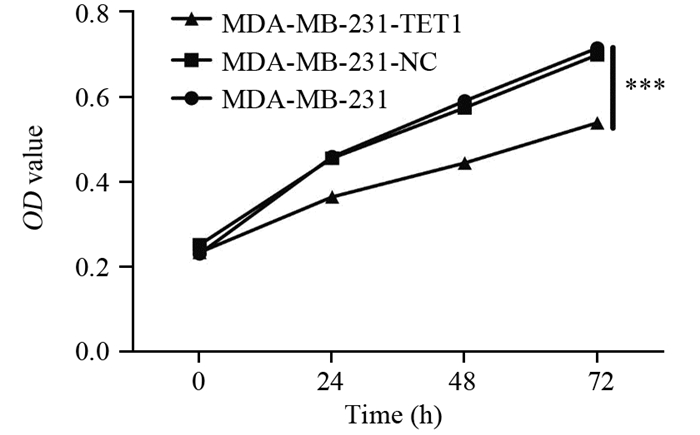
|
| TET1 inhibited the proliferation of MDA-MB-231 in vitro. CCK-8 assay showed that the overexpression of TET1 suppressed MDA-MB-231 cell growth; Cells (1×103) were seeded into 96-well plates and the viable cell number was evaluated as the value of absorbance at 450nm; n=6, ***: P < 0.001, compared with control groups 图 3 TET1过表达对MDA-MB-231细胞增殖能力的影响 Figure 3 Effects of TET1 overexpression on proliferation ability of MDA-MB-231 cells |
MDA-MB-231-TET1细胞G2/M期延长,阴性对照组细胞G2/M期占(4.30±1.36)%,而MDA-MB-231-TET1细胞G2/M期占(13.24±3.64)%,差异有统计学意义(P=0.002),见图 4。提示MDA-MB-231-TET1细胞G2/M期阻滞。
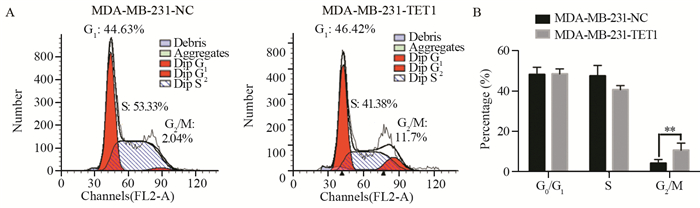
|
| A: the overexpression of TET1 inhibited cell cycle progression and DNA synthesis in MDA-MB-231 cells. The overexpression of TET1 arrested cell cycle progression by accumulating cells in G2/M phase; B: the histogram was made by Prism 6; n=3; **: P < 0.01 图 4 TET1过表达对MDA-MB-231细胞细胞周期的影响 Figure 4 Effects of TET1 overexpression on MDA-MB-231 cell proliferation |
与阴性对照组和空白对照组细胞相比,MDA-MB-231-TET1迁移能力显著降低,统计学分析显示,MDA-MB-231-TET1与阴性及空白对照组的迁移能力比较,差异具有统计学意义(P < 0.001),见图 5。
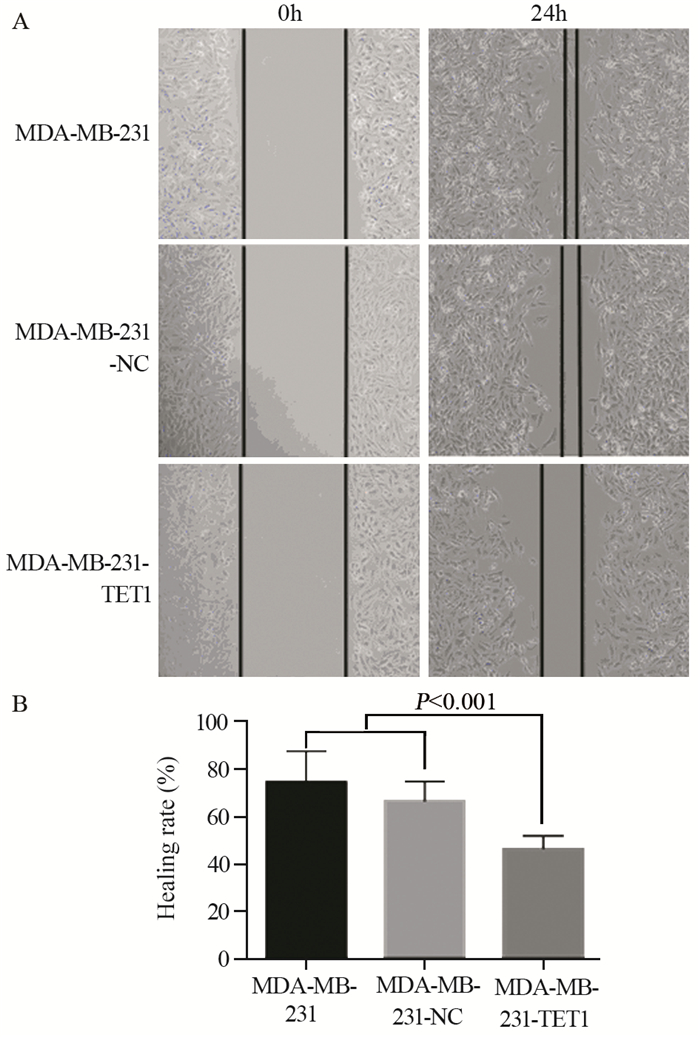
|
| A:the migration ability of MDA-MB-231 cells and MDA-MB-231 cells transfected with TET1 or not were tested by wound healing assay (SP ×100); B: the histogram was made by Prism 6; n=3 图 5 TET1过表达对MDA-MB-231细胞迁移的影响 Figure 5 Effects of TET1 overexpression on migration ability of MDA-MB-231 cells |
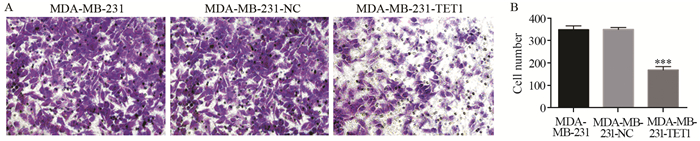
与阴性对照组和空白对照组细胞相比,MDA-MB-231-TET1侵袭能力显著降低,差异有统计学意义(P < 0.001),见图 6。
|
| A: the effect of TET1 expression on the migration of MDA-MB-231 cells; transwell assay was performed on MDA-MB-231 cells transfected with TET1 or control; B: the histogram was made by Prism 6; n=3; ***: P < 0.001, compared with control groups 图 6 TET1过表达对MDA-MB-231细胞侵袭的影响 Figure 6 Effects of TET1 overexpression on invasion ability of MDA-MB-231 cells |
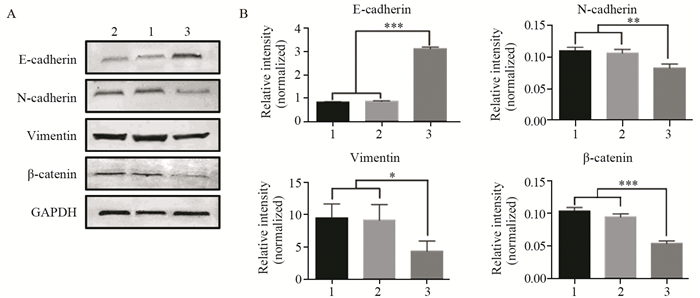
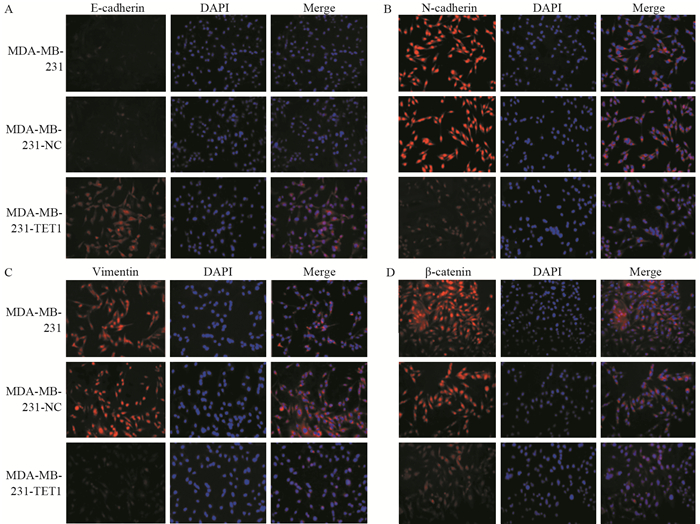
Western blot结果显示:与阴性对照组和空白对照组细胞相比,MDA-MB-231-TET1组细胞E-cadherin表达明显升高(P < 0.001),N-cadherin(P=0.003)、Vimentin表达降低(P=0.041),黏附蛋白的辅助因子β-catenin表达降低(P < 0.001),见图 7。免疫荧光染色检测结果显示:与阴性对照组和空白对照组细胞相比,MDA-MB-231-TET1组细胞E-cadherin表达明显升高,N-cadherin、Vimentin表达降低,MDA-MB-231-TET1组、阴性对照组和空白对照组细胞的细胞质和细胞核均有β-catenin表达,但相较于MDA-MB-231-TET1组细胞,阴性对照组和空白对照组细胞的细胞核内β-catenin表达升高,提示其向核内转移,见图 8。
|
| 1: MDA-MB-231; 2: MDA-MB-231-NC; 3: MDA-MB-231-TET1; A: the effects of TET1 overexpression on the expression of EMT-related molecular markers in MDA-MB-231 cells were detected by Western blot; B: the histograms were made by Prism 6; n=3; ***: P < 0.001; **: P < 0.01; *: P < 0.05 图 7 TET1过表达对EMT相关分子标志物表达的影响 Figure 7 Effects of TET1 overexpression on expression of EMT-related molecular markers |
|
| A, B, C, D: the expression and location of EMT markers in MDA-MB-231 cells before and after TET1 transfection detected by immunofluorescence; n=3 图 8 TET1过表达对MDA-MB-231细胞EMT相关分子标志物表达的影响 Figure 8 Effects of TET1 overexpression on expression of EMT-related molecular markers in MDA-MB-231 cells |
TET家族是一种α-酮戊二酸和Fe2+依赖的双加氧酶,能够将5-甲基胞嘧啶(5mC)转化为5-羟甲基胞嘧啶(5hmC),并进一步转化为5-甲酰胞嘧啶(5fC)和5-羧基胞嘧啶(5caC),启动DNA去甲基化程序[1-3]。研究证实,TET家族蛋白能使DNA甲基化介导的基因沉默重新激活,参与肿瘤的增殖及侵袭转移过程[6-8]。相较于正常组织,TET蛋白在乳腺癌及肝癌组织中的表达均有不同程度地降低,但TET1的表达最低[5]。Neri等[9]发现,通过沉默TET1在正常结肠上皮细胞中的表达,可使细胞周期进程加快,然而重新激活TET1的表达后,结肠细胞的增殖则被抑制;而在肾癌786-O细胞中,下调TET1的表达明显抑制细胞增殖,并使细胞阻滞于G0/G1期[10]。因此,TET1对肿瘤细胞增殖能力的影响作用不一,本研究结果发现TET1过表达可明显降低乳腺癌MDA-MB-231细胞增殖速度,TET1过表达可促使细胞周期中的G2/M期阻滞,表明TET1可通过阻滞G2/M期抑制乳腺癌细胞增殖。
肿瘤的转移是一个多基因、多步骤、多因素的过程,经历脱离原发肿瘤、进入血液循环、趋化“归巢”及黏附“定居”等多个阶段后,最终形成新的转移灶[11-12]。上皮间质转化可诱导肿瘤细胞脱离原发部位,通过血液循环或淋巴循环转移至远处。在此过程中,肿瘤细胞的上皮型标志物E-cadherin表达降低,间质型标志物Vimentin及N-cadherin表达增加,使肿瘤细胞获得间质细胞所具有的迁移能力[13-15]。EMT的发生受精密的信号通路网络调控,其中Wnt/β-catenin信号通路被认为是促进EMT发生的重要信号通路之一,分泌型Wnt蛋白是该通路的启动因子,其进入细胞后可抑制由Axin、APC和GSK-3β组成的复合物的活性,导致β-catenin无法被GSK-3β磷酸化而在细胞质内聚集并向核内转移,入核后直接结合转录因子TCF/LEF并激活其活性,启动下游靶基因的表达,进而促使EMT的发生[16-18]。在正常情况下,成熟细胞内的β-catenin大多数与E-cadherin结合于细胞膜上表达,细胞质内游离的β-catenin保持在较低水平,故目前研究学者把β-catenin的核内移现象作为Wnt/β-catenin信号通路开通的标志[19-20]。最近研究显示,Liu等[21]在研究肺癌A549细胞EMT过程时发现,TET1表达明显上调时,DNA甲基转移酶(DNMT)1、3a及3b表达明显降低。然而,Tsai等[22]提出,TET1能作为乏氧诱导因子HIF-1α和HIF-2α的转录共激活因子,上调乏氧应答基因的表达来促进乏氧诱导EMT的发生。这均提示TET1可能参与到肿瘤的EMT过程中。因此,本研究首先检测TET1对乳腺癌MDA-MB-231细胞迁移、侵袭能力的影响,结果发现过表达TET1的细胞迁移速度明显慢于阴性对照组及空白对照组,同时,其侵袭能力也明显低于阴性对照组及空白对照组。由此说明,TET1抑制乳腺癌细胞的迁移、侵袭能力。进一步定量检测EMT相关分子标志物在各组细胞中的表达,结果发现,过表达TET1可明显升高上皮样标志物E-cadherin蛋白的表达,抑制间质样标志物N-cadherin及Vimentin蛋白表达,抑制黏附蛋白的辅助分子β-catenin蛋白表达,而β-catenin在过表达TET1的细胞中表达均一,但在阴性对照组及空白对照组细胞的细胞核中β-catenin表达高于胞质,提示β-catenin向核内转移。这均与EMT机制相符,由此说明TET1可以通过调控EMT参与乳腺癌的侵袭及转移,通过促进乳腺癌细胞向上皮样表型转化,升高E-cadherin的表达增强细胞间的黏附能力,降低β-catenin在细胞质内积累进而阻止β-catenin入核,从而抑制EMT发生。类似研究也证实了此推论[9],TET1可降低β-catenin在结肠癌细胞的细胞核中的表达,通过抑制Wnt/β-catenin通路进而抑制肿瘤细胞的增殖。
关于TET1如何调控肿瘤的增殖、侵袭转移的相关机制尚未完全清楚,近年研究证实,高表达TET1的乳腺癌患者预后较好[23-24]。在乳腺癌细胞中,TET1可通过对金属蛋白酶组织抑制剂(TIMP)基因的启动子去甲基化,维持TIMP2及TIMP3的表达来抑制肿瘤转移[8]。而Sun等[24]却提出,同样在乳腺癌中,高迁移率蛋白(HMGA2)是TET1重要的上游调控因子,促进TET1通过对其自身及HOXA7和HOXA9的启动子进行去甲基化来上调其表达,抑制肿瘤侵袭、转移并改善乳腺癌患者的预后。有学者研究乏氧时发现,TET1还能通过去甲基化上调胰岛素诱导基因1(INSIG1)的表达,促进代谢及EMT[22]。这提示TET1不仅具有去甲基化的活性,还可能在乏氧条件下参与到肿瘤细胞代谢的调控中。
综上所述,上调TET1蛋白表达能够通过阻滞G2/M期来抑制乳腺癌MDA-MB-231细胞的增殖,并且能够抑制乳腺癌MDA-MB-231细胞的迁移、侵袭,其机制可能与通过Wnt/β-catenin通路抑制EMT的发生相关,为研究TET1与肿瘤的关系提供了新的研究思路。深入探讨TET1对肿瘤增殖及侵袭的作用机制可能为癌症治疗提供新的靶点。
| [1] | Jin C, Lu Y, Jelinek J, et al. TET1 is a maintenance DNA demethylase that prevents methylation spreading in differentiated cells[J]. Nucleic Acids Res, 2014, 42(11): 6956–71. DOI:10.1093/nar/gku372 |
| [2] | Zhao H, Chen T. Tet family of 5-methylcytosine dioxygenases in mammalian development[J]. J Hum Genet, 2013, 58(7): 421–7. DOI:10.1038/jhg.2013.63 |
| [3] | Tan L, Shi YG. Tet family proteins and 5-hydroxymethylcytosine in development and disease[J]. Development, 2012, 139(11): 1895–902. DOI:10.1242/dev.070771 |
| [4] | Cimmino L, Abdel-Wahab O, Levine RL, et al. TET family proteins and their role in stem cell differentiation and transformation[J]. Cell Stem Cell, 2011, 9(3): 193–204. DOI:10.1016/j.stem.2011.08.007 |
| [5] | Yang H, Liu Y, Bai F, et al. Tumor development is associated with decrease of TET gene expression and 5-methylcytosine hydroxylation[J]. Oncogene, 2013, 32(5): 663–9. DOI:10.1038/onc.2012.67 |
| [6] | Chen HF, Wu KJ. Epigenetics, TET proteins, and hypoxia in epithelial-mesenchymal transition and tumorigenesis[J]. BioMedicine(Taipei), 2016, 6(1): 1–8. |
| [7] | Jeschke J, Collignon E, Fuks F. Portraits of TET-mediated DNA hydroxymethylation in cancer[J]. Curr Opin Genet Dev, 2016, 36: 16–26. DOI:10.1016/j.gde.2016.01.004 |
| [8] | Hsu C H, Peng KL, Kang ML, et al. TET1 suppresses cancer invasion by activating the tissue inhibitors of metalloproteinases[J]. Cell Rep, 2012, 2(3): 568–79. DOI:10.1016/j.celrep.2012.08.030 |
| [9] | Neri F, Dettori D, Incarnato D, et al. TET1 is a tumour suppressor that inhibits colon cancer growth by derepressing inhibitors of the WNT pathway[J]. Oncogene, 2015, 34(32): 4168–76. DOI:10.1038/onc.2014.356 |
| [10] | 谢素红, 翁文浩, 李智. TET1对肾癌786-O细胞增殖的影响及其相关机制[J]. 肿瘤, 2012, 32(12): 962–8. [ Xie SH, Weng WH, Li Z. The effect of TET1 on the proliferation of renal cancer 786-O cells and its related mechanism[J]. Zhong Liu, 2012, 32(12): 962–8. DOI:10.3781/j.issn.1000-7431.2012.12.003 ] |
| [11] | Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms[J]. Cell, 2011, 147(2): 275–92. DOI:10.1016/j.cell.2011.09.024 |
| [12] | Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization[J]. Nat Rev Cancer, 2009, 9(4): 274–84. DOI:10.1038/nrc2622 |
| [13] | De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression[J]. Nat Rev Cancer, 2013, 13(2): 97–110. DOI:10.1038/nrc3447 |
| [14] | Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells[J]. Science, 2013, 342(6159): 1234850. DOI:10.1126/science.1234850 |
| [15] | Gheldof A, Berx G. Cadherins and epithelial-to-mesenchymal transition[J]. Prog Mol Biol Transl Sci, 2013, 116(1): 317–36. |
| [16] | Yan D, Avtanski D, Saxena NK, et al. Leptin-induced epithelial-mesenchymal transition in breast cancer cells requires β-catenin activation via Akt/GSK3-and MTA1/Wnt1 protein-dependent pathways[J]. J Biol Chem, 2012, 287(11): 8598–612. DOI:10.1074/jbc.M111.322800 |
| [17] | Arend RC, Londoño-Joshi AI, Straughn JM, et al. The Wnt/β-catenin pathway in ovarian cancer: a review[J]. Gynecol Oncol, 2013, 131(3): 772–9. DOI:10.1016/j.ygyno.2013.09.034 |
| [18] | Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions[J]. J Clin Invest, 2009, 119(6): 1429–37. DOI:10.1172/JCI36183 |
| [19] | Iwai S, Yonekawa A, Harada C, et al. Involvement of the Wnt-β-catenin pathway in invasion and migration of oral squamous carcinoma cells[J]. Int J oncol, 2010, 37(5): 1095–103. |
| [20] | Gavert N, Ben-Ze'ev A. Epithelial-mesenchymal transition and the invasive potential of tumors[J]. Trends Mol Med, 2008, 14(5): 199–209. DOI:10.1016/j.molmed.2008.03.004 |
| [21] | Liu F, Zhou Y, Zhou D, et al. Whole DNA methylome profiling in lung cancer cells before and after epithelial-to-mesenchymal transition[J]. Diagn Pathol, 2014, 9: 66. DOI:10.1186/1746-1596-9-66 |
| [22] | Tsai YP, Chen HF, Chen SY, et al. TET1 regulates hypoxia-induced epithelial-mesenchymal transition by acting as a co-activator[J]. Genome Biol, 2014, 15(12): 513. DOI:10.1186/s13059-014-0513-0 |
| [23] | Yang L, Yu SJ, Hong Q, et al. Reduced expression of TET1, TET2, TET3 and TDG mRNAs are associated with poor prognosis of patients with early breast cancer[J]. PloS One, 2015, 10(7): e0133896. DOI:10.1371/journal.pone.0133896 |
| [24] | Sun M, Song CX, Huang H, et al. HMGA2/TET1/HOXA9 signaling pathway regulates breast cancer growth and metastasis[J]. Proc Natl Acad Sci U S A, 2013, 110(24): 9920–5. DOI:10.1073/pnas.1305172110 |
 2017, Vol. 44
2017, Vol. 44


