

2. 赤峰学院医学部,内蒙古 赤峰 024000;
3. 内蒙古人类遗传病研究重点实验室,内蒙古 赤峰 024000;
4. 赤峰学院附属医院神经内科, 内蒙古 赤峰 024005
2. Chifeng University Health Science Center, Chifeng, Inner Mongolia 024000, China;
3. Key Laboratory of Human Genetic Diseases in Inner Mongolia, Chifeng, Inner Mongolia 024000, China;
4. Dept of Neurology, the Affiliated Hospital of Chifeng University, Chifeng, Inner Mongolia 024005, China
传统中药多年来一直用于预防和治疗多种疾病,现在又重新激发了全球研究者的兴趣,而用于治疗各种神经系统疾病的中草药提取物也越来越受到关注。神经退行性疾病的预防和治疗对传统医学来说是一个挑战,需要更多的研究,因为多靶点、多途径和多层次疗效,副作用较少,使传统中药在这一领域显示出独特的优势和潜力。近年来,对中药单体的研究已成为中医药研究抗病机制的主要途径。中药单体及其活性成分在多种疾病中的作用机制正在逐步被发现,这为中药复方应用研究提供了理论依据。
川芎嗪(tetramethylpyrazine, TMP)是中药川芎(Ligusticum wallichii Franch)的重要活性成分之一,其化学名为2,3,5,6-川芎嗪(2,3,5,6-tetramethylpyrazine,C8H12N2) [1]。最近研究显示,TMP具有神经保护活性,在帕金森病(Parkinson’s disease, PD)中的神经保护作用取得了一定进展,本文对TMP在PD中的神经保护作用及其机制进行了综述,表明TMP是治疗PD的潜在药物。
1 川芎嗪的来源和药理学特性川芎嗪也称为2,3,5,6-川芎嗪,属于TMP酰胺类生物碱,是从川芎中(Ligusticum chuanxiong Hort)提取的主要生物活性化合物之一,已在中国和东南亚国家临床用于预防与治疗神经退行性疾病和心脑血管疾病[2-3]。在传统中医药中,川芎嗪又名天然四甲基吡嗪,是从伞形科藁本属植物川芎中分离提纯的生物碱单体,是中药川芎的主要有效成分,是活血行气祛瘀的一种中药活性生物碱。川芎又是药膳中常用的药材之一。
TMP最早于1957年分离出来,自20世纪70年代以来,因其对心肌梗死和脑梗死的作用而被越来越多地研究[4]。
在过去几十年中,研究人员探索了TMP在各种疾病中的药理作用。以往的研究表明,TMP具有多种药理活性,包括抗癌、抗炎、钙拮抗、自由基清除、抗氧化、抗血小板和抗凋亡等[5-6]。系统给予TMP可通过抑制体内炎症反应来预防缺血和脊髓损伤引起的神经元丢失。TMP可能通过减少炎症细胞活化和促炎介质的产生来抑制体内的炎症事件[7]。
2 在PD中的神经保护作用机制近年来,TMP在治疗神经系统疾病中作用引起学者关注。自2007年王丹巧等[18]首次证实TMP在PD中具有神经保护作用以来,TMP在PD中的应用研究越来越多。TMP能够通过多种机制在多种PD的体内、外模型中具有神经保护作用,见Tab 1。
| Ref | Compound | Compound dose | Experimental model | Effects | Signaling |
| [8] | CXC195 | 10 mg·kg-1, i.p., for 14 days | 6-OHDA/Male C57BL/6 mice | ↑The content of DA in the STR; ↓ cleaved caspase-3 and Bax; ↑Bcl-2; |
↓pAkt;↑ GSK-3β |
| [9] | TN-2 | 100~400 μmol·L-1, pretreatment |
6-OHDA/PC12 cell | ↑Cell viability; ↓ LDH leakage; ↓ Apoptosis; ↓ NO; ↓ iNOS |
↓NF-κB;↑PKCα/PI3-K/Akt |
| [9] | TN-2 | 48 h with 100~400 μmol·L-1 | 6-OHDA/Zebrafifish | ↓ IL-1β, TNF-α and COX-2 mRNA | NA |
| [9] | TN-2 | 30 mg·kg-1, once daily for 14 days. |
6-OHDA/SD rats | ↓ Microglial/astrocyte activation | NA |
| [10] | T-006 | 10~30 μmol·L-1 | PC12/A53T-α-syn cell | ↑α-syn degradation; ↑LMP7 protein | ↑PKA/Akt/mTOR/p70S6K |
| [10] | T-006 | 3 mg·kg-1 | A53T-α-syn transgenic mice | ↑α-syn degradation; ↑LMP7 protein | ↑PKA/Akt/mTOR/p70S6K |
| [11] | T-006 | 1~10 mg·kg-1 | MPTP/C57BL/6 Mice | ↑Locomotor behavior; ↑nigra dopaminergic neurons; ↑ striatal dopamine levels | ↑Akt/GSK3β |
| [11] | T-006 | 1~10 mg·kg-1 | 6-OHDA/Female SD rats | ↑Locomotor behavior; ↑nigra dopaminergic neurons; ↑ striatal dopamine levels; ↑neurogenesis; ↑BDNF; ↑CREB | ↑Akt/GSK3β |
| [11] | T-006 | 0.1~10 μmol·L-1 | MPP+/CGNs | ↑Cell viability; ↓ LDH release; ↓ apoptosis; ↑MMP; ↑complex I activity; ↑ATP content | ↑Akt/GSK3/MFF2D; ↑Nrf2 |
| [11] | T-006 | 3~30 μmol·L-1 | 6-OHDA//CGNs | ↑Cell viability; ↓ LDH release; | NA |
| [12] | T-006 | 10~30 μmol·L-1 | 6-OHDA/PC12 | ↑MMP; restored the energy metabolism; ↑mitochondrial biogenesis | ↑PKA/Akt/GSK-3β;↑CREB/PGC-1α/NRF-1/ TFAM |
| [12] | T-006 | 3 mg·kg-1 gavage for 16 days | 6-OHDA/C57BL/6 mice | ↑TH-positive neurons in the SNpc; ↑dopaminergic nerve fibers in the striatum; ↑ striatal dopamine levels; ↑motor coordination and rotational behavior. |
↑PKA/Akt/GSK-3β |
| [13] | DT-010 | 3~30 μmol·L-1 | 6-OHDA/PC12 | ↑Cell survival; ↓ROS; ↑Bcl-2; ↓Bax; ↓apoptosis; MEF2D |
↑PI3K/Akt/GSK3β |
| [13] | DT-010 | 3~30 μmol·L-1 | 6-OHDA/PCGN | ↑Cell survival; ↓apoptosis | ↑PI3K/Akt/GSK3β |
| [14] | TN-2 | 20~60 mg·kg-1, i.p. for 14 days | MPTP/male C57BL/6 mice | ↑TH-positive neurons in the SNpc; ↑striatal dopamine level; ↑SOD activity; ↑GSH concentration; ↓MDA | NA |
| [14] | TN-2 | 200~400 μmol·L-1 | MPP+/PCGNs | ↑Cell viability↓apoptosis; ↑PGC- 1α and β/Tfam | NA |
| [14] | TN-2 | 200~400 μmol·L-1 | MPP+/SH-SY5Y | ↑Cell survival; ↓ LDH leakage; ↓apoptosis; ↓ROS; ↑MMP; ↑Bcl-2;↓Bax; ↓ cytochrome c release; ↓ caspase-9 activation; ↓ caspase-3 activation; ↑PGC- 1α and β/Tfam | NA |
| [15] | TMP | 20 mg·kg-1·d-1, i.p.for 7 days | MPTP/male Wistar rats | ↓Motor deficit; ↑TH expression; ↑striatal dopamine level; ↑Bcl-2;↓Bax; ↓ cytochrome c release; ↓ caspase-3 activation; ↓TBARS level; ↑GSH level, |
↑Nrf2/GCLC |
| [16] | TMP | 3~30 mg·kg-1·d-1, i.p.for 5 days | MPTP/male C57B/6 mice | ↑Behavioral performance; ↓TNF-α; ↓IL-1β | NA |
| [17] | TMP | 10, 20, and 40 mg·kg-1, i.p. for 4 weeks | Rotenone/Male SD rats | ↑Midbrain and striatal TH expression; ↑striatal dopamine content; ↓ motor deficits; ↓α-synuclein; ↓Bax/Bcl2 ratio(striatal); ↓ caspase-3 activation; ↓ iNOS, COX2, GFAP and DJ-1expression (midbrain and striatum); | ↓NF-кB |
| [7] | TBN | 50~500 μmol·L-1 | MPP+/SH-SY5Y | ↓ROS | NA |
| [7] | TBN | 100~500 μmol·L-1 | MPTP/Zebrafifish | ↑TH-positive neurons. | NA |
| [7] | TBN | 20~80 mg·kg-1·d-1, orally twice daily for 14 days | MPTP/Male C57BL/6 mice | ↑SOD activity; ↑GSH; ↑TH-positive neurons; ↑Striatal DA |
NA |
| [7] | TBN | 5~45 mg·kg-1·d-1, orally twice daily for 14 days | 6-OHDA/male SD rats | ↑TH-positive DA neurons in the SNpc | NA |
| TMP, Tetramethylpyrazine; CXC195, TMP Analogue; 6-OHDA, 6-hydroxydopamine; STR, Striatum; TN-2, tetramethylpyrazine bis-nitrone; T-006, tetramethylpyrazine derivative; LMP7, 20S proteasome subunit β5i;MEF2D, myocyte enhancer factor 2D;PGC1α, peroxisome proliferator-activated receptor γ(PPARγ) co-activator 1α; Nrf1/2, NF-E2-related factor 1/2;BDNF, brain-derived neurotrophic factor; CREB, cAMP responsive element-binding protein; CGNs, Cerebellar granule neurons; DT-010, a novel analogue in which TMP; PCGN, primary cerebellar granule neurons; TN-2, Tetramethylpyrazine Bis-nitrone; Tfam, mitochondrial transcription factor; GCLC, glutathione cysteineligase catalytic subunit; TBARS, Thiobarbituric acid reactive substances; GSH, glutathione; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; TBN, 2-[[(1, 1-Dimethylethyl)oxidoimino]-methyl]-3, 5, 6-trimethylpyrazine, a novel nitrone derivative of tetramethylpyrazine | |||||
α-突触核蛋白(α-synuclein, α-syn)是PD患者脑内多巴胺能神经元内路易小体的主要成分,错误折叠的α-syn在神经元内积聚。编码α-syn的SNCA基因突变及其拷贝数的变化与某些形式家族性PD有关[19]。研究显示,在细胞PD模型中,一种TMP新的衍生物T-006具有促进α-syn降解的作用[10]。T-006抑制可溶性和非可溶性形式α-syn的积累,并对A53T-α-syn转基因小鼠中α-syn诱导的神经毒性具有抑制作用。机制研究发现T-006以蛋白酶体依赖和自噬非依赖的方式促进α-syn降解。T-006通过上调20S蛋白酶体亚单位β5i(20S proteasome subunit β5i, LMP7)的表达从而增强蛋白酶体活性。T-006激活PKA/Akt/mTOR/p70S6K通路,触发LMP7表达,增强糜蛋白酶样蛋白酶体活性(chymotrypsin-like proteasomal activity)。T-006是一种有效的蛋白酶体激活剂,是预防和治疗PD及相关疾病的潜在治疗药物[10]。另外研究发现,TMP在鱼藤酮诱导的PD大鼠模型中,能够减少中脑和纹状体内α-syn蛋白的表达,表明TMP具有抑制α-syn生成的功能[17]。TMP能够在PD中阻止α-syn的聚集,主要通过抑制α-syn寡聚体形成来实现。
2.2 抗氧化损伤作用氧化应激损伤是PD的重要特征之一,抑制氧化应激成为治疗PD的重要靶点之一[20]。TMP的新型硝基衍生物TBN对1-甲基-4-苯基吡啶(MPP+)诱导的SH-SY5Y细胞和原代培养的多巴胺能神经元,以及对1-甲基-4-苯基-1, 2, 3, 6-四氢吡啶(MPTP)诱导的斑马鱼多巴胺能神经元丢失具有显著的神经保护作用[7]。在MPTP诱导的小鼠和6-羟基多巴胺(6-OHDA)诱导的大鼠PD模型中,小鼠MPTP损伤后3 d和大鼠6-OHDA损伤后7 d给药TBN(每天灌胃给药两次,持续14 d),TBN能增加多巴胺能神经元的数量,同时改善6-OHDA损伤PD大鼠运动障碍[7]。TBN抑制MPP+诱导的SH-SY5Y细胞内活性氧(ROS),提高MPTP处理小鼠黑质的超氧化物歧化酶(SOD)活性和谷胱甘肽(GSH)浓度[7]。这些数据表明,TBN通过减少ROS和增加细胞抗氧化防御能力,保护和拯救多巴胺能神经元免受MPP+和MPTP/6-OHDA诱导的损伤[7]。后续研究也证实,TMP的抗氧化应激损伤作用,TMP能抑制MPTP导致的TBARS水平升高,降低GSH水平,提示TMP在PD模型中具有抗氧化作用[15]。TMP的新型硝基衍生物TN-2也能够抑制MPP+诱导的SH-SY5Y细胞中ROS产生增加[14]。TMP的新型类似物DT-010能够在MPP+诱导的PC12细胞和原代小脑颗粒神经元中减少ROS[13]。综上所述,TMP能够在PD体内外模型中中抑制神经毒素诱导的氧化应激损伤。
2.3 抗凋亡作用细胞凋亡被认为是PD神经元死亡的主要机制。在PD的背景下,患者和实验模型的大量证据支持内在凋亡在多巴胺能神经退行性变中的作用[20]。抑制凋亡成为治疗PD的重要靶点之一[21]。TMP能显著减弱MPTP诱导的多巴胺能神经元损伤,表现为运动障碍的改善、TH表达的增强、多巴胺及其代谢物DOPAC含量的增加,同时,通过Bax的上调、Bcl-2的下调、细胞色素c(Cyt C)的释放和Caspase-3激活抑制MPTP诱导的线粒体凋亡死亡途径的激活[15]。而TMP的衍生物TN-2能显著逆转MPTP诱导的小鼠黑质多巴胺能神经元的丢失和纹状体多巴胺水平的降低[14]。此外,TN-2抑制MPP+诱导的原代小脑颗粒神经元和SH-SY5Y细胞的神经元损伤/凋亡。TN-2阻止线粒体膜电位的丧失,阻止线粒体细胞色素c的释放,抑制Caspase-3和Caspase-9的激活[14],这些结果表明,TN-2可以通过抑制线粒体依赖性凋亡来保护多巴胺能神经元免受MPTP/MPP+诱导的神经毒性。TMP新型类似物DT-010能够在MPP+诱导的PC12细胞和原代小脑颗粒神经元中减少凋亡发生[13]。在鱼藤酮诱导的大鼠PD模型中,TMP显著减弱鱼藤酮诱导的中脑和纹状体Caspase-3激活以及Bax/Bcl-2比值的升高[17]。TMP的另一种衍生物CXC195,能够在6-OHDA诱导的PD小鼠模型中,改善6-OHDA诱导的PD小鼠DA神经退行性病变,主要与其抑制Caspase-3激活和Bax水平升高,并增加Bcl-2水平有关[8]。综上所述,TMP及其衍生物能够在PD体内外模型中通过抑制神经毒素诱导的凋亡,发挥神经保护作用。
2.4 抗炎作用慢性神经炎症在PD的进展过程中发挥重要作用,PD患者脑内受累脑区内发现小胶质细胞和星形胶质细胞的激活。在PD患者脑内、脑脊液和血液中存在高浓度的促炎性因子(如IL-1β、IL-6和TNF-α)的水平升高。小胶质细胞和星形胶质细胞激活产生的促炎性因子导致突触功能障碍和突触的丢失,而慢性炎症也可以导致路易体的形成进而参与PD发病机制。因此,抑制慢性炎症成为PD潜在的治疗靶点[22-23]。研究显示,TN-2对6-OHDA诱导的PC12细胞凋亡具有强大的神经保护作用,在6-OHDA处理的斑马鱼中,TN-2可防止6-OHDA诱导的多巴胺能神经元丢失,并抑制包括IL-1β、TNF-α和COX-2在内的促炎基因的mRNA表达,同时TN-2显著减弱6-OHDA大鼠模型中的小胶质细胞/星形胶质细胞激活[9],进一步机制研究发现,TN-2通过抑制NF-κB活性进而抑制细胞内一氧化氮(nitric oxide, NO)的过度产生和诱导型一氧化氮合酶(inducible nitric oxide synthase, iNOS)的蛋白表达,而PKCα/PI3-K/Akt通路参与了TN-2的神经保护作用。总之,TN-2通过调节NF-κB介导的神经炎症和PKCα/PI3-K/Akt通路,抑制6-OHDA诱导的神经毒性损伤[9]。在MPTP诱导的PD小鼠模型中,TMP还降低小鼠黑质和纹状体中IL-1β和TNF-α细胞因子的上调[16]。最近研究印证了TMP的抗炎作用,在鱼藤酮诱导的大鼠PD模型中,TMP下调中脑和纹状体中神经炎症标记物NF-κB、iNOS、COX2和GFAP的表达[17]。综上所述,TMP及其衍生物能够在PD体内外模型中通过抗炎发挥神经保护作用。
2.5 调控DA神经递质系统PD最主要的病理改变是中脑黑质多巴胺能神经元的变性死亡,由此而引起纹状体DA含量显著性减少而致病。因此,恢复纹状体DA含量成为治疗PD的重要靶点。TMP抑制MPTP诱导的大鼠多巴胺能神经元损伤,促进TH表达,并可升高SN内多巴胺及其代谢物[3, 4-二羟基苯乙酸(3, 4-dihydroxyphenyl acetic acid,DOPAC]的含量[15]。进一步研究发现,在鱼藤酮诱导的大鼠PD模型中,TMP (20 mg·kg-1)能够显著抑制鱼藤酮诱导的中脑黑质TH表达,并增加纹状体内多巴胺含量[17]。TN-2能显著逆转MPTP诱导的小鼠黑质多巴胺能神经元的丢失和纹状体多巴胺水平的降低[14]。在6-OHDA和MPTP损伤小鼠中,T-006治疗后神经功能缺陷得以恢复,运动协调性和旋转行为得到改善,同时,T-006促进黑质多巴胺能神经元的存活和促进纹状体内多巴胺的含量[11-12]。综上所述,TMP及其衍生物具有升高脑内DA,但其机制是否与抑制DA降解仍不清楚。
2.6 调控Nrf2/ARE抗氧化防御体系通常PD中存在内源性抗氧化防御体系的耗竭,从而加重氧化应激损伤[24]。因此,恢复抗氧化防御体系成为治疗PD的重要靶标[25-26]。TMP能抑制MPTP导致的TBARS水平升高和GSH水平降低,提示TMP在PD模型中具有抗氧化作用。TMP的抗氧化作用归因于其能够上调MPTP诱导的Nrf2和GCLC表达减少, 表明在MPTP诱导的PD模型中,TMP能够阻止Nrf2和GCLC的下调,维持氧化还原平衡,抑制神经元细胞凋亡,从而减轻多巴胺能神经元的损伤[15]。而TN-2改善了MPTP诱导的脑超氧化物歧化酶活性和谷胱甘肽浓度的降低,以及脑丙二醛的增加[14]。而TBN上调MPTP处理小鼠黑质的超氧化物歧化酶(SOD)活性和谷胱甘肽(GSH)浓度[7]。综上所述,TMP及其衍生物具有调控Nrf2/ARE抗氧化防御体系。
3 结语综上所述,TMP通过多种机制在PD中发挥其神经保护作用。其可以在PD的细胞模型和动物模型中具有神经保护作用。TMP及其衍生物促进受损伤神经细胞和其他细胞的修复等作用,同时它们通过促进脑内DA升高,调节脑内病变区的神经递质平衡,发挥控制帕金森病震颤症状的作用,控制症状、抑制病情发展、促进疾病恢复方面显示出一定的特点,而且具有良好的安全性。鉴于TMP在缺血性中风、胸胁刺痛、跌打肿痛、头痛、高血压、冠状粥样硬化性心脏病和缺血性脑病等的临床使用,TMP对PD治疗的临床实验和可能的疗效值得期待。TMP在PD中的神经保护作用及其机制总结见Fig 1。
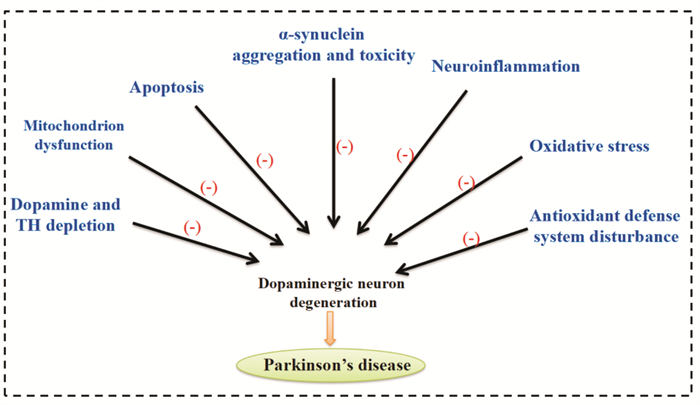
|
| Fig 1 Schematic representation of TMP-mediated neuroprotection in PD |
| [1] |
Yan Y, Zhao J, Cao C, et al. Tetramethylpyrazine promotes SH-SY5Y cell differentiation into neurons through epigenetic regulation of Topoisomerase Ⅱβ[J]. Neuroscience, 2014, 278: 179-93. doi:10.1016/j.neuroscience.2014.08.010 |
| [2] |
黄文东, 杨永飞, 陈建文, 等. 丹参素与川芎嗪对心血管系统的协同作用[J]. 中国药理学通报, 2013, 29(3): 432-6. Huang W D, Yang Y F, Chen J W, et al. Synergism of tanshinol and tetramethylpyrazine on cardiovascular system in rats[J]. Chin Pharmacol Bull, 2013, 29(3): 432-6. doi:10.3969/j.issn.1001-1978.2013.03.029 |
| [3] |
刘丹, 尹东, 曾姝, 等. 川芎嗪对LPS致心肌细胞损伤的影响及机制研究[J]. 中国药理学通报, 2012, 28(11): 1531-5. Liu D, Yin D, Zeng S, et al. Effects of TMPZ on LPS-nduced cardiac cell damage and involved mechanism[J]. Chin Pharmacol Bull, 2012, 28(11): 1531-5. |
| [4] |
Zhao Y, Liu Y, Chen K. Mechanisms and clinical application of tetramethylpyrazine (an interesting natural compound isolated from Ligusticum Wallichii): current status and perspective[J]. Oxid Med Cell Longev, 2016, 2016: 2124638. |
| [5] |
Li J, Gong X. Tetramethylpyrazine: an active ingredient of Chinese herbal medicine with therapeutic potential in acute kidney injury and renal fibrosis[J]. Front Pharmacol, 2022, 13: 820071. doi:10.3389/fphar.2022.820071 |
| [6] |
Yang S, Wu S, Dai W, et al. Tetramethylpyrazine: a review of its antitumor potential and mechanisms[J]. Front Pharmacol, 2021, 12: 764331. doi:10.3389/fphar.2021.764331 |
| [7] |
Guo B, Xu D, Duan H, et al. Therapeutic effects of multifunctional tetramethylpyrazine nitrone on models of Parkinson's disease in vitro and in vivo[J]. Biol Pharm Bull, 2014, 37(2): 274-85. doi:10.1248/bpb.b13-00743 |
| [8] |
Chen L, Cheng L, Wei X, et al. Tetramethylpyrazine analogue CXC195 protects against dopaminergic neuronal apoptosis via activation of PI3K/Akt/GSK3β signaling pathway in 6-OHDA-induced Parkinson's disease mice[J]. Neurochem Res, 2017, 42(4): 1141-50. doi:10.1007/s11064-016-2148-x |
| [9] |
Xu D P, Zhang K, Zhang Z J, et al. A novel tetramethylpyrazine bis-nitrone (TN-2) protects against 6-hydroxyldopamine-induced neurotoxicity via modulation of the NF-κB and the PKCα/PI3-K/Akt pathways[J]. Neurochem Int, 2014, 78: 76-85. doi:10.1016/j.neuint.2014.09.001 |
| [10] |
Zhou H, Shao M, Guo B, et al. Tetramethylpyrazine analogue T-006 promotes the clearance of alpha-synuclein by enhancing proteasome activity in Parkinson's disease models[J]. Neurotherapeutics, 2019, 16(4): 1225-36. doi:10.1007/s13311-019-00759-8 |
| [11] |
Chen H, Cao J, Zha L, et al. Neuroprotective and neurogenic effects of novel tetramethylpyrazine derivative T-006 in Parkinson's disease models through activating the MEF2-PGC1α and BDNF/CREB pathways[J]. Aging (Albany NY), 2020, 12(14): 14897-917. |
| [12] |
Zhou H, Shao M, Yang X, et al. Tetramethylpyrazine analogue T-006 exerts neuroprotective effects against 6-Hydroxydopamine-induced Parkinson's disease in vitro and in vivo[J]. Oxid Med Cell Longev, 2019, 2019: 8169125. |
| [13] |
Hu S, Wang L, Mak S, et al. Potent protection against MPP+-induced neurotoxicity via activating transcription factor MEF2D by a novel derivative of naturally occurring Danshensu/Tetramethylpyrazine[J]. Neuromolecular Med, 2016, 18(4): 561-72. doi:10.1007/s12017-016-8399-5 |
| [14] |
Xu D, Duan H, Zhang Z, et al. The novel tetramethylpyrazine bis-nitrone (TN-2) protects against MPTP/MPP+-induced neurotoxicity via inhibition of mitochondrial-dependent apoptosis[J]. J Neuroimmune Pharmacol, 2014, 9(2): 245-58. doi:10.1007/s11481-013-9514-0 |
| [15] |
Lu C, Zhang J, Shi X, et al. Neuroprotective effects of tetramethylpyrazine against dopaminergic neuron injury in a rat model of Parkinson's disease induced by MPTP[J]. Int J Biol Sci, 2014, 10(4): 350-7. doi:10.7150/ijbs.8366 |
| [16] |
Zhao H, Xu M L, Zhang Q, et al. Tetramethylpyrazine alleviated cytokine synthesis and dopamine deficit and improved motor dysfunction in the mice model of Parkinson's disease[J]. Neurol Sci, 2014, 35(12): 1963-7. doi:10.1007/s10072-014-1871-9 |
| [17] |
Michel H E, Tadros M G, Esmat A, et al. Tetramethylpyrazine ameliorates rotenone-induced Parkinson's Disease in rats: involvement of its anti-Inflammatory and anti-apoptotic actions[J]. Mol Neurobiol, 2017, 54(7): 4866-78. doi:10.1007/s12035-016-0028-7 |
| [18] |
王丹巧, 王巍, 景富春, 等. 川芎嗪对帕金森病大鼠脑内灌流左旋多巴引起的脑氧化损伤的作用[J]. 中国中西医结合杂志, 2007, 27(7): 629-32. Wang D Q, Wang W, Jing F C, et al. Effects of tetramethylpyrazine on brain damage induced by intracerebral perfusion of L-DOPA in rat with Parkinson's disease[J]. Chin J Integr Tradit West Med, 2007, 27(7): 629-32. |
| [19] |
袁惠莉, 汪璇, 张丽娟, 等. 中药在防治帕金森病中的作用及研究进展[J]. 中国药理学通报, 2010, 26(7): 850-4. Yuan HL, Wang X, Zhang L J, et al. Mechanisn and research progress of Chinese Traditional Medicine in the prevention and treatment of Parkinson's disease[J]. Chin Pharmacol Bull, 2010, 26(7): 850-4. |
| [20] |
Dionísio PA, Amaral JD, Rodrigues C. Oxidative stress and regulated cell death in Parkinson's disease[J]. Ageing Res Rev, 2021, 67: 101263. doi:10.1016/j.arr.2021.101263 |
| [21] |
Parkinson's disease. Pathogenesis and clinical aspects[M]. Brisbane (AU): Codon Publications, 2018.
|
| [22] |
Lee Y, Lee S, Chang S C, Lee J. Significant roles of neuroinflammation in Parkinson's disease: therapeutic targets for PD prevention[J]. Arch Pharm Res, 2019, 42(5): 416-25. doi:10.1007/s12272-019-01133-0 |
| [23] |
Hassanzadeh K, Rahimmi A. Oxidative stress and neuroinflammation in the story of Parkinson's disease: could targeting these pathways write a good ending?[J]. J Cell Physiol, 2018, 234(1): 23-32. |
| [24] |
Hemmati-Dinarvand M, Saedi S, Valilo M, et al. Oxidative stress and Parkinson's disease: conflict of oxidant-antioxidant systems[J]. Neurosci Lett, 2019, 709: 134296. doi:10.1016/j.neulet.2019.134296 |
| [25] |
Wang Y, Gao L, Chen J, et al. Pharmacological modulation of Nrf2/HO-1 signaling pathway as a therapeutic target of Parkinson's disease[J]. Front Pharmacol, 2021, 12: 757161. doi:10.3389/fphar.2021.757161 |
| [26] |
王玉敏, 崔其福, 赵伟丽, 等. Nrf2/ARE/HO-1通路是治疗帕金森病的作用新靶点[J]. 基础医学与临床, 2014, 34(8): 1125-8. Wang Y M, Cui Q F, Zhao W L, et al. Nrf2/ARE/HO-1 signaling pathway is neuroproteetive target for Parkinson's disease[J]. Basic Clin Med, 2014, 34(8): 1125-8. |

