

人类抗原R(human antigen R,HuR)是由胎致死性异常视觉样1(embryonic lethal abnormal vision-like 1,ELAVL1)基因编码的蛋白产物,因此也被称为ELAV样蛋白1。HuR在人体细胞中普遍表达,主要通过与含AREs的目的mRNA结合调节mRNA稳定性这种转录后调控机制来参与多种生理和病理过程。
近十年来针对HuR的功能研究表明,除了这种经典的调控机制HuR还可通过与非编码RNA(non-coding RNA,ncRNA)相互作用来参与基因表达的调控,包括长链非编码RNA(long non-coding RNAs,lncRNA)、环状RNA(circular RNA,circRNA)、微小RNA(microRNA,miRNA)和穹窿体RNA(Vault RNA,vtRNA)等。研究表明,HuR和这些调节性的lncRNA的相互作用在多种疾病的发生和发展中起了关键作用。其中,HuR在肿瘤中的作用研究最多,已经有综述进行归纳总结,该综述仅关注HuR-ncRNA相互作用在肿瘤以外的疾病中的作用。
1 HuR-ncRNA相互作用模式及其对基因表达的影响 1.1 HuR与miRNA的相互作用miRNA的长度通常为22nt左右,可以通过5′端与mRNA互补结合来调控mRNA的表达。编码miRNA的基因被RNA聚合酶II转录为含发夹结构的较长初级转录产物pri-miRNA,通过切割后形成约60 nt的pre-miRNA并转移到细胞质,最终再次切割形成短的双链RNA结构,即成熟的miRNA。miRNA可通过其两条链的其中一条(引导链),与Argonaute(Ago)蛋白结合,形成RNA诱导的沉默复合物(RNA-induced silencing complex,RISC),与mRNA的3'非翻译区(3'UTR)结合并通过降解或翻译抑制来抑制它们的使用来调节基因的表达[1]。
文献报道显示,HuR是多种miRNA的调节靶点,如miR-9a-5p[2]、miR-122[3]、miR-325-3p[4]等,可以通过与HuR基因的3'UTR结合抑制HuR的表达。同时某些miRNA还可以与HuR的目标mRNA结合阻止HuR对mRNA的稳定作用,如miR-146a[5]、miR-195[6]、miR-637[7]等。反之,HuR也可以影响miRNA的功能,对于含有AREs区域的mRNA,HuR与miRNA竞争性的与其结合,拮抗miRNA对基因表达的抑制作用,如miR-195[8]、miR-29a-3p[9]、miR-21[10]等。HuR还可以调控细胞中miRNA的含量,包括增加或抑制miRNA表达、促进miRNA通过细胞外囊泡(extracellular vesicles,EVs)的外排等,从而影响受miRNA调控的靶基因的表达。此外, HuR可以与miRNA协作来调控基因的表达,如miR-21可以招募HuR和Ago1,结合在目的mRNA的3'UTR区域增加基因的表达[11];let-7可与HuR协作与Myc mRNA结合抑制Myc的表达[12](Tab 1)。综上所述,HuR与miRNA可通过多种机制相互竞争或者协作来调控基因的时空表达。
| Mechanism | miRNA | Gene |
| HuR and miRNAs competitively bind to mRNA | miR-195 | PHB1 |
| adenosine-methylated form of miR-125a-3p | IGSF11 | |
| miR-146a | TNF-a | |
| miR-195 | DCLK1 | |
| miR-637 | CRP33 | |
| miR-29a-3p | HMGB1 | |
| miR-21 | PDCD4 | |
| miR-29 | CB1 | |
| miR-330 | STAT3 | |
| miR-633 | BMP1 | |
| miR-21 | PDCD4 | |
| miR181 | TNF-a | |
| miR-21、miR-142-3p | ITGA4、CAV1 | |
| miR-1192 | HMGB1 | |
| miR-200b | VEGF | |
| miRNAs inhibit HuR expression | miR-9a | - |
| miR-122 | - | |
| miR-325-3p | - | |
| miR-204-5p | - | |
| miR-33a | - | |
| miR-378a-3p | - | |
| miR-18a-3p | - | |
| miR-125b | - | |
| miR-291b-3p | - | |
| miR-33a | - | |
| miR-146a | - | |
| miR-23c | - | |
| miR-29b | - | |
| miR-570-3p | - | |
| miR-146 | - | |
| HuR promotes the expression of miRNAs | miR-122 | - |
| has-let-7c | - | |
| HuR inhibits the expression of miRNAs | miR-7a-1 | - |
| miR-16 | - | |
| miR-466i | - | |
| miR-409、miR-335 | - | |
| miR-29b | - | |
| miR-675 | - | |
| miR-7 | - | |
| HuR cooperates with miRNA | miR-26 | RGS4 |
| miR-21、miR-181b | NFI-A | |
| miR-200a | c-JUN | |
| let-7 | MYC | |
| miR-3134 | SOX9、VEGFA、RGFR | |
| HuR promotes the export of miRNAs via extracellular vesicles (EVs) | let-7a、miR-122 | |
| miR-122 | ||
| miR-125b | ||
| let-7i-5p | ||
| let-7a |
LncRNA的长度一般大于200 nt,其转录过程与编码基因相似,转录起始位点和基因内部的组蛋白甲基化特征也与编码基因一致。但lncRNA的外显子较少,总体表达水平较低。功能方面LncRNA可以与miRNA或与其他RNA相互作用调控基因的表达,其中吸附miRNA的lncRNA可通过竞争性内源RNA(competing endogenous RNA,ceRNA)机制解除miRNA对mRNA表达的抑制作用。同时还可以直接与蛋白质结合影响染色质结构、转录因子和RBPs的功能,从转录水平影响基因的表达(Tab 2)。
| Mechanism | lncRNA | Gene |
| lncRNAs promote the binding of HuR to mRNA | lnc00969 | HER2 |
| SNHG3 | CSNK2A1 | |
| ASMTL-AS1 | VEGF | |
| MRPL39-2:1 | CTNNB1 | |
| CASC11 | HDAC4 | |
| RMRP | COX2 | |
| CERS6 antisense RNA 1 | MCU | |
| DNMBP-AS1 | LCLAT1 | |
| TUG1 | COX2 | |
| NKILA | FOXA1 | |
| lncRNAs promote HuR expression via ceRNA mechanism | linc00942(miR-671-5p) | |
| HOTAIR (miR-326) | ||
| MALAT1(miR-191-3p) | ||
| lncRNAs interact with both HuR and miRNAs | linc01857(miR-19b-3p) | MYCN |
| RP1-59D14.5(miR-147a) | CK1 | |
| TUG1(miR-29b-3p) | MYC | |
| MGPF(miR-135a-5p) | MYOG |
LncRNA可以作为ceRNA拮抗miRNA对HuR表达的抑制作用,如HOTAIR[13]、LINC00943[14]、MALAT1[15]。还可以直接与HuR相结合,招募HuR来稳定目的基因的mRNA促进其表达,如lnc00969[16]、ASMTL-AS1[17]、CASC11[18]。这种作用可能还伴随着作为ceRNA吸附miRNA缓解对基因表达的抑制作用[19-21]。综上所述,lncRNA可以作为ceRNA缓解miRNA对HuR表达的抑制,还可以直接与HuR结合为HuR和mRNA的结合提供便利。
1.3 HuR与circRNA的相互作用CircRNA是一类特殊的IncRNA,其因单链共价闭合的RNA分子形成环状结构而得名。CircRNA通常是通过反向剪接产生的,但其确切的生成机制仍未完全阐明。对于circRNA的功能学研究还不完善,它与HuR的相互作用基本与一般lncRNA一致。一方面可以通过海绵作用吸附miRNA发挥ceRNA功能,影响HuR或者HuR所调控的mRNA的表达。另一方面可以直接与HuR结合促进或抑制HuR发挥稳定mRNA的作用(Tab 3)。作为ceRNA的circRNA可以直接吸附miRNA解除其对HuR表达的抑制,如miR-338-3p[22]和miR-182-5p[23]。CircRNA可以直接与HuR结合促进或者抑制HuR与目的mRNA的结合,还可能同时吸附miRNA来改变基因的表达,如RYK[24]、SEMA6A[25]、circ0104103[26]。多数情况下,circRNA与HuR的结合可以促进HuR与mRNA的结合,增加目的基因的表达,在此过程中circRNA可能发挥了支架作用。而circRNA也可能结合HuR后抑制其功能,如PPFIA1-L和-S可以与HuR结合后解除HuR对RAB36 mRNA的稳定作用,降低RAB36的表达[27]。
| Mechanism | lncRNA | Gene |
| circRNA promotes the binding of HuR to mRNA | RHBDD1 | SCD |
| ATP9A | NUCKS1 | |
| NOLC1 | NOLC1 | |
| circ0001944 | PARP1 | |
| EXOC6B | RBMS1 | |
| circRNA inhibits the binding of HuR to mRNA | PPFIA1 | RAB36 |
| ZNF367 | LRP5 | |
| circRNA increases HuR expression via ceRNA mechanism | WHSC1(miR-338-3p) | |
| HECTD1(miR-182-5p) | ||
| circRNAs promote the binding of HuR to mRNA and simultaneously increases HuR expression via ceRNA mechanism | RYK(miR-330-5p) | VLDLR |
| SEMA6A(miR-520h) | PRRG4 | |
| circ0104103(miR-373-5p) | LACTB | |
| circ0060551(miR-3121-3p) | TPD52 |
鉴于HuR-ncRNA相互作用在调控基因表达中的多重功能作用,多个文献研究报道了其在心血管疾病中的作用,包括心脏重构、动脉粥样硬化等。心脏重构往往是心脏过度纤维化并伴随心肌细胞死亡所造成的心脏结构功能变化。生信分析结果显示,ELAVL1是5个主要参与细胞焦亡导致的心脏重构的基因之一。在缺氧再复氧处理的CFs可以分泌富含miR-33a的EVs,抑制受体细胞中的HuR从而降低NLRP3的表达,缓解心肌细胞焦亡[28]。高糖处理心肌细胞后可以刺激HuR的表达,抑制HuR后可以缓解TNF-α导致的心肌细胞焦亡[29]。
HuR还可以通过调节内皮细胞功能障碍、炎症和血栓形成等过程参与动脉粥样硬化的发生和发展。在巨噬细胞和血管平滑肌细胞中IL-19可以通过miR-133a抑制HuR的表达,IL-19敲除的LDLR-/-小鼠的脾脏和主动脉弓中HuR表达上升,加重小鼠动脉粥样硬化[30]。经氧化LDL刺激的巨噬细胞可以分泌富含miR-146a的EVs,抑制受体巨噬细胞中的HuR,阻止巨噬细胞的迁移[31]。
虽然在以上总结的研究提示,HuR可能促进动脉粥样硬化的发生和发展,但有研究表明,HuR有保护内皮细胞的功能。在心脏微血管内皮细胞中,lncRNA CASC11可以与HuR协作促进HDAC4的表达,缓解内皮细胞炎症和损伤[18]。在小鼠内皮细胞中过表达HuR可以逆转H2O2导致的内皮细胞凋亡和功能障碍[32]。
以上文献表明HuR-ncRNA相互作用可以通过心肌细胞铁死亡、成纤维细胞EVs生成、巨噬细胞和内皮细胞炎症反应等参与心血管疾病的发生和发展。
2.2 肠道炎症性疾病除了肿瘤和心血管疾病外,HuR在炎性肠病中的作用也被广泛研究。在肠道上皮细胞Caco-2中,vault RNA2-1可以与HuR结合而抑制HuR对claudin 1和occludin mRNA的稳定作用,抑制两个蛋白的表达而干扰肠道上皮细胞的功能[33]。CircRNA HECTD1可以通过吸附miR-182-5p解除其对HuR表达的抑制作用,介导LPS导致的Caco-2细胞发生炎症和自噬[23]。在Tuft细胞(肠道上皮细胞)中,miR-195可以与HuR竞争mRNA的结合造成肠上皮细胞功能障碍[6]。综上所述,HuR-ncRNA的相互作用可以通过调节上皮细胞炎症、凋亡,参与炎性肠病的发生和发展。
2.3 肾脏相关疾病文献报道显示,HuR-ncRNA相互作用还参与糖尿病肾病以及其他病因导致的肾脏损伤的发病。在急性肾衰模型中,HK-2细胞(肾近曲小管细胞)来源的EVs通过向周围细胞传递miR-122来抑制HuR,从而缓解HuR介导的细胞焦亡[3]。在高糖处理的人微血管内皮细胞中,过表达miR-192-5p后可以抑制HuR的表达,从而缓解高糖导致的内皮细胞增生和迁移。在高糖刺激的HK-2细胞中,miR-30e-5p可以通过抑制HuR缓解高糖导致的细胞焦亡[34]。在同一细胞中,lncRNA MALAT1可以吸附miR-23c从而解除其对HuR的抑制作用,促进HuR介导的NLRP3表达,导致高糖引起的细胞焦亡[35]。
2.4 其他疾病和病理过程此外, HuR-ncRNA相互作用还参与阿尔茨海默、帕金森病等神经损伤疾病的发生。过表达miR-9a-5p可以通过抑制HuR表达缓解外伤造成的脑损伤[2]。LncRNA NKILA可以与HuR协作增加FPXA1的表达,促进阿尔茨海默病的进展[36]。在SK-N-SH细胞中,linc00943可以吸附miRNA解除其对HuR的抑制作用,加重MPP+刺激的细胞损伤[14]。在MPP+刺激的SH-SY5Y神经细胞中,lncRNA HOTAIR可以通过缓解miR-326对HuR的抑制作用,导致NLRP3的表达介导细胞焦亡[13]。
HuR还可能参与细胞的应急反应,包括饥饿刺激、缺氧刺激和热休克刺激等[37]。在饥饿刺激的肝细胞中,HuR可以替代Ago与miRNA结合,促进自身的泛素化后通过EVs排除。在缺氧刺激的子宫内膜基质细胞中,HuR的表达下降失去对DNMT1 mRNA的保护作用[38]。
3 总结HuR可以通过与ncRNA相互作用调节基因的表达,通过介导炎症、细胞焦亡、细胞增殖和自噬等生理病理过程,参与心脏重构、动脉粥样硬化、炎性肠病、糖尿病肾病、帕金森病等疾病的发生和发展(Fig 1)。针对HuR与ncRNA相互作用的机制问题将有助于对疾病治疗靶点的探讨,促进开辟新的药物治疗,为疾病防治带来希望。
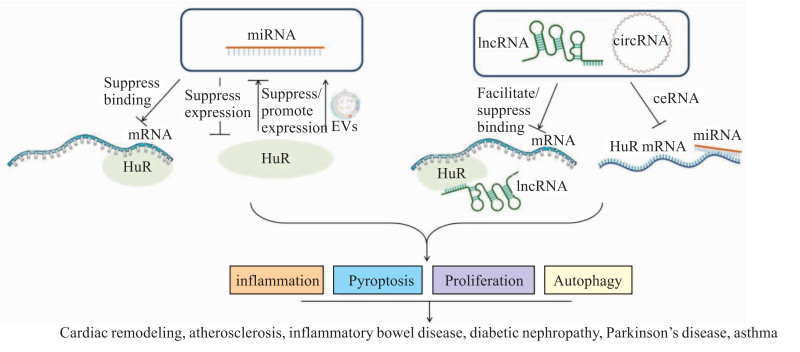
|
| Fig 1 Role of HuR-ncRNA interaction in diseases |
| [1] |
秦玮, 罗志洁, 荣瑞雪, 等. 非编码RNA调控内皮间质转化在疾病中的研究进展[J]. 中国药理学通报, 2024, 40(7): 1215-21. Qin W, Luo Z J, Rong R X, et al. Research progress of non-coding RNAs in regulating endothelial to mesenchymal transition in diseases[J]. Chin Pharmacol Bull, 2024, 40(7): 1215-21. doi:10.12360/CPB202210064 |
| [2] |
Feng C, Tian Q, Tang X, et al. MicroRNA-9a-5p disrupts the ELAVL1/VEGF axis to alleviate traumatic brain injury[J]. Exp Neurol, 2024, 375: 114721. doi:10.1016/j.expneurol.2024.114721 |
| [3] |
Zhu B, He J, Ye X, et al. Role of cisplatin in inducing acute kidney injury and pyroptosis in mice via the exosome miR-122/ELAVL1 regulatory axis[J]. Physiol Res, 2023, 72(6): 753-65. |
| [4] |
Huang Z, Luo Y, Chen C, et al. MiR-325-3p reduces proliferation and promotes apoptosis of gastric cancer cells by inhibiting human antigen R[J]. Can J Gastroenterol Hepatol, 2023, 2023: 6882851. |
| [5] |
Ganguly S, Ghoshal B, Banerji I, et al. Leishmania survives by exporting miR-146a from infected to resident cells to subjugate inflammation[J]. Life Sci Alliance, 2022, 5(6): e202101229. doi:10.26508/lsa.202101229 |
| [6] |
Kwon M S, Chung H K, Xiao L, et al. MicroRNA-195 regulates Tuft cell function in the intestinal epithelium by altering translation of DCLK1[J]. Am J Physiol Cell Physiol, 2021, 320(6): C1042-54. doi:10.1152/ajpcell.00597.2020 |
| [7] |
Kim Y, Noren Hooten N, Dluzen D F, et al. Posttranscriptional regulation of the inflammatory marker C-reactive protein by the RNA-binding protein HuR and microRNA 637[J]. Mol Cell Biol, 2015, 35(24): 4212-21. doi:10.1128/MCB.00645-15 |
| [8] |
Xiao L, Warner B, Mallard C G, et al. Control of Paneth cell function by HuR regulates gut mucosal growth by altering stem cell activity[J]. Life Sci Alliance, 2023, 6(11): e202302152. doi:10.26508/lsa.202302152 |
| [9] |
Guo H, Zhang L, Wang Y, et al. Mechanisms of HuR in regulation of epithelial cell apoptosis in rat ulcerative colitis[J]. Cell Signal, 2021, 82: 109957. doi:10.1016/j.cellsig.2021.109957 |
| [10] |
Kumar R, Poria D K, Ray P S. RNA-binding proteins La and HuR cooperatively modulate translation repression of PDCD4 mRNA[J]. J Biol Chem, 2021, 296: 100154. doi:10.1074/jbc.RA120.014894 |
| [11] |
Bah I, Alkhateeb T, Kumbhare A, et al. Corrigendum to: "huR promotes miRNA-mediated upregulation of NFI-A protein expression in MDSCs during murine sepsis" (Mol Immunol., 2019, july, 123, 97-105)[J]. Mol Immunol, 2020, 127: 56. doi:10.1016/j.molimm.2020.08.012 |
| [12] |
Gunzburg M J, Sivakumaran A, Pendini N R, et al. Cooperative interplay of let-7 mimic and HuR with MYC RNA[J]. Cell Cycle, 2015, 14(17): 2729-33. doi:10.1080/15384101.2015.1069930 |
| [13] |
Zhang Q, Huang X M, Liao J X, et al. LncRNA HOTAIR promotes neuronal damage through facilitating NLRP3 mediated-pyroptosis activation in Parkinson's disease via regulation of miR-326/ELAVL1 axis[J]. Cell Mol Neurobiol, 2021, 41(8): 1773-86. doi:10.1007/s10571-020-00946-8 |
| [14] |
Zhang X, Luan N, Shi J. A novel linc00943/miR-671-5p/ELAVL1 ceRNA crosstalk regulates MPP(+) toxicity in SK-N-SH cells[J]. Metab Brain Dis, 2022, 37(7): 2349-62. doi:10.1007/s11011-022-01034-0 |
| [15] |
Wang Y, Xiao X, Zhou T, et al. Novel mechanisms for osteogenic differentiation of human aortic valve interstitial cells[J]. J Thorac Cardiovasc Surg, 2020, 159(5): 1742-53 e7. doi:10.1016/j.jtcvs.2019.05.051 |
| [16] |
Liu C, Lu C, Yixi L, et al. Exosomal linc00969 induces trastuzumab resistance in breast cancer by increasing HER-2 protein expression and mRNA stability by binding to HuR[J]. Breast Cancer Res, 2023, 25(1): 124. doi:10.1186/s13058-023-01720-6 |
| [17] |
Cai C, Zhi Y, Xie C, et al. Ursolic acid-downregulated long noncoding RNA ASMTL-AS1 inhibits renal cell carcinoma growth via binding to HuR and reducing vascular endothelial growth factor expression[J]. J Biochem Mol Toxicol, 2023, 37(8): e23389. doi:10.1002/jbt.23389 |
| [18] |
Hu K, Huang M J, Ling S, et al. LncRNA CASC11 upregulation promotes HDAC4 to alleviate oxidized low-density lipoprotein-induced injury of cardiac microvascular endothelial cells[J]. Kaohsiung J Med Sci, 2023, 39(8): 758-68. doi:10.1002/kjm2.12687 |
| [19] |
Nie Z, Xu M, Zhou L, et al. LncSNHG3 drives breast cancer progression by epigenetically increasing CSNK2A1 expression level[J]. Aging (Albany NY), 2023, 15(12): 5734-50. |
| [20] |
Tian Y, Ai M, Liu C, et al. Upregulated long non-coding RNA lnc-MRPL39-2:1 induces the growth and invasion of nasopharyngeal carcinoma by binding to HuR and stabilizing beta-catenin mRNA[J]. Int J Biol Sci, 2023, 19(8): 2349-65. doi:10.7150/ijbs.79115 |
| [21] |
Xia H, Shanshan X, Sumeng L, et al. LncRNA RMRP aggravates LPS-induced HK-2 cell injury and AKI mice kidney injury by upregulating COX2 protein via targeting ELAVL1[J]. Int Immunopharmacol, 2023, 116: 109676. doi:10.1016/j.intimp.2022.109676 |
| [22] |
李颖, 李玉杰, 于敏, 等. circ-WHSC1通过靶向作用于miR-338-3p/ELAVL1轴影响鼻咽癌细胞的生物学特征和放射敏感性[J]. 中华肿瘤杂志, 2022, 44(11): 1175-85. Li Y, Li Y J, Yu M, et al. [circ-WHSC1 affects the growth, metastasis and radiotherapy sensitivity of nasopharyngeal carcinoma cells by targeting miR-338-3p/ELAVL1 axis][J]. Chin J Oncol, 2022, 44(11): 1175-85. |
| [23] |
Xu Y, Tian Y, Li F, et al. Circular RNA HECTD1 mitigates ulcerative colitis by promoting enterocyte autophagy via miR-182-5p/HuR axis[J]. Inflamm Bowel Dis, 2022, 28(2): 273-88. doi:10.1093/ibd/izab188 |
| [24] |
Wang Y, Wang B, Cao W, et al. TGF-beta-activated circRYK drives glioblastoma progression by increasing VLDLR mRNA expression and stability in a ceRNA- and RBP-dependent manner[J]. J Exp Clin Cancer Res, 2024, 43(1): 73. doi:10.1186/s13046-024-03000-3 |
| [25] |
Liu Y, Xin Y, Shang X, et al. CircSEMA6A upregulates PRRG4 by targeting miR-520h and recruiting ELAVL1 to affect cell invasion and migration in papillary thyroid carcinoma[J]. Arch Endocrinol Metab, 2024, 68: e210541. doi:10.20945/2359-4292-2021-0541 |
| [26] |
Tan P, Sun H, Xu M, et al. Circular RNA circ0104103 inhibits colorectal cancer progression through interactions with HuR and miR-373-5p[J]. Cancer Sci, 2023, 114(4): 1396-409. doi:10.1111/cas.15695 |
| [27] |
Ji H, Kim T W, Lee W J, et al. Two circ PPFIA 1s negatively regulate liver metastasis of colon cancer via miR-155-5p/CDX1 and HuR/RAB36[J]. Mol Cancer, 2022, 21(1): 197. doi:10.1186/s12943-022-01667-w |
| [28] |
Liu N, Xie L, Xiao P, et al. Cardiac fibroblasts secrete exosome microRNA to suppress cardiomyocyte pyroptosis in myocardial ischemia/reperfusion injury[J]. Mol Cell Biochem, 2022, 477(4): 1249-60. doi:10.1007/s11010-021-04343-7 |
| [29] |
Jeyabal P, Thandavarayan R A, Joladarashi D, et al. MicroRNA-9 inhibits hyperglycemia-induced pyroptosis in human ventricular cardiomyocytes by targeting ELAVL1[J]. Biochem Biophys Res Commun, 2016, 471(4): 423-9. doi:10.1016/j.bbrc.2016.02.065 |
| [30] |
Ray M, Gabunia K, Vrakas C N, et al. Genetic deletion of IL-19 (interleukin-19) exacerbates atherogenesis in IL-19(-/-)x Ldlr(-/-) double knockout mice by dysregulation of mRNA stability protein HuR (human antigen R)[J]. Arterioscler Thromb Vasc Biol, 2018, 38(6): 1297-308. doi:10.1161/ATVBAHA.118.310929 |
| [31] |
Nguyen M A, Karunakaran D, Geoffrion M, et al. Extracellular vesicles secreted by atherogenic macrophages transfer microRNA to inhibit cell migration[J]. Arterioscler Thromb Vasc Biol, 2018, 38(1): 49-63. doi:10.1161/ATVBAHA.117.309795 |
| [32] |
Sui X, Yu S, Dou L, et al. MiR-291b-3p mediated ROS-induced endothelial cell dysfunction by targeting HuR[J]. Int J Mol Med, 2018, 42(5): 2383-92. |
| [33] |
Ma X X, Xiao L, Wen S J, et al. Small noncoding vault RNA2-1 disrupts gut epithelial barrier function via interaction with HuR[J]. EMBO Rep, 2023, 24(2): e54925. doi:10.15252/embr.202254925 |
| [34] |
Lv J, Hao Y N, Wang X P, et al. Bone marrow mesenchymal stem cell-derived exosomal miR-30e-5p ameliorates high-glucose induced renal proximal tubular cell pyroptosis by inhibiting ELAVL1[J]. Ren Fail, 2023, 45(1): 2177082. doi:10.1080/0886022X.2023.2177082 |
| [35] |
Li X, Zeng L, Cao C, et al. Long noncoding RNA MALAT1 regulates renal tubular epithelial pyroptosis by modulated miR-23c targeting of ELAVL1 in diabetic nephropathy[J]. Exp Cell Res, 2017, 350(2): 327-35. doi:10.1016/j.yexcr.2016.12.006 |
| [36] |
Zhou Y, Wang Y, Wang Y, et al. LncRNA NKILA exacerbates alzheimer's disease progression by regulating the FOXA1-mediated transcription of TNFAIP1[J]. Neurochem Res, 2023, 48(9): 2895-910. doi:10.1007/s11064-023-03944-6 |
| [37] |
Kraynik S M, Gabanic A, Anthony S R, et al. The stress-induced heat shock protein 70.3 expression is regulated by a dual-component mechanism involving alternative polyadenylation and HuR[J]. Biochim Biophys Acta, 2015, 1849(6): 688-96. doi:10.1016/j.bbagrm.2015.02.004 |
| [38] |
Hsiao K Y, Wu M H, Chang N, et al. Coordination of AUF1 and miR-148a destabilizes DNA methyltransferase 1 mRNA under hypoxia in endometriosis[J]. Mol Hum Reprod, 2015, 21(12): 894-904. doi:10.1093/molehr/gav054 |

