

2. 山东第一医科大学附属中心医院,山东 济南 250013
2. Central Hospital Affiliated to Shandong First Medical University, Jinan 250013, China
疼痛在2020的新定义是一种与实际或潜在的组织损伤相关的不愉快的感觉和情绪情感体验,或相似的经历。外界刺激可引起细胞外液中K+、5-HT、缓激肽、前列腺素等炎症介质刺激痛觉感受器产生电信号,经有关神经元传至较高级的疼痛中枢──丘脑、大脑皮质以及其他脑区,引起疼痛的感觉和反应[1]。疼痛分类方式较多,根据疼痛机制可分为伤害性疼痛(躯体疼痛和内脏疼痛)和非伤害性疼痛(神经疼痛和心理疼痛);根据疼痛持续时间可分为急性疼痛和慢性疼痛;根据引起疼痛的原因可区分出炎性痛、癌痛等。目前全球有40%左右的人面临着疼痛的威胁,若不加以控制,可造成一系列生理、心理病理变化。临床常用的镇痛药有非甾体类抗炎镇痛药、阿片类镇痛药和抗抑郁抗惊厥类药,但存在副作用大、疗效不佳、易产生耐受性等问题。随着科学探索的发展,人们逐渐认识到天然产物具有更好的疗效。
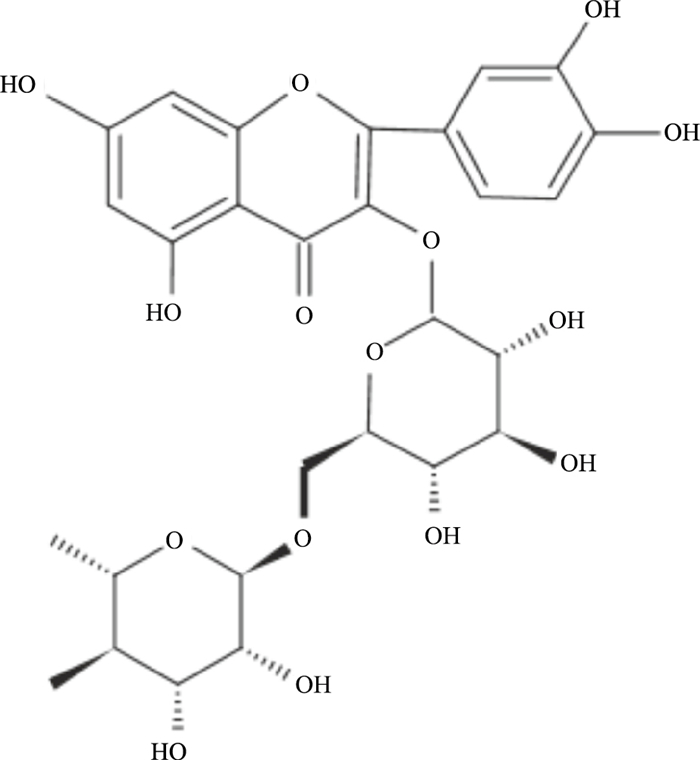
芦丁异名芸香苷又称维生素P、络通,分子式为C27H30O16(Fig 1),在芸香科植物芸香Ruta graveolens L.及豆科植物槐Scphora japonica L.的花蕾槐米中含量丰富[2]。芦丁具有抗炎、抗氧化、血管保护和神经保护等多种药理活性,在1995年被报道可以产生优异的镇痛效果。天然产物应用于镇痛,具有副作用小、成瘾性低和不易产生耐受等优点。芦丁片及复方芦丁片已投入临床使用,在抗出血方面发挥重要作用,但尚无镇痛相关应用。近年来,关于芦丁镇痛的相关研究逐渐增多。本文通过检索中国知网及Pubmed,基于镇痛发生机制归纳总结芦丁的镇痛作用(Tab 1)。同时,指出芦丁作为天然产物应用于临床的局限性及改进措施。
|
| Fig 1 Chemical structure of rutin |
| Animal | Evaluation | Effects | Ref |
| Male Balb/c mice | Acetic acid-induced writhing test | Modulate the opiodergic system | 3 |
| Swiss albino mice | Acetic acid-induced writhing test, hot plate test | Modulate the opioid receptors and inhibit prostaglandin synthesis | 4 |
| Male Wistar rats | Formalin test | Modulate the vlPAG descending circuit partly mediated by an opioidergic mechanism | 5 |
| Male Imprinting Control Region (ICR) mice | Acetic acid-induced writhing test, hot plate test, formalin test | Activate the GABAergic system and opioid receptors, and inhibit pain hypersensitivity by reducing the release of PGE2 and endogenous inflammatory mediators | 6 |
| Wistar rats | Hot plat test, tail flick test, tail immersion test, formalin test | Activate COX-2 protein and mu-opioid receptors | 7 |
| Male ICR mice Male BALB/c mice | Hot plat test, tail immersion test, acetic acid-induced writhing test, formalin test | Activate CB1R and PPAR, and inhibit TRPA1 to modulate the release of inflammatory cytokine and signal transduction of Ca2+ and K+ ion channels | 8~9 |
| Male BALB/c mice | Hot plat test, tail immersion test, acetic acid-induced writhing test, formalin test | Activate α2-adrenergic receptor to terminate pain signal transduction | 10 |
| Male Swiss mice | Chemical (acetic-acid, formalin, capsaicin, cinnamaldehyde and glutamate tests), thermal (tail-flick and hot-plate test) models of pain and intratecal administration of excitatory amino acids and pro-inflammatory cytokine-induced pain behaviour in mice | Inhibit glutamatergic ionotropic receptors and TRPV1, and reducing the release of pro-inflammatory cytokine such as IL-1β and TNF-α | 11 |
| Male guinea pigs | Ileal spasm model | Maintain Ca2+ homeostasis and block of voltage dependent L-type Ca2+ channels | 12-14 |
| Male Swiss mice | Acetic acid-induced writhing test, H2O2, carrageenan and algogen- induced pain model | Block TRPA1 receptor activation to inhibit pain signaling and release of pro-inflammatory cytokine | 15 |
| Male Swiss mice | Acetic acid-induced writhing test, hot-plate test, rota-rod test | Inhibit TNF-α concentration, neutrophil recruitment and mechanical hypernociception | 17 |
| Male Wistar rats | Formalin test | Inhibit the release of pro-inflammatory cytokine | 18 |
| Male Kunming mice | Formalin test | Inhibit proinflammatory cytokines, iNOS and NF-κB pathway-related proteins | 19 |
| Male SD rats | mechanical, heat and cold sensation test | Decrease IL-6, TNF-α, and caspase-3 expression in dorsal root ganglions | 20 |
| Male Swiss mice | G-CSF induced | Activate NO-cGMP-PKG-ATP-KATP signaling pathway, inhibit NF-κB and trigger the Nrf2/HO-1 pathway | 21 |
| Female Wistar rats | Induction of acute gastric lesions by ethanol or indometacin | Scaveng free radicals, and inhibit the release of pro-inflammatory cytokine | 22 |
| Male Swiss mice | Tail immersion test, von Frey test | Decrease peroxidation inducible nitric oxide synthase and nitrotyrosine in the spinal cord, and prevent painful peripheral neuropathy | 23 |
| Male Wistar rats | Formalin test | Inhibit lipid peroxidation and inflammation | 24 |
镇痛药物可与阿片受体结合,影响K+和Ca2+传递,减少神经末梢P物质等的释放,阻断痛觉冲动传导从而产生镇痛作用。研究发现,芦丁通过调控阿片能系统、扑热息痛和非甾体抗炎药通过下调p38-MAPK-NF-κB和前列腺素,协同产生镇痛作用[3]。Selvaraj等[4]发现, 银杏叶提取物可显著减少小鼠扭体次数、延长热板实验潜伏舔足时间并且可被阿片受体拮抗剂纳洛酮拮抗,分子对接结合高效液相发现,芦丁为发挥抗内脏痛及热痛的活性物质。Hernandez-Leon等[5]发现芦丁抑制了神经性和炎性疼痛,在中脑导水管周围灰质腹外侧(ventrolateral column of the periaqueductal gray, vlPAG)中应用阿片受体拮抗剂纳曲酮可阻止芦丁对炎性疼痛的抑制,证明芦丁通过阿片受体介导的vlPAG下行回路发挥镇痛作用。Ovalle-Magallanes等[6]研究发现芦丁可以抑制内脏痛、神经源性和炎性痛,并且这种作用可以被GABA受体拮抗剂氟马西尼和阿片受体拮抗剂纳曲酮所抵消,这证实芦丁的镇痛作用与激活GABA系统和阿片受体有关。Tewari等[7]发现掌叶榕果提取物具有显著镇痛活性,分子对接实验显示,其重要活性成分芦丁对环氧合酶-2(cyclooxygenase-2, COX-2)蛋白和μ-阿片受体活性位点具有高亲合力。
1.1.2 激动大麻素受体大麻素受体主要有两种亚型:大麻素受体Ⅰ(cannabinoid receptor type 1, CB1)和大麻素受体Ⅱ(cannabinoid receptor type 2, CB2),受体激活后可以抑制致痛介质的释放,降低神经元的活化,产生有效的抑制炎性痛、慢性痛和神经病理性痛的作用。Su等[8]研究发现, 芦丁显著增加小鼠脑内CB1受体相关蛋白(CB1 cannabinoid receptor interacting proteins a, CRIP1a)的表达,CRIP1a是CB1的辅助蛋白,可以调节CB1受体介导的电压依赖性钙通道的紧张性抑制。另有研究发现,沙棘叶提取物在小鼠热板、甩尾、醋酸扭体和福尔马林实验中显示出较强的抗炎性痛、内脏痛和躯体痛的作用,CB1受体及过氧化物酶体增殖物激活受体α(peroxisome proliferator-activated receptorα, PPARα)拮抗剂可以逆转其作用。液质联合分析发现沙棘叶提取物的主要成分包括芦丁、熊果苷及槲皮素等[9],表明芦丁极有可能可以激活CB1受体调控炎症介质及细胞因子发挥镇痛作用。
1.1.3 激动α2肾上腺素受体(alpha-2 adreno-receptor, α2-AR)α2-AR的激活可减少环磷酸腺苷(cyclic adenosine monophosphate, cAMP)的释放,进而抑制神经元的兴奋性,终止疼痛信号的传导发挥镇痛作用。研究发现,α2-AR激动剂法多咪定表现出强大的抑制炎性、术后和神经性疼痛的活性。Jaffal等[10]发现辣木提取物表现出明显的抗热痛及炎性痛的活性并且此作用可被α2肾上腺素受体拮抗剂育亨宾所阻断,其主要活性成分芦丁为α2肾上腺素受体的配体之一。
1.2 芦丁通过调控离子通道受体发挥镇痛作用疼痛的产生依赖于上传到中枢神经系统的疼痛信号,神经递质及离子通道调控初级传入神经的电活动。离子型谷氨酸受体与离子通道偶联,在介导中枢和外周神经系统对疼痛信号的传导中起着重要作用,谷氨酸受体活化会诱导NO和cGMP增加,参与外周炎症引起的痛觉神经元敏化。有研究表明,远志水醇提取物表现出明显的抑制化学疼痛的活性,且只有从水醇提取物中分离的芦丁对谷氨酸诱导的疼痛有剂量抑制关系,表明芦丁镇痛与与抑制离子型谷氨酸受体有关[11]。
电压依赖性钙通道(voltage-gated calcium channels, VGCC)是细胞内钙离子浓度的调节器,影响信号转导,是目前疼痛治疗的热点。维拉帕米(L-钙通道阻滞剂)、ziconotide及相关复合物(N-钙通道阻断剂)可以降低Ca2+超载,改善痛觉超敏,其中ziconotide已被美国FDA批准用于临床。有研究发现芦丁可以降低MPP+诱导的Ca2+浓度增加[12]。早在1996年就有研究推测芦丁的镇痛机制可能与钙离子通道有关[13]。痉挛主要是平滑肌剧烈收缩引起的肌肉不协调及剧烈疼痛,改善痉挛与镇痛密切相关。万寿菊水提取物可以缓解高浓度KCl引起的内脏痉挛,机制与阻断L型电压依赖性钙通道有关,其活性成分主要为芦丁和槲皮素[14]。Gilani等发现,红茶提取物通过激活K+通道和阻滞Ca2+通道解除胃肠道痉挛,其活性成分芦丁表现出良好的Ca2+通道阻滞作用。由以上研究可推测芦丁可能通过阻断电压依赖性钙通道,调节Ca2+浓度从而阻断痛觉信号转导发挥镇痛作用。
瞬时感受器电位A1离子通道(transient receptor potential cation channel, member A1, TRPA1)又称辣椒素受体,是一种非选择性的阳离子通道,可以选择性激活感觉神经元传导疼痛信号,参与神经病理性疼痛的形成。Trevisan等[15]发现,芦丁能抑制醋酸实验引起的内脏疼痛,并能减轻H2O2和TRPA1激动剂引起的伤害性反应,机制与抑制TRPA1激活从而抑制信号转导和促炎因子的释放进而抑制痛觉敏化有关。
1.3 芦丁通过抑制炎症反应发挥镇痛作用机体组织损伤时,K+、5-HT、乙酰胆碱、炎症因子等异常活性物质会激活痛感受器,造成痛觉敏化,降低痛觉阈值导致疼痛产生。芦丁可以调控炎症过程,抑制炎性疼痛。大量实验证明,芦丁可抑制肿瘤坏死因子-α(tumor necrosis factor-α, TNF-α)、白细胞介素(interleukin, IL)、趋化因子等炎症因子的转录从而抑制炎症反应[16]。似絮菊假蓬提取物芦丁通过抑制酸感应离子通道和内源性炎症介质释放,产生抗神经源性和炎性疼痛的作用[6]。天蚕总黄酮和芦丁对小鼠关节炎疼痛有明显改善作用,机制与抑制中性粒细胞聚集及TNF-α合成从而提高小鼠痛阈值有关[17]。三叶刺五加乙酸乙酯提取物的活性成分主要为芦丁,在福尔马林实验和耳廓肿胀实验中表现出明显的抗炎性痛的作用,机制与抑制IL-1β等促炎介质释放进而降低痛觉敏化有关[18]。对黑桑果实中的总黄酮和芦丁的作用进行研究,发现两者均可改善福尔马林引起的炎性疼痛,机制与抑制促炎因子、iNOS和NF-κB通路相关蛋白的表达有关[19]。芦丁对糖尿病大鼠的机械性痛觉超敏、热痛觉超敏和冷痛觉超敏均有显著的抑制作用,机制与抑制NF-κB的激活,减少背根神经节中IL-6和TNF-α的产生有关[20]。Thacyana等[21]研究发现, 芦丁通过激活NO-cGMP-PKG-ATP-KATP信号通路和Nrf2/HO-1通路,抑制NF-kB通路,抑制粒细胞集落刺激因子(granulocyte colony stimulating factor, G-CSF)诱导的机械性疼痛。
1.4 芦丁通过改善氧化应激损伤发挥镇痛作用机体内自由基堆积会形成局部负电位,刺激痛神经末梢,引发神经冲动。芦丁是清除自由基的强氧化剂,通过增加过氧化氢酶(catalase,CAT)、谷胱甘肽过氧化物酶(glutathione peroxidase,GPX)和超氧化物歧化酶(superoxide dismutase,SOD)等抗氧化应激酶的活性,清除自由基、抑制过氧化、保护损伤组织,从而发挥镇痛作用。有研究观察了远志水醇提取物和芦丁对大鼠胃急性损伤的保护作用,发现两者均可以清除自由基、抑制炎症介质释放、上调细胞保护因子,从而产生镇痛作用[22]。Azevedo等[23]研究发现,奥沙利铂能诱导小鼠脊髓过氧化,增加小鼠热伤害性和机械伤害性反应,而芦丁和槲皮素可以抑制背角区域硝基酪氨酸和iNOS表达、抑制脂质过氧化,从而改善周围神经性病变,达到镇痛目的。此外,芦丁可以逆转福尔马林致痛实验第1和第2相的神经源性和炎性痛,机制主要与抗氧化和抗炎有关[24]。
2 基于芦丁代谢和剂型的临床应用展望近年来药物开发的热点已聚焦于对天然产物的利用,天然产物具有活性高、作用机制多样及毒副作用较小的特点。天然产物芦丁镇痛活性较好,但是它具有的难溶于水、易分解和生物利用度低的缺陷,限制了它的临床应用。为了使芦丁更好发挥临床镇痛活性,需了解芦丁的代谢过程并对芦丁的结构及剂型进行优化。
2.1 芦丁的代谢芦丁具有丰富的官能团,生物活性强但是稳定性不足,体内体外均易代谢且代谢情况较为复杂。体外代谢研究发现,芦丁可被肠道菌群代谢为槲皮素、苯酚衍生物、甲基化产物、槲皮素-3-氧葡萄糖苷及无色花青素等。芦丁口服后存在多个代谢途径,其中大部分被代谢为槲皮素进而生成槲皮素的葡萄糖醛酸、硫酸和甲基结合代谢产物,少部分被胃肠道吸收后以原型物和甲基化、乙酰化产物存在[25]。槲皮素是芦丁的主要体内代谢产物,已有研究表明槲皮素具有明显的镇痛活性。静脉给药后,芦丁在大鼠体内的代谢过程符合二室模型,0.08 h达到最大血药浓度且Cmax为(22.81±5.32) mg·kg-1。比较芦丁混悬液和芦丁纳米乳制剂的药代动力学数据,发现芦丁纳米乳的AUC和Cmax分别是混悬液的1.8和1.9倍,芦丁纳米乳和芦丁混悬液Tmax分别为2 h和4 h,芦丁纳米粒生物利用度增加了约两倍[26]。由此可见,虽然芦丁的应用存在局限,但是对芦丁的结构进行修饰及开发芦丁新剂型可以改善生物利用度和疗效。
2.2 芦丁衍生物及剂型为了更好的发挥芦丁活性,以期应用于临床,芦丁的醚类衍生物、酯类衍生物及金属配合物得以研发。曲克芦丁是典型的芦丁醚类衍生物,曲克芦丁能溶于水,易被人体吸收,已经广泛用于临床。研究表明曲克芦丁可以通过AMPK/SIRT1通路抑制疼痛因子和神经小胶质细胞的激活从而抑制神经性疼痛[27]。Mecenas等[28]发现,芦丁乙酸酯的抗氧化活性有所提升且细胞毒性降低,并且可以减少巨噬细胞中活性氧的含量。芦丁可与铁、铜、锌、镁及稀土元素等金属形成配合物,结构稳定、药理活性增加。Ikeda等[29]发现的芦丁-锌金属配合物,抗氧化活性显著升高,细胞毒性降低。
另一种提高芦丁生物利用度的方法是研发新剂型。目前研究最多的是芦丁的各种固体分散体制剂,可以提高芦丁的溶出速率和口服生物利用度。Shanmugam等[30]采用溶剂蒸发法将芦丁制备成纳米粒体系,芦丁的溶解度、溶出速率显著提升,在家兔体内的药代动力学显示芦丁的生物利用度提高了4.24倍。此外,芦丁HPMC控释片、β-环糊精包合物等均可改变芦丁的释放速度,提高生物利用度。
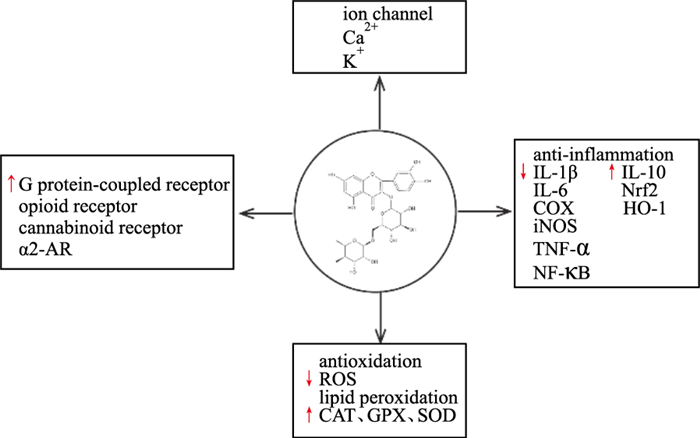
3 结语芦丁具有明确的镇痛活性,但其临床应用尚未起步。文献证实,芦丁对神经性疼痛、慢性痛特别是炎性痛有明显活性,可能的机制包括调节阿片受体、大麻素受体、α2-AR等疼痛信号相关受体和离子通道的活性,减少疼痛介质的释放,抑制外周及中枢敏化,降低神经信号传导;也可以直接抑制炎症介质释放、清除自由基,抑制痛觉神经末梢的敏化(Fig 2)。但芦丁产生镇痛作用的明确通路及确切靶点尚需深入研究。
|
| Fig 2 Analgesic mechanism of rutin |
目前,对芦丁镇痛活性的开发仍然很薄弱,芦丁片及芦丁的衍生物曲克芦丁片、曲克芦丁胶囊主要用于血管保护及静脉炎。文献表明,芦丁水溶性差、生物利用度低的缺陷,可通过修饰芦丁结构及制备新剂型进行改善。以天然产物芦丁为先导化合物开发生物利用度高、代谢稳定、溶解度好的镇痛制剂具有重要意义。
| [1] |
苏漫, 朱清, 李俊旭. 镇痛药物作用靶点的研究现状[J]. 中国药理学通报, 2018, 34(2): 161-5. Su M, Zhu Q, Li J X. Research progress on targets for analgesics[J]. Chin Pharmacol Bull, 2018, 34(2): 161-5. |
| [2] |
占今舜, 钟小军, 杨群, 等. 芦丁的生物活性功能及其在反刍动物生产中的应用[J]. 动物营养学报, 2019, 31(7): 2952-7. Zhan J S, Zhong X J, Yang Q, et al. Bio-active functions of rutin and its application in ruminant production[J]. Chin J Anim Nutr, 2019, 31(7): 2952-7. |
| [3] |
Zapata-Morales J R, Alonso-Castro A J, Munoz-Martinez G S, et al. In vitro and in vivo synergistic interactions of the flavonoid rutin with paracetamol and with non-steroidal anti-inflammatory drugs[J]. Arch Med Res, 2021, 52(6): 611-9. doi:10.1016/j.arcmed.2021.03.007 |
| [4] |
Selvaraj G, Kaliamurthi S, Thirungnasambandam R, et al. Anti-nociceptive effect in mice of thillai flavonoid rutin[J]. Biomed Environ Sci, 2014, 27(4): 295-9. |
| [5] |
Hernandez-Leon A, Fernandez-Guasti A, Gonzalez-Trujano M E. Rutin antinociception involves opioidergic mechanism and descending modulation of ventrolateral periaqueductal grey matter in rats[J]. Eur J Pain, 2016, 20(2): 274-83. doi:10.1002/ejp.720 |
| [6] |
Ovalle-Magallanes B, Deciga-Campos M, Mata R. Antinociceptive and hypoglycaemic evaluation of Conyza filaginoides (D.C.) hieron asteraceae[J]. J Pharm Pharmacol, 2015, 67(12): 1733-43. doi:10.1111/jphp.12477 |
| [7] |
Tewari D, Gupta P, Bawari S, et al. Himalayan ficus palmata L. Fruit extract showed in vivo central and peripheral analgesic activity involving COX-2 and mu opioid receptors[J]. Plants (Basel), 2021, 10(8): 1685. |
| [8] |
Su K Y, Yu C Y, Chen Y W, et al. Rutin, a flavonoid and principal component of saussurea involucrata, attenuates physical fatigue in a forced swimming mouse model[J]. Int J Med Sci, 2014, 11(5): 528-37. doi:10.7150/ijms.8220 |
| [9] |
Jaffal S M, Oran S A, Alsalem M. Anti-nociceptive effect of Arbutus andrachne L. methanolic leaf extract mediated by CB1, TRPV1 and PPARs in mouse pain models[J]. Inflammopharmacology, 2020, 28(6): 1567-77. doi:10.1007/s10787-020-00746-y |
| [10] |
Jaffal S M, Al-Najjar B O, Abbas M A, et al. Antinociceptive action of moringa peregrina is mediated by an interaction with alpha2-adrenergic receptor[J]. Balkan Med J, 2020, 37(4): 189-95. |
| [11] |
Lapa F R, Gadotti V M, Missau F C, et al. Antinociceptive properties of the hydroalcoholic extract and the flavonoid rutin obtained from Polygala paniculata L. in mice[J]. Basic Clin Pharmacol Toxicol, 2009, 104(4): 306-15. doi:10.1111/j.1742-7843.2008.00365.x |
| [12] |
Enogieru A B, Haylett W L, Miller H C, et al. Attenuation of endoplasmic reticulum stress, impaired calcium homeostasis, and altered Bioenergetic functions in MPP(+)-exposed SH-SY5Y cells pretreated with rutin[J]. Neurotox Res, 2019, 36(4): 764-76. doi:10.1007/s12640-019-00048-4 |
| [13] |
宋必卫, 田薇, 陈志武, 等. 芦丁镇痛作用部位和机制的研究[J]. 安徽医科大学学报, 1996, 31(1): 1-3. Song B W, Tian W, Chen Z W, et al. Central analgesic activity and possible mechanism of rutin[J]. Acta Univ Med Anhui, 1996, 31(1): 1-3. |
| [14] |
Ventura-Martínez R, Ángeles-López GE, Rodríguez R, et al. Spasmolytic effect of aqueous extract of Tagetes erecta L. flowers is mediated through calcium channel blockade on the guinea-pig ileum[J]. Biomed Pharmacother, 2018, 103(2018): 1552-6. |
| [15] |
Trevisan G, Rossato M F, Hoffmeister C, et al. Antinociceptive and antiedematogenic effect of pecan (Carya illinoensis) nut shell extract in mice: A possible beneficial use for a by-product of the nut industry[J]. J Basic Clin Physiol Pharmacol, 2014, 25(4): 401-10. |
| [16] |
Kauss T, Moynet D, Rambert J, et al. Rutoside decreases human macrophage-derived inflammatory mediators and improves clinical signs in adjuvant-induced arthritis[J]. Arthritis Res Ther, 2008, 10(1): R19. |
| [17] |
Teixeira F M, Coelho M N, Jose-Chagas F, et al. Oral treatments with a flavonoid-enriched fraction from Cecropia hololeuca and with rutin reduce articular pain and inflammation in murine zymosan-induced arthritis[J]. J Ethnopharmacol, 2020, 260: 112841. doi:10.1016/j.jep.2020.112841 |
| [18] |
Chen Z, Cheng S, Lin H, et al. Antibacterial, anti-inflammatory, analgesic, and hemostatic activities of Acanthopanax trifoliatus (L.) merr[J]. Food Sci Nutr, 2021, 9(4): 2191-202. doi:10.1002/fsn3.2190 |
| [19] |
Chen H, Yu W, Chen G, et al. Antinociceptive and antibacterial properties of anthocyanins and flavonols from fruits of black and non-black mulberries[J]. Molecules, 2017, 23(1): 4. doi:10.3390/molecules23010004 |
| [20] |
Tian R, Yang W, Xue Q, et al. Rutin ameliorates diabetic neuropathy by lowering plasma glucose and decreasing oxidative stress via Nrf2 signaling pathway in rats[J]. Eur J Pharmacol, 2016, 771(2016): 84-92. |
| [21] |
Carvalho T T, Mizokami S S, Ferraz C R, et al. The granulopoietic cytokine granulocyte colony-stimulating factor (G-CSF) induces pain: Analgesia by rutin[J]. Inflammopharmacology, 2019, 27(6): 1285-96. doi:10.1007/s10787-019-00591-8 |
| [22] |
Lapa F R, Freitas C S, Baggio C H, et al. Gastroprotective activity of the hydroalcoholic extract obtained from Polygala paniculata L. in rats[J]. J Pharm Pharmacol, 2007, 59(10): 1413-9. |
| [23] |
Azevedo M I, Pereira A F, Nogueira R B, et al. The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy[J]. Mol Pain, 2013, 9: 53. |
| [24] |
Hasanein P, Emamjomeh A, Chenarani N, et al. Beneficial effects of rutin in diabetes-induced deficits in acquisition learning, retention memory and pain perception in rats[J]. Nutr Neurosci, 2020, 23(7): 563-74. |
| [25] |
伍明江, 吴晓磊, 张德芹, 等. UPLC-Q-TOF/MS鉴定芦丁在大鼠体内的代谢产物[J]. 中国实验方剂学杂志, 2017, 23(17): 91-7. Wu M J, Wu X L, Zhang D Q, et al. Identification of metabolites of rutin in rats by UPLC-Q-TOF/MS[J]. Chin J Exp Tradit Med Formulae, 2017, 23(17): 91-7. |
| [26] |
Sharma S, Rabbani S A, Narang J K, et al. Role of rutin nanoemulsion in ameliorating oxidative stress: Pharmacokinetic and pharmacodynamics studies[J]. Chem Phys Lipids, 2020, 228(2020): 104890. |
| [27] |
Gui Y, Li A, Chen F, et al. Involvement of AMPK/SIRT1 pathway in anti-allodynic effect of troxerutin in CCI-induced neuropathic pain[J]. Eur J Pharmacol, 2015, 769(2015): 234-41. |
| [28] |
Mecenas A S, Adao M C, Sangenito L S, et al. Rutin derivatives obtained by transesterification reactions catalyzed by Novozym 435: Antioxidant properties and absence of toxicity in mammalian cells[J]. PLoS One, 2018, 13(9): e203159. |
| [29] |
Ikeda N E, Novak E M, Maria D A, et al. Synthesis, characterization and biological evaluation of Rutin-zinc(Ⅱ) flavonoid-metal complex[J]. Chem Biol Interact, 2015, 239(2015): 184-91. |
| [30] |
Ramaswamy S, Dwarampudi L P, Kadiyala M, et al. Formulation and characterization of chitosan encapsulated phytoconstituents of curcumin and rutin nanoparticles[J]. Int J Biol Macromol, 2017, 104(Pt B): 1807-12. |

