| 三维动脉自旋标记技术在胶质瘤术后复发诊断中的价值 |
2. 贵州省人民医院放射科,贵州 贵阳 550002;
3. 贵州省德江县人民医院放射科,贵州 德江 565206
胶质瘤是最常见的脑原发肿瘤,治疗以手术为主[1];低级别胶质瘤预后相对较好,但仍有50%~75%的患者死于肿瘤进展[2],高级别胶质瘤治疗后存在复发可能。MRI平扫及增强扫描对肿瘤复发的判断主要依据是否出现新的强化区域,具有一定的局限性,与肿瘤治疗后假性进展、放射性损伤难以鉴别。三维动脉自旋标记技术(three-dimensional arterial spin labeling,3D-ASL)是近年新出现的MRI灌注功能成像技术,无需外源性对比剂即可了解组织灌注信息,已应用于脑胶质瘤术前分级、脑缺血性疾病的诊断等。3D-ASL灌注测量参数具有良好的可靠性和一致性,对胶质瘤的灌注评估可代替动态磁敏感灌注成像[3]。现收集贵州省人民医院2015年5月至2017年12月期间胶质瘤术后定期行MRI增强扫描及3D-ASL检查的15例患者,回顾性分析术后放化疗随访中出现新增异常强化区域的范围变化、测定强化区灌注参数,即脑血流量值(cerebral blood flow,CBF)及脑血流量相对比值(relative cerebral blood flow,rCBF),分析灌注参数与术后复发的关系,以探讨3D-ASL对胶质瘤术后复发的诊断价值。
1 资料与方法 1.1 一般资料本组15例,其中男9例,女6例;年龄36~67岁,中位年龄42岁。纳入标准:①胶质瘤术后定期复查MRI增强扫描及3D-ASL;②无对比剂过敏史,无肾功能受损,无明显心脏疾病;③除去囊变、坏死、钙化或出血后的病灶强化成分最短径>1.5 cm,经第2次手术切除或活检证实复发。排除标准:运动伪影使图像质量降低及无法配合检查的患者。15例中,WHO Ⅲ级7例(46.7%),4例为间变型星形细胞瘤,3例间变少突胶质细胞瘤;Ⅳ级8例(53.3%),均为胶质母细胞瘤。患者检查前均签署知情同意书。随访9个月,在术后1周内行MRI增强扫描,之后每隔3个月(术后第3、6、9个月)复查MRI增强扫描及3D-ASL检查。
1.2 仪器与方法采用GE Discovery 750W 3.0 T MRI扫描仪及头颅8通道相控阵线圈。所有患者均先行头部常规MRI扫描,再行3D-ASL全脑灌注成像,最后行T1WI增强扫描。扫描范围覆盖全脑。扫描序列及参数:T1WI TE 36 ms,TR 1 748 ms,层厚6 mm;T2WI TE 118 ms,TR 4 213 ms,层厚6 mm,矩阵512×512;3D-ASL TE 33 ms,TR 2 187 ms,激发后采集时间2.0 s,层厚4 mm,矩阵512×512;SE-T1WI TE 33 ms,TR 2 187 ms,层厚4 mm。增强扫描对比剂采用顺磁性对比剂Gd-DTPA,剂量0.2 mmol/kg体质量,注射流率3 mL/s。
1.3 图像分析及后处理将3D-ASL原始图像导入GE AW 4.6工作站Functool软件中进行校正、降噪及后处理。通过3D-ASL与T1 BRAVO解剖像融合,以确保所选的ROI与增强扫描图像强化范围一致。由2名高年资主治医师及1名副主任医师分别确定复查MRI增强扫描图像强化区域范围,测量、记录强化范围大小,并根据MRI增强扫描图像、3D-ASL图像及两者联合图像判断是否复发;避开术后坏死、出血、囊变及大血管区域,分3次测定可疑复发区域内的CBF值并取平均值,测定对侧正常脑组织CBF值,ROI取10~30 mm2;为校正不同年龄、心率对灌注值的影响,将病变区CBF除以正常对侧CBF得到rCBF。
1.4 统计学分析采用SPSS 19.0软件进行统计分析,计量资料以x±s表示。对胶质瘤术后复发区域3次复查的CBF、rCBF值分别行方差分析;并对第3、6、9个月CBF、rCBF值行两两配对t检验。对常规MRI增强扫描、3D-ASL及两者联合的诊断准确率行χ2检验,以P<0.05为差异有统计学意义。
2 结果15例术后第3、6、9个月MRI增强扫描出现异常强化病灶分别为5、11、13例,其中第6个月病灶强化扩大者4例,第9个月新增2例强化病灶。3D-ASL示术后第3、6、9个月时分别有8、12、13例呈相对高灌注。
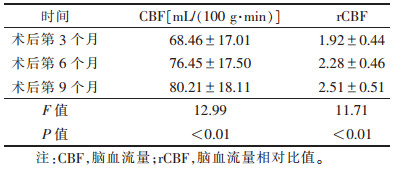
术后第3、6、9个月CBF、rCBF值行方差分析,差异均有统计学意义(均P<0.01);其中第3个月与第6个月、第3个月与第9个月CBF值比较,差异均有统计学意义(均P<0.05);第6个月与第9个月CBF值比较,差异无统计学意义(P > 0.05)。第3个月与第9个月rCBF值比较,差异有统计学意义(P<0.01)(表 1)。
| 表 1 15例胶质瘤术后CBF、rCBF值变化(x±s) |
 |
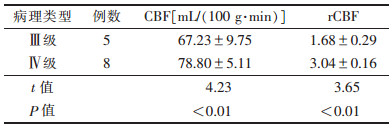
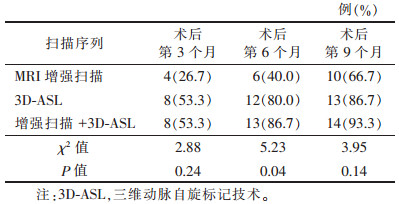
术后第9个月,13例复发高灌注组中,Ⅳ级胶质瘤复发病变灌注参数CBF、rCBF值与Ⅲ级比较差异均有统计学意义(均P<0.01),提示胶质瘤级别越高,复发时病灶的灌注血流越丰富(表 2)。MRI增强扫描与3D-ASL对术后第6个月复发的诊断准确率比较,差异有统计学意义(P<0.05)(表 3)。
| 表 2 13例高灌注不同病理类型的CBF、rCBF值(x±s) |
 |
| 表 3 MRI增强扫描与3D-ASL对术后复发诊断正确率比较 |
 |
典型胶质瘤术后复发的MRI增强扫描及3D-ASL表现见图 1,2。
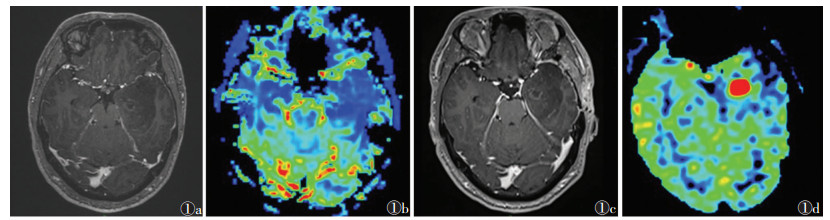 |
| 图 1 男,48岁,胶质母细胞瘤,Ⅳ级 图 1a,1b 术后第3个月复查MRI增强扫描及三维动脉自旋标记技术(3D-ASL),术区轻度环状强化,无明显高灌注 图 1c,1d 术后第6个月复查,术区可见环形强化轻度增高,并呈高灌注改变,活检病理证实为复发 |
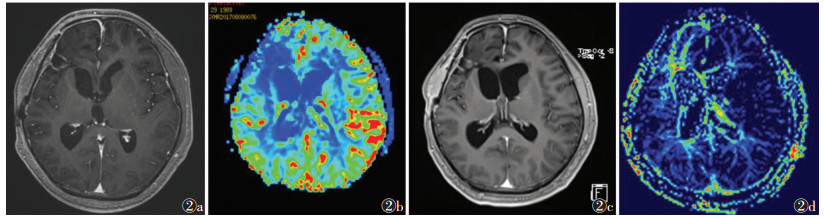 |
| 图 2 男,50岁,间变型胶质细胞瘤,Ⅲ级 图 2a,2b 术后第6个月复查MRI增强扫描及3D-ASL,可见术区环形强化,未见明显高灌注 图 2c,2d 术后第9个月复查,新出现结节样强化,并呈高灌注表现,第2次手术证实为复发 |
3 讨论
脑胶质瘤以手术为首选治疗方法,但手术常难以完全切除,存在残留和复发可能;高级别胶质瘤术后行放疗及联合化疗的综合治疗,但20%~40%的患者会复发。Sanghera等[4]进行的一项大样本研究表明,约26%的胶质母细胞瘤患者术后复发;复发患者2、5年无进展生存期分别占11%和4%[5]。目前缺乏对胶质瘤复发统一的治疗方案,但研究[6-7]表明复发患者能从早期放射治疗中获益,因此复发患者的早期诊断具有重要意义。脑胶质瘤术后复发的常规MRI增强扫描表现与放疗后脑损伤、假性进展类似,鉴别困难[8]。随着fMRI广泛应用于胶质瘤术前分级及术后评估,多模态影像学无创检查成为胶质瘤术后复查的重要手段。
ASL是一种无创性MRI灌注成像技术,无需对比剂即能获得组织灌注信息。既往研究[9]表明,ASL能够评估肿瘤血管,在胶质瘤灌注程度及术前胶质瘤的分级上能够取代动态磁敏感对比增强灌注成像技术,3D-ASL是近几年在ASL技术上发展而来的伪连续式动脉自转标记(pseudocontinuous arterial spin labeling,pCASL)技术,具有成像速度快、质量高、可重复性较好,以及可定量测量CBF的优势,可应用于缺血性卒中、脑肿瘤等领域。本研究采用3D-ASL技术及MRI增强扫描对15例高级别胶质瘤术后肿瘤复发区域的CBF、rCBF值分析发现,3D-ASL较MRI增强扫描对肿瘤复发与治疗效应的鉴别更具有诊断价值,在术后随访过程中不断出现强化区域的增大和(或)新强化区域,与Xu等[9]的研究结果接近。有学者[10]报道,应用11C-胆碱鉴别肿瘤复发和放射性坏死比18F-FDG PET更敏感,但PET-CT费用昂贵,复查成本高。白雪菲等[12]采用PWI及DWI分析胶质瘤术后复发患者发现,PWI中相对脑血容量(rCBV)、rCBF值对胶质瘤复发有鉴别价值。Sunwoo等[12]应用ASL鉴别胶质母细胞瘤及转移瘤,发现其肿瘤内及瘤周的CBF值有明显差异。
肿瘤的生成依赖于血供的形成。本研究中增强扫描无明显强化但3D-ASL表现为高灌注者,其原因可能在于钆对比剂无法进入正常血-脑脊液屏障,因此对于无血-脑脊液屏障破坏的肿瘤患者,MRI增强扫描肿瘤可无强化。但3D-ASL为内源性对比剂,不依赖于血-脑脊液屏障是否完整,能较客观地评价肿瘤血供情况。因此,MRI增强扫描联合3D-ASL更有助于评价肿瘤复发。Thomas等[13]亦认为,胶质瘤复发病变内也常混合治疗后进展,单纯应用MRI增强扫描对胶质瘤复发的诊断效能不高。另有研究[14-16]表明,ASL检查能够评估肿瘤灌注情况,间接反映肿瘤毛细血管密度,在评估胶质瘤灌注程度及术前胶质瘤的分级上能够取代动态磁敏感对比增强灌注成像,且对复发后治疗效果评估也有一定价值[17]。研究[18-20]表明,利用3D-ASL技术对胶质瘤患者术前肿瘤区域CBF值的测量并进行标准化后发现低级别与高级别胶质瘤间存在明显差异,能够很好地鉴别两者。还有研究[21-22]表明,因无需对比剂,ASL更能准确反映肿瘤内灌注,且测得肿瘤实质的rCBF与血管内皮生长因子、微血管密度表达程度呈正相关,可间接反映肿瘤新生血管。本研究在第9个月观察终点时,13例明显强化;术后第3~9个月,53.3%~86.7%的复发患者出现灌注增加;通过强化区域CBF的测量及rCBF的计算,发现肿瘤复发后CBF显著增加,第3、6、9个月平均CBF值为(68.46±17.01)、(76.45±17.50)、(80.21±18.11)] mL/(100 g·min),且随着复查时间延长,CBF、rCBF有明显增加趋势;该趋势与肿瘤内新生毛细血管增加相关或由于肿瘤复发的时间点不同所致,需进一步研究,但在术后复查过程中CBF、rCBF值的高低,以及不断升高的变化趋势为肿瘤复发的诊断提供了重要依据。15例中不同级别胶质瘤复发灌注参数CBF、rCBF差异均有统计学意义(均P<0.01),提示肿瘤恶性程度与病灶血流量有明显相关性,这与既往研究[20]一致。但当术后患者病变区CBF值> 78.8 mL/(100 g·min)时应高度考虑肿瘤复发。本研究发现,3D-ASL判断肿瘤复发准确率高于常规MRI增强扫描,尤其联合两者图像时,能够明显提高肿瘤复发的诊断准确率。
综上所述,3D-ASL技术能可靠测量胶质瘤术后患者的CBF及rCBF值,对评价胶质瘤术后复发具有重要诊断价值;动态追踪CBF及rCBF值变化规律有利于评价胶质瘤术后放化疗的疗效;联合MRI增强扫描更有利于提高胶质瘤复发的诊断准确率。但本研究存在不足之处:样本量偏少、复查时间间隔过长,其中2例肿瘤复发患者3D-ASL呈低灌注,是否与后标记延迟时间设定及肿瘤非颈内动脉供血有关,需进一步追踪复查。随访超过9个月复发患者未纳入,今后将增大样本量进一步研究。
| [1] |
Dolecek TA, Propp JM, Stroup NE, et al. CBTRUS statistical re- port:primary brain and central nervous system tumors diagnosed in the United States in 2005-2009[J]. Neuro-Oncology, 2013, 15: 1-56. |
| [2] |
Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter me- thylation status can predict the incidence and outcome of pseu- doprogression after concomitant radiochemotherapy in newly dia- gnosed glioblastoma patients[J]. J Clin Oncol, 2008, 26: 2192-2197. |
| [3] |
Westen DV, Petersen ET, Wirestam R, et al. Correlation between arterial blood volume obtained by arterial spin labelling and ce- rebral blood volume in intracranial tumours[J]. MAGMA, 2011, 24: 211-223. |
| [4] |
Sanghera P, Perry J, Sahgal A, et al. Pseudoprogression following chemoradiotherapy for glioblastoma multiforme[J]. Can J Neurol Sci, 2010, 37: 36-42. |
| [5] |
Tugcu B, Postalci LS, Gunaldi O, et al. Efficacy of clinical prog- nostic factors on survival in patients with glioblastoma[J]. Turk-ish Neuro, 2010, 20: 117-125. |
| [6] |
Zemlin A, Martens B, Wiese B, et al. Timing of re-irradiation in recurrent high-grade gliomas:a single institution study[J]. J Ne-uro-Oncol, 2018, 138: 571-579. |
| [7] |
Combs SE, Kessel KA, Hesse J, et al. Moving second courses of ra- diotherapy forward:early re-irradiation after surgical resection for recurrent gliomas improves efficacy with excellent tolerability[J]. Neurosurgery, 2018, 83: 1241-1248. |
| [8] |
Brandsma D, Stalpers L, Taal W, et al. Clinical features, mechanis- ms, and management of pseudoprogression in malignant gliomas[J]. Lancet Oncol, 2008, 9: 453-461. |
| [9] |
Xu Q, Liu Q, Ge H, et al. Tumor recurrence versus treatment effects in glioma:a comparative study of three dimensional pse- udo-continuous arterial spin labeling and dynamic susceptibility contrast imaging[J]. Medicine, 2017, 96: e9332. |
| [10] |
刘续磊, 陶荣杰. 应用11C-胆碱PET/CT鉴别恶性胶质瘤复发与坏死的研究进展[J]. 现代肿瘤医学, 2007, 15(11): 1692-1694. |
| [11] |
白雪菲, 牛广明, 韩晓东, 等. PWI和DWI技术在鉴别脑胶质瘤复发与放射性脑损伤中的价值[J]. 磁共振成像, 2014, 5(1): 7-10. |
| [12] |
Sunwoo L, Yun TJ, You SH, et al. Differentiation of glioblastoma from brain metastasis:qualitative and quantitative analysis us- ing arterial spin labeling MR imaging[J]. PLoS One, 2016, 11: e0166662. |
| [13] |
Thomas A, Rosenblum M, Karimi S, et al. Radiographic patterns of recurrence and pathologic correlation in malignant gliomas treated with bevacizumab[J]. Cns Oncol, 2018, 7: 7. |
| [14] |
White CM, Pope WB, Zaw T, et al. Regional and voxelwise co- mparisons of blood flow measurements between dynamic suscep-tibility contrast magnetic resonance imaging(DSC-MRI) and ar-terial spin labeling(ASL) in brain tumors[J]. J Neuroimaging, 2014, 24: 23-30. |
| [15] |
Wolf RL, Wang J, Wang S, et al. Grading of CNS neoplasms us- ing continuous arterial spin labeled perfusion MR imaging at 3 Tesla[J]. J Magn Reson Imaging, 2005, 22: 475-482. |
| [16] |
Cebeci H, Aydin O, Ozturkisik E, et al. Assesment of perfusion in glial tumors with arterial spin labeling; comparison with dyn- amic susceptibility contrast method[J]. Eur J Radiol, 2014, 83: 1914-1919. |
| [17] |
江晶晶, 赵凌云, 姚义好, 等. 三维动脉自旋标记灌注成像在星形细胞瘤术前分级中的应用[J]. 放射学实践, 2014, 29(8): 896-900. |
| [18] |
Miyoshi F, Shinohara Y, Kambe A, et al. Utility of intravoxel in- coherent motion magnetic resonance imaging and arterial spin labeling for recurrent glioma after bevacizumab treatment[J]. Acta Radiol, 2018, 59: 1372-1379. |
| [19] |
戴辉, 王星宇, 张体江. 三维动脉自旋标记技术在脑胶质瘤分级诊断中的应用价值[J]. 实用医学杂志, 2016, 32(24): 4044-4047. |
| [20] |
赵倩倩, 杨秋霞, 许桂晓, 等. 全脑3D动脉自旋标记成像在颅脑肿瘤诊断中的应用价值[J]. 中华医学杂志, 2017, 97(23): 1801-1804. |
| [21] |
王子文, 杨本强, 刘文源, 等. 胶质瘤3D ASL灌注指数与VEGF MVD表达相关性研究[J]. 中国肿瘤临床, 2016, 43(13): 557-561. |
| [22] |
Ningning D, Haopeng P, Xuefei D, et al. Perfusion imaging of brain gliomas using arterial spin labeling:correlation with histo- pathological vascular density in MRI-guided biopsies[J]. Neur- oradiology, 2017, 59: 51-59. |
 2020, Vol. 18
2020, Vol. 18


