| 青花菜C3H型锌指蛋白基因BoCCCH2的克隆与表达 |
2. 丽水学院生态学院,浙江 丽水 323000
2. College of Ecology, Lishui University, Lishui 323000, Zhejiang, China
Zinc finger proteins are kinds of important transcription factors in eukaryotic organisms, which involve in various biological activities, such as replication, transcription, translation, repair, metabolism and signaling. According to the number and order of cysteine and histidine residues, zinc finger proteins were classified into several different types, such as C2H2, C2C2, C2C2C2, C2HC and C3H. For example, C3H-type ones contain one to six typical motifs with three cysteine residues and one histidine residue. However, their functions are little known, and no gene has been reported in broccoli.
In this study, a C3H-type zinc finger protein gene BoCCCH2 was isolated from broccoli, and later the expression patterns in different organs as well as leaves infected by H. parasitica and B. cinerea were studied.
Results indicated that BoCCCH2 contained no intron, and the full length of coding sequence was 1 740 bp encoding 579 amino acids. The deduced protein sequence contained two ANK domains and two CCCH zinc finger structures, respectively, and the CCCH zinc finger types were C—X8—C—X5—C—X3—H and C—X5—C—X4—C—X3—H. Reverse transcription-polymerase chain reaction results showed that the BoCCCH2 was expressed in roots, leaves, stalks, young siliques, flower buds and flowers, with highest level in roots. Expression levels increased when challenged by both H. parasitica and B. cinerea. When infected by H. parasitica, expression levels increased after 24 h, and decreased after 72 h, while infected by B. cinerea, the highest level was detected after 6 h, and slowed down in 12 h. Homologous sequences were downloaded from NCBI (National Center for Biotechnology Information) website, including Citrus sinensis, Gossypium raimondii, Populus trichocarpa, Ricinus communis, Prunus persica, P. mume, Malus domestica, Fragaria vesca, Phaseolus vulgaris, Glycine soja, B. rapa, Camelina sativa, Capsella rubella, Arabidopsis thaliana and Eutrema salsugineum. Phylogenetic analysis results revealed that BoCCCH2 was grouped with homogeneous sequences from other Cruciferae plants with bootstrap confidence of 100%, and sequences from Leguminosae, Euphorbiaceae and Rosaceae were found on different clades.
In conclusion, these results indicate that the BoCCCH2 might play an important role in defense responses challenged by either H. parasitica or B. cinerea. Cloning and expression analysis of BoCCCH2 provide evidence for further studies on gene function.
青花菜(Brassica oleracea var. italica)为十字花科(Cruciferae)甘蓝类蔬菜,是甘蓝(B. oleracea)的一个变种,迄今已有2 500多年的栽培历史[1]。青花菜以花蕾群和花茎为食用部位,可生食也可蒸煮,色泽碧绿,营养丰富,风味独特,深受人们的喜爱。浙江是我国青花菜主产省,在台州、宁波和温州等沿海地区均有大面积栽植,是当地菜农的重要收入来源。霜霉病和灰霉病是青花菜在生长过程中常见的2种病害,分别由寄生霜霉菌(Hyaloperonospora parasitica)和灰葡萄孢菌(Botrytis cinerea)引起,这2种病害在苗期和成株期均可发生,危害叶片和花球[2]。我国的青花菜种质资源十分匮乏,常规育种受到很大的限制,已成为青花菜良种培育的重要制约因素[3]。分子育种是种质创新的重要手段之一,具有成本低、目的性强和效率高等优点,近年来在青花菜育种中已有一些报道[4, 5, 6]。合适的靶标基因是基因工程育种的基础,转录因子通过结合特定的启动子,控制一系列下游基因的活动;导入一个抗病转录因子基因就相当于转入多个抗病相关基因,从而提高综合抗病能力[7]。
锌指蛋白是真核生物中一类重要的转录因子,参与细胞的诸多生命活动,包括复制、转录、翻译、修复、物质代谢、信号转导和RNA编辑等[8, 9]。锌指结构由一段小分子肽链组成,可与Zn2+结合形成指形结构,该结构能与核酸、蛋白质及一些小分子结合,从而发挥特定的功能[10]。根据锌指结构中半胱氨酸(C)和组氨酸(H)的数量及排列位置,可分为C2H2、CCCC、C2HC、C2C2、C2C2C2C2和C3H等类型[11, 12]。C3H型锌指蛋白通常含1~6个锌指结构,每个锌指结构由3个半胱氨酸和1个组氨酸组成,它们在种子萌发、抗病免疫反应、盐胁迫响应、叶片衰老和干旱胁迫反应等方面起着重要作用[13, 14, 15, 16]。目前,有关青花菜C3H型锌指蛋白基因方面的研究尚未见报道;因此,本研究在克隆BoCCCH2的基础上,利用反转录聚合酶链反应(reverse transcription-polymerase chain reaction,RT-PCR)方法研究该锌指基因在不同器官及在霜霉菌和灰葡萄孢菌侵染下叶片中的表达模式,旨在为开展该基因的功能鉴定奠定基础。
1 材料与方法 1.1 实验材料青花菜Bo0112在室内栽植,其生育期为75 d,花球坚实,花蕾深绿色,侧枝少,具有较强的霜霉病和灰霉病抗性。于花期采集根、叶、花茎、花蕾、开放的花和嫩角果,用于DNA和RNA提取。霜霉菌病叶采自浙江省临海市上盘镇青花菜基地,用无菌ddH2O小心冲洗叶片上的白色霉层,将孢子溶液稀释后备用,待青花菜长至2叶1心时用喷雾法接种[17]。灰葡萄孢菌也采自上盘镇青花菜基地,利用分离法获得菌株,在无菌室中把菌株接种到马铃薯葡萄糖琼脂(potato-dextrose agar,PDA)培养基上,25 ℃培养6 d,在菌落边缘用打孔器打出直径为5 mm的琼脂块,反贴于叶片正面,对照为不接菌的PDA培养基。分别采集接种霜霉菌和灰葡萄孢菌0、6、12、24、36和72 h时的叶片,置于-80 ℃备用。
从NCBI数据库下载BoCCCH2的同源序列,分别来自橙(Citrus sinensis,登录号KDO66907.1)、雷蒙德氏棉(Gossypium raimondii,KJB08973.1)、毛果杨(Populus trichocarpa,EEE84607.2)、蓖麻(Ricinus communis,EEF36773.1)、桃(Prunus persica,EMJ09531.1)、梅(Prunus mume,XP_008220916.1)、苹果(Malus domestica,XP_008384793.1)、野草莓(Fragaria vesca,XP_004294290.1)、菜豆(Phaseolus vulgaris,ESW14199.1)、野生大豆(Glycine soja,KHN40076.1)、不结球白菜(Brassica rapa,XP_009120380.1)、亚麻荠(Camelina sativa,XP_010483463.1)、荠菜(Capsella rubella,EOA13094.1)、拟南芥(Arabidopsis thaliana,AED97077.1)和山萮菜(Eutrema salsugineum,ESQ42513.1)。
1.2 DNA、RNA提取和cDNA合成基因组DNA的提取采用十二烷基磺酸钠法;RNA提取采用TRIzol法;cDNA合成试剂盒购自TaKaRa公司,第1链和第2链的合成根据说明书进行。
1.3 基因克隆采用的上、下游引物分别为BoC3HUP:5′-ATGGGAGATGACGAGCTGT-3′和BoC3HDN:5′-TCAAGCAACGGTTTGTTCT-3′。反应总体积为25 μL,含1×PCR缓冲液,0.8 U Taq DNA聚合酶(北京鼎国昌盛生物技术有限责任公司),0.3 μmol/L dNTPs(上海生工生物工程股份有限公司),各0.2 μmol/L上、下游引物,40 ng叶片基因组DNA或cDNA模板,最后加无菌ddH2O至25 μL。PCR程序:94 ℃预变性5 min;94 ℃变性30 s,53.3 ℃退火55 s,72 ℃延伸115 s,31个循环;72 ℃延伸10 min。
PCR产物经1.2%琼脂糖凝胶电泳,割取含目的条带的胶块,利用DNA凝胶回收试剂盒(碧云天生物技术研究所)回收,实验操作根据说明书进行。取2 μL PCR回收产物克隆到pGEM-T Easy载体(Promega公司,美国),于4 ℃冰箱中连接过夜,用热激法将连接产物转入DH5α大肠杆菌感受态细胞(北京鼎国昌盛生物技术有限责任公司)中,各取3个阳性克隆用于测序。
1.4 基因表达分析根据测序结果设计RT-PCR引物。上、下游引物分别为BoC3HRTUP:5′-TATGATCGCTGCCT TGTTT-3′和BoC3HRTDN:5′-AGTTCATTGCT GCGTTCTAT-3′,以各器官及经霜霉菌和灰葡萄孢菌处理的叶片cDNA为模板进行PCR扩增,反应体系及所采用的试剂同1.3节。PCR程序:94 ℃预变性5 min;94 ℃变性30 s,56.5 ℃退火55 s,72 ℃延伸80 s,33个循环;72 ℃延伸10 min。以肌动蛋白基因为内标,上、下游引物分别为5′-TCTCGATGGAAGAGCTGGTT-3′和5′-GATCC TTACCGAGGGAGGTT-3′。PCR程序:94 ℃预变性5 min;94 ℃变性30 s,55.6 ℃退火45 s,72 ℃延伸60 s,32个循环;72 ℃延伸10 min。PCR结束后,产物在1%琼脂糖凝胶上电泳、拍照和记录。
1.5 生物信息学分析BoCCCH2的编码蛋白用Primer premier 5.0软件翻译;BoCCCH2及其同源序列比对采用ClustalX 1.81软件;系统进化树的构建采用Mega 3.1软件,建树方法为邻接法,自举检验次数为1 000。
2 结果与分析 2.1 BoCCCH2的克隆与序列分析分别以叶片基因DNA和cDNA为模板,用BoC3HUP/BoC3HDN引物进行PCR扩增,经割胶、回收、连接、转化和测序,获得相应的基因序列。测序结果(图 1)表明:BoCCCH2的基因组DNA和cDNA长度均为1 740 bp,该基因没有内含子,分别以ATG和TGA作为起始及终止密码子;BoCCCH2编码579个氨基酸,推导的蛋白质具2个ANK结构域,分别位于57~87和92~124处,另有2个不同类型的CCCH锌指结构,分别为C—X8—C—X5—C—X3—H和C—X5—C—X4—C—X3—H,位于249~267和284~299处。
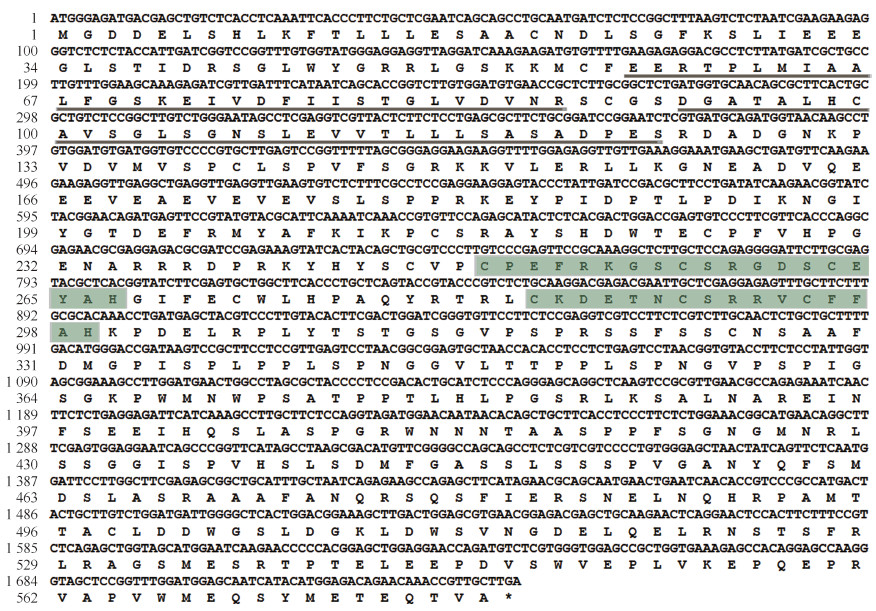 |
| 图1 青花菜BoCCCH2编码区及推导的氨基酸序列 Fig. 1 Complete coding sequence and deduced amino acid sequence of BoCCCH2 from Brassica oleracea var. italica |
为研究BoCCCH2在不同器官及经霜霉菌和灰葡萄孢菌侵染叶片中的表达模式,以肌动蛋白基因为内标,用BoC3HRTUP/BoC3HRTDN引物对进行RT-PCR分析。结果(图 2)表明:BoCCCH2在根、叶、花茎、嫩角果、花蕾和花中均有表达;在根中的表达量最大,条带最亮,在叶片、花茎和花中的表达量较小,条带亮度较弱。
 |
| 图2 BoCCCH2基因在不同器官中的表达 Fig. 2 Expression patterns of BoCCCH2 in different organs |
RT-PCR结果表明:对照叶片中BoCCCH2的表达量没有明显变化(图 3E);在霜霉菌侵染下,6 h和12 h时与0 h没有区别,24 h后表达量增加,但增幅较小(图 3A);叶片经灰葡萄孢菌侵染后,6~36 h的表达量增加,其中6 h时的表达量最大,之后逐渐减少,72 h时的表达量与0 h相仿(图 3C)。
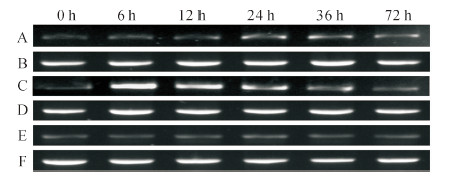 |
| 图3 霜霉菌和灰葡萄孢菌侵染下BoCCCH2在叶片中的表达 Fig. 3 Expression patterns of BoCCCH2 in leaves challenged by Hyaloperonospora parasitica and Botrytis cinerea |
利用ClustalX 1.81软件对BoCCCH2及其同源序列比对结果表明:桃(EMJ09531.1)和梅(XP_008220916.1)的相似性最大,达97%;其次为不结球白菜(XP_009120380.1)和BoCCCH2,相似性达94%;而菜豆(ESW14199.1)和不结球白菜、菜豆和BoCCCH2及野生大豆(KHN40076.1)和不结球白菜的序列相似性最低,仅为43%;BoCCCH2与不结球白菜的相似性最高,与亚麻荠(XP_010483463.1)、荠菜(EOA13094.1)、拟南芥(AED97077.1)及山萮菜(ESQ42513.1)等十字花科植物的相似性分别为77%、78%、78%和79%,而与其他植物的相似性较低,为43%~47%。
从图 4可以看出:BoCCCH2及15条同源序列均有2个CCCH锌指结构,它们是蛋白序列中最为保守的区域;第1个CCCH锌指结构在+296、+298、+299和+302位存在差异,第2个锌指结构较第1个保守,有3处出现差异,分别位于+328、+329和+331;BoCCCH2、不结球白菜和山萮菜的2个锌指结构序列完全一致,类似现象也出现在拟南芥、荠菜和亚麻荠、菜豆和大豆、苹果和草莓及桃和梅的组合中;另外,雷蒙德氏棉与十字花科植物第2个锌指结构序列完全一致。
 |
| 图4 BoCCCH2及其同源序列锌指模体的比较 Fig. 4 Comparisons of zinc finger motifs among BoCCCH2 and its homologous sequences |
利用Mega 3.1软件构建系统发育树。结果(图 5)表明:16条蛋白序列可分为4类,BoCCCH2先与不结球白菜聚为一组,再与山萮菜、拟南芥、亚麻荠和荠菜等十字花科植物组成Ⅳ类,支持率达100%;豆科的菜豆和野生大豆聚为一类(Ⅲ),支持率为100%;蔷薇科的野草莓、苹果、梅和桃聚为一类(Ⅱ),支持率也是100%;橙、雷蒙德氏棉、毛果杨和蓖麻处于同一分支,归为Ⅰ类,但支持率低,仅51%。
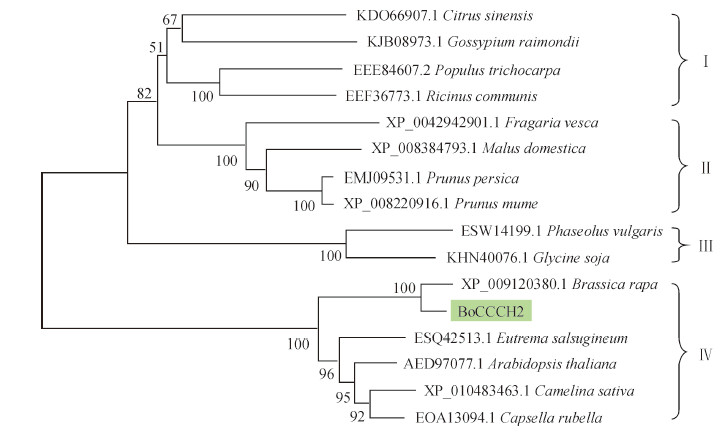 |
| 图5 用邻接法构建的BoCCCH2及其同源序列的系统进化树 Fig. 5 Phylogenetic tree of BoCCCH2 and its homologous sequences constructed using neighbor-joining method |
锌指蛋白广泛存在于真核生物中,在转录调控中起着重要作用。有关C2H2型锌指蛋白的研究最多,而对C3H型的报道相对较少[8, 18],与青花菜锌指蛋白相关的研究很少。GAO等[19]在研究种子和幼苗硫代葡萄糖苷代谢时,利用RNA-Seq鉴定出1 633个转录因子基因,其中C3H与C2H2锌指蛋白基因的数量分别为69和65。C3H型锌指结构由3个半胱氨酸和1个组氨酸构成,参与植物生长发育、物质代谢和逆境响应[13, 20, 21]。根据半胱氨酸和组
氨酸之间的氨基酸残基数,C3H型锌指结构共有序列的最初定义是C—X6-14—C—X4-5—C—X3—H[22],在对拟南芥和水稻的C3H进行全基因组鉴定时发现了一些新类型,重新定义为C—X4-15—C—X4-6—C—X3—H[23]。锌指蛋白通常含1~6个锌指结构,但也有例外,如玉米的ZmC3H3含7个C3H,类型为C—X17—C—X6—C—X3—H,除此之外,还有C—X8—C—X5—C—X3—H、C—X7—C—X5—C—X3—H、C—X7—C—X4—C—X3—H、C—X5—C—X4—C—X3—H、C—X7—C—X6—C—X3—H和C—X8—C—X4—C—X3—H[24]。在本研究中BoCCCH2具2个锌指结构,分别为C—X8—C—X5—C—X3—H和C—X5—C—X4—C—X3—H,它们均为常见类型,拟南芥C3H14和C3H15各具2个锌指结构,都是C—X8—C—X5—C—X3—H类型[23];在毛果杨的211个C3H锌指结构中,C—X8—C—X5—C—X3—H有96个,C—X7—C—X5—C—X3—H有76个[25],而在拟南芥的148个C3H锌指结构中,C—X8—C—X5—C—X3—H和C—X7—C—X5—C—X3—H类型的C3H分别为78和43个。
锚蛋白重复序列(ankyrin repeats,ANK)结构域在蛋白质-蛋白质互作中起着重要作用,它们参与转录起始、细胞周期调控、细胞骨架、离子运输和信号转导[26]。玉米C3H锌指蛋白ZmC3H4、ZmC3H10、ZmC3H43和ZmC3H63均带有2个ANK结构域[24];在毛果杨的91个C3H锌指蛋白中,PtC3H32、PtC3H33、PtC3H34、PtC3H35、PtC3H36、PtC3H37、PtC3H38、PtC3H39、PtC3H81、PtC3H82、PtC3H90和PtC3H91等12个蛋白具ANK[25];而在拟南芥中,有5个C3H型锌指蛋白具ANK,它们是AtC3H30、AtC3H56、AtC3H66、AtC3H47和AtC3H29[23],其中AtC3H29又名ZFAR1,它发生突变后,对灰葡萄孢菌的敏感性增加[27]。在本研究中,推导的BoCCCH2具2个ANK结构域,分别位于57~87和92~124处,其功能尚需进一步研究。
C3H型锌指蛋白基因参与植物生长发育,如毛果杨的34个CCCH在根中大量表达,24个在新叶中高表达,43个在雌花序或雄花序中表达[25];与毛果杨不同,拟南芥中的大部分CCCH在根、叶、花序和种子中均有表达[23];拟南芥的C3H14和C3H15在花茎、花和角果中表达量最高,它们与细胞次生壁加厚相关[23]。在本研究中,BoCCCH2在根中的表达量最高,而在花茎、叶、嫩角果、花蕾和花中的表达量相对较低。C3H型锌指蛋白参与逆境胁迫响应,陆地棉(Gossypium hirsutum)GhTZF1的表达受聚乙二醇、盐、茉莉酸甲酯和过氧化氢的诱导,该基因的过量表达可增加拟南芥的耐旱性和延缓因干旱引起的衰老[16]。本研究青花菜BoCCCH2的表达受霜霉菌和灰葡萄孢菌的诱导,但表达量和表达模式存在一定差异,暗示该基因与2种病菌的抗病反应相关。
对青花菜BoCCCH2基因的克隆与表达分析,为该基因在抗病反应中的功能鉴定奠定了基础。下一步我们将开展载体构建和转基因研究,以明确BoCCCH2在霜霉病和灰霉病抗性反应中的功能。
| [1] | BUCK P A. Origin and taxonomy of broccoli. Economic Botany, 1956, 10(3):250-253. |
| [2] | 任典东, 汪恩国, 王永才.台州西兰花主要病虫发生为害规律研究.蔬菜, 2013(2):65-67. REN D D, WANG E G, WANG Y C. Occurrence of broccoli key diseases and insect pests in Taizhou. Vegetables, 2013(2):65-67. (in Chinese) |
| [3] | 兰梅, 黄丽, 许彬, 等.青花菜单倍体育种研究进展.云南农业大学学报(自然科学版), 2012, 27(4):579-584. LAN M, HUANG L, XU B, et al. Advances in haploid breeding of broccoli (Brassica oleracea var. italica). Journal of Yunnan Agricultural University (Natural Science), 2012, 27(4):579-584. (in Chinese with English abstract) |
| [4] | MORA-AVILES M A, EARLE E D. Expression of pathogenesis-related genes in transgenic broccoli and canola plants expressing the Trichoderma harzianum-endochitinase gene. Revista Chapingo Serie Horticultura, 2004, 10(2):141-146. |
| [5] | CAO J, TANG J D, STRIZHOV N, et al. Transgenic broccoli with high levels of Bacillus thuringiensis Cry1C protein control diamondback moth larvae resistant to Cry1A or Cry1C. Molecular Breeding, 1999, 5(2):131-141. |
| [6] | ZHAO J Z, COLLINS H L, TANG J D, et al. Development and characterization of diamondback moth resistance to transgenic broccoli expressing high levels of Cry1C. Applied and Environmental Microbiology, 2000, 66(9):3784-3789. |
| [7] | 金慧, 栾雨时.转录因子在植物抗病基因工程中的研究进展.中国生物工程杂志, 2010, 30(10):94-99. JIN H, LUAN Y S. Progress on transcription factor in gene engineering of diseases resistances in plants. China Biotechnology, 2010, 30(10):94-99. (in Chinese with English abstract) |
| [8] | KRISHNA S S, MAJUMDAR I, GRISHIN N V. Structural classification of zinc fingers: Survey and summary. Nucleic Acids Research, 2003, 31(2):532-550. |
| [9] | SUN T, SHI X, FRISO G, et al. A zinc finger motif-containing protein is essential for chloroplast RNA editing. PLoS Genetics, 2015, 11(3):e1005028. |
| [10] | KLUG A, SCHWABE J W. Protein motifs 5. Zinc fingers. The FASEB Journal, 1995, 9(8):597-604. |
| [11] | IUCHI S. Three classes of C2H2 zinc finger proteins. Cellular and Molecular Life Sciences, 2001, 58(4):625-635. |
| [12] | LI W T, HE M, WANG J, et al. Zinc finger protein (ZFP) in plants. Plant Omics Journal, 2013, 6(6):474-480. |
| [13] | BOGAMUWA S P, JANG J C. Tandem CCCH zinc finger proteins in plant growth, development and stress response. Plant and Cell Physiology, 2014, 55(8):1367-1375. |
| [14] | KONG Z, LI M, YANG W, et al. A novel nuclear-localized CCCH-type zinc finger protein, OsDOS, is involved in delaying leaf senescence in rice. Plant Physiology, 2006, 141(4):1376-1388. |
| [15] | LEE S J, JUNG H J, KANG H, et al. Arabidopsis zinc finger proteins AtC3H49/AtTZF3 and AtC3H20/AtTZF2 are involved in ABA and JA responses. Plant and Cell Physiology, 2012, 53(4):673-686. |
| [16] | ZHOU T, YANG X Y, WANG L C, et al. GhTZF1 regulates drought stress responses and delays leaf senescence by inhibiting reactive oxygen species accumulation in transgenic Arabidopsis. Plant Molecular Biology, 2014, 85(1/2):163-177. |
| [17] | JIANG M, MIAO L X, HE C M. Overexpression of an oil radish superoxide dismutase gene in broccoli confers resistance to downy mildew. Plant Molecular Biology Reporter, 2012, 30(4):966-972. |
| [18] | SHI Z H, ZHANG C, XU X F, et al. Overexpression of AtTTP affects ARF17 expression and leads to male sterility in Arabidopsis. PLoS One, 2015, 10(3):e0117317. |
| [19] | GAO J, YU X, MA F, et al. RNA-seq analysis of transcriptome and glucosinolate metabolism in seeds and sprouts of broccoli (Brassica oleracea var. italic). PLoS One, 2014, 9(2):e88804. |
| [20] | TAKATSUJI H. Zinc-finger transcription factors in plants. Cellular and Molecular Life Sciences, 1998, 54(6):582-596. |
| [21] | LIN P C, POMERANZ M C, JIKUMARU Y, et al. The Arabidopsis tandem zinc finger protein AtTZF1 affects ABA- and GA-mediated growth, stress and gene expression responses. The Plant Journal, 2011, 65(2):253-268. |
| [22] | BERG J M, SHI Y. The galvanization of biology: A growing appreciation for the roles of zinc. Science, 1996, 271:1081-1085. |
| [23] | WANG D, GUO Y H, WU C A, et al. Genome-wide analysis of CCCH zinc finger family in Arabidopsis and rice. BMC Genomics, 2008, 9:44. |
| [24] | PENG X J, ZHAO Y, CAO J G, et al. CCCH-type zinc finger family in maize: Genome-wide identification, classification and expression profiling under abscisic acid and drought treatments. PLoS One, 2012, 7(7):e40120. |
| [25] | CHAI G, HU R, ZHANG D, et al. Comprehensive analysis of CCCH zinc finger family in poplar (Populus trichocarpa). BMC Genomics, 2012, 13(1):253. |
| [26] | CHAKRABARTY B, PAREKH N. Identifying tandem ankyrin repeats in protein structures. BMC Bioinformatics, 2014, 15(1):6599. |
| [27] | ABUQAMAR S, CHEN X, DHAWAN R, et al. Expression profiling and mutant analysis reveals complex regulatory networks involved in Arabidopsis response to Botrytis infection. The Plant Journal, 2006, 48(1):28-44. |
 2016, Vol. 42
2016, Vol. 42


