

2. 甘肃省中医药防治慢性疾病重点实验室,甘肃 兰州 730000;
3. 甘肃省高校重大疾病分子医学与中医药防治研究省级重点实验室,甘肃 兰州 730000
2. Gansu Provincial Key Laboratory of Traditional Basic Medical College, Chinese Medicine for Prevention and Treatment of Chronic Diseases, Lanzhou 730000, China;
3. Provincial-Level Key Laboratory for Molecular Medicine of Major Disease and Treatment with Traditional Chinese Medicine Research in Gansu Colleges and Universities, Lanzhou 730000, China
癌症作为一类常见的慢性消耗性疾病,严重危害人类生命与健康。据统计,2020年全球新增癌症病例1 930万例,癌症死亡人数近1 000万例[1]。由于大多数癌症具有异质性、耐药性以及易复发转移的特点,使得临床治愈这类疾病十分困难。因此,解析癌症发生发展的分子机制、鉴定新的诊断标志物和分子治疗靶标,是解决癌症早期诊断、治疗及改善患者预后问题的有效策略。人类全基因组测序表明,大约70%的基因组序列能够转录成RNA,其中只有2%的RNA编码蛋白质,剩余98%的RNA属非编码RNA(non-coding RNA, ncRNA)。在这些ncRNAs中,长链非编码RNA(long non-coding RNA, lncRNA)因其具有丰富的生物学功能而受到广泛关注。LncRNAs是一类由RNA聚合酶Ⅱ转录产生的、长度大于200个碱基的非编码RNA分子,主要参与染色质修饰、基因组修饰、核内运输、转录干扰、转录激活等多种生物学过程。尽管lncRNA不编码任何功能性蛋白,但可通过充当诱饵分子、引导分子、信号分子以及支架分子分别与DNA、RNA、蛋白质相互作用,从而参与疾病的发生与发展。近年来许多研究表明,lncRNAs的异常表达是引起癌症的重要因素之一。细胞骨架调节因子RNA (cytoskeleton regulator RNA, CYTOR)是近年发现的一种致癌性lncRNA,它在多种类型癌症中均高表达,并与癌症临床特征密切相关[2-4]。研究证实,CYTOR可通过靶向特定基因、调控癌症信号通路等多种途径来影响人类不同类型癌症细胞的生物学行为,在癌症进展中起着关键调控分子的作用[2, 4]。因此,深入解读lncRNAs CYTOR在人类癌症中的分子调控机制及其相关生物学功能将会为癌症预防、诊断及分子靶向治疗提供新的科学理论依据。
1 LncRNA CYTOR简述LncRNA CYTOR,又名LINC00152,最初在肝癌中被发现,呈差异低甲基化。基于NCBI在线数据库信息显示,CYTOR作为一种基因间lncRNA位于人类2号染色体p11.2区域,存在5个转录本,包括NR_024204.2、NR_024205.3、NR_024206.2、NR_146460.1、NR_146461.1。CYTOR在细胞内外均有表达,细胞内主要定位于细胞骨架和细胞核中。近年来研究发现,CYTOR在结直肠癌、乳腺癌、肺癌、肝癌、胃癌等多种类型癌症中均高表达,主要通过竞争性结合微小RNA(microRNA,miRNA)、调节致癌转录因子的活性、激活癌症相关信号通路、维持蛋白质稳定性以及其他方式促进人类癌症进程[2-6]。更为重要的是,基于已有的临床样本数据及在线数据库信息分析表明,CYTOR高表达与许多癌症病理分级、临床分期、淋巴结转移、患者总体生存期较短呈正相关[2, 4, 6]。
2 CYTOR在人类癌症中的调控机制癌症是一类由基因突变以及表观遗传改变等多种因素引起的慢性疾病,主要表现为癌细胞无限增殖、逃避凋亡、入侵周围组织及器官等[7]。近年多项研究表明,lncRNA CYTOR参与调控癌细胞增殖、凋亡、侵袭、迁移及放化疗敏感性等多种生物学过程,而lncRNA CYTOR在癌症中调控因子的功能是通过复杂的分子作用机制实现的(Tab 1)。
| Cancer type | Cell line | Expression | Functional effects | Related gene | Reference |
| Hepatocellular Carcinoma | HepG2,SNU449,HCCLM3,MCC97H,Hep3B,Huh7 | Upregulated | Proliferation, invasion, migration,apoptosis | miR-215,miR-139,miR-193a/b-3p,CDK13,CCND1 PIK3CA | [3, 12, 13] |
| Lung cancer | A549,95D,H358,H1299,H158 | Upregulated | Proliferation, invasion, migration,radiosensitivity | miR-195 | [5] |
| Breast cancer | MCF-7,BT-549,MDA-MB-468,MDA-MB-231 | Upregulated | Proliferation, invasion, migration, chemosensitivity | miR-125a-5p,SRF,DNMTs PTEN,KLF5 | [6, 18, 28, 29] |
| Gastric cancer | AGS,BGC-823,SGC7901,MGC-803,MKN45 | Upregulated | Tumorigenesis, Proliferation, invasion, migration, apoptosis, Aerobic, glycolysis | miR-193a/b-3p,miR-139-5p,MCL1,PRKAA1 Bcl-2,p-ERK-1/2,p-MEK1/2,c-fos | [2, 9, 17, 27] |
| Colorectal cancer | SW480,HCT116,HEK293,Caco2,RKO,HT29,SW620,LoVo,HCT8 | Upregulated | Proliferation, invasion, migration, chemosensitivity | miR-632,miR-185-3p,miR-378a-5p,NCL,Sam68,β-Catenin,SERPINE1 | [4, 16, 19, 21, 22] |
| Esophageal cancer | EC109,KYSE150,TE-1,TE13,Eca109 | Upregulated | Proliferation, invasion, migration, apoptosis | miR-153-3p,FYN,EZH2,PI3K,AKT | [10, 23, 24] |
| Ovarian cancer | HO-8910,SKOV3,A2780,ES-3 | Upregulated | Proliferation, apoptosis | miR-125b,MCL-1,BCL6 | [8, 26] |
| Cervical cancer | CaSki, C-33A | Upregulated | Proliferation, apoptosis | miR-216b-5p,HOX1A | [11] |
| Papillary thyroid cancer | TPC-1,K1,IHH4 | Upregulated | Proliferation, invasion, migration | miR‐497,BDNF | [14] |
| Nasopharyngeal carcinoma | CNE-1,6-10B | Upregulated | Proliferation, invasion, migration | miR-613,ANXA2 | [15] |
| Bladder cancer | T24,HT-1197 | Upregulated | Proliferation, invasion, migration | Wnt/β-Catenin | [25] |
| Oral squamous cell cancer | CAL-27,SCC4 | Upregulated | invasion, migration, chemosensitivity | FOXD1,miR-1252-5p, miR-3148,LPP | [20] |
miRNA是一类由内源基因编码的长度约为22个核苷酸的非编码RNA分子,主要通过与靶信使RNA特异性结合,从而抑制转录后基因的表达。而lncRNA可通过应答元件(miRNA response elements, MRE)与miRNA竞争性结合从而解除miRNA对靶信使RNA的抑制作用,促进其表达。近年已有多项研究报道CYTOR通过与多种癌症细胞中的miRNA如miR-193a/b-3p、miR-613、miR-139等竞争性结合,正调控癌基因的表达,发挥促癌作用(Fig 1)。
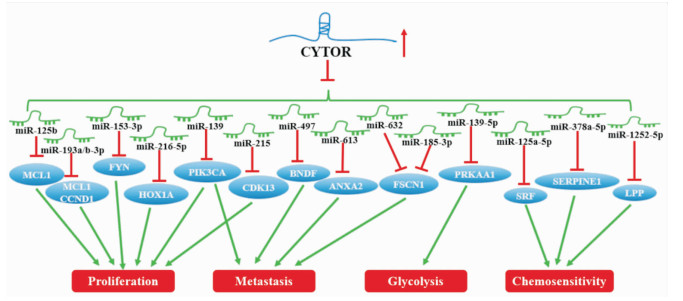
|
| Fig 1 CYTOR induced oncogene expression through competitive binding to microRNA |
CYTOR可通过与miRNA竞争性结合来调控癌细胞增殖相关基因的表达,从而介导多种癌细胞的增殖。在卵巢癌及胃癌细胞中,高表达的CYTOR可作为miR-125b、miR-193a-3p的分子海绵通过上调髓样细胞白血病因子1(myeloid cell leukemia-1, MCL1)的表达来介导细胞的增殖[8-9]。与此类似,在食管癌组织及细胞系中,CYTOR可通过充当miR-153-3p的竞争性海绵上调FYN(Src家族酪氨酸激酶)来促进细胞的增殖、抑制细胞凋亡[10]。而宫颈癌中的CYTOR亦可通过与miR-216b-5p竞争性结合提高同源异形盒A1(homeobox A1,HOXA1)的表达从而促进细胞的增殖、抑制细胞凋亡[11]。CYTOR与miRNA竞争性结合对肝癌细胞增殖也有促进作用。高表达的CYTOR可通过解除miR-139对磷脂酰肌醇-3-激酶催化σ亚基(phosphatidylinosiol-3 kinase catalytic alpha, PIK3CA)的抑制, 从而介导细胞的增殖、侵袭及迁移[3]; 相反,通过敲除肝癌细胞中CYTOR基因,可以释放miR-215,使细胞周期蛋白依赖激酶13(cyclin dependent kinase 13,CDK13)的表达降低,从而抑制细胞的增殖[12]。另外,肝癌中的CYTOR还能与miR-193a/b-3p竞争性结合,通过诱导细胞周期蛋白D1(cyclin D1,CCND1)的表达来促进细胞的增殖[13]。CYTOR亦可通过与miRNA竞争性结合调控癌细胞转移相关基因的表达,从而驱动癌细胞的侵袭和迁移。研究表明,若敲除甲状腺癌中CYTOR可促使miR-497表达水平增加,下调靶基因脑源性神经营养因子(brain derived neurotrophic factor,BDNF)的表达,从而抑制细胞的侵袭和迁移[14]。在鼻咽癌中,CYTOR可作为miR-613的分子海绵通过靶向膜联蛋白A2(annexin A2,ANXA2)来介导细胞的增殖、侵袭及迁移[15]。Yes相关蛋白1 (Yes1 associated transcriptional regulator,YAP1)是Hippo信号通路中关键转录因子,在癌症转移中发挥重要作用。最新研究发现,YAP1可促进结直肠癌中CYTOR的转录,使其作为miR-632、miR-185-3p的海绵上调筋膜肌动蛋白束蛋白1(fascin actin-bundling protein 1,FSCN1)的表达从而介导细胞的增殖、侵袭和迁移[16]。CYTOR与miRNA竞争性结合还可参与调节癌细胞能量代谢以及药物敏感性。研究表明,高表达的CYTOR能够抑制miR-139-5p的表达,进而激活蛋白激酶AMP活化α1催化亚单位(protein kinase AMP-activated catalytic subunit alpha 1,PRKAA1),促进胃癌细胞有氧糖酵解[17]。而在乳腺癌中,CYTOR通过竞争性结合miR-125a-5p促进血清反应因子(serum response factor,SRF)的表达,从而降低了人乳腺癌细胞对他莫昔芬的敏感性[18]。类似地,结肠癌中的CYTOR可通过海绵miR-378a-5p上调serpin E家族成员1(serpin family E member 1,SERPINE1)的表达从而使结肠癌细胞对奥沙利铂的敏感性下降[19]。此外,CYTOR可通过竞争性结合miR-1252-5p和miR-3148来上调LPP(Lim domain containing preferred translocation partner in lipoma)的表达,从而减少了口腔鳞状细胞癌对顺铂的敏感性[20]。以上结果提示,CYTOR作为miRNA的分子海绵具有调控癌细胞增殖、侵袭、迁移、能量代谢、化疗敏感性等多种生物学行为的功能。
2.2 调节转录因子的活性转录因子是一类能够特异性地结合特定基因序列、保证靶基因以特定的强度在特定时间与空间表达的蛋白质分子[37]。据报道,目前已发现Kruppel样转录因子5(Kruppel like factor 5,KLF5)、核因子κB(nuclear factor kappa-B,NF-κB)等294个致癌性转录因子,约占已知致癌基因的20%。lncRNA与转录因子之间相互作用形式已被多次报道,即lncRNA可调节转录因子的活性并募集远离染色质的转录因子入核内促进基因表达; 被激活的转录因子又可促进相应lncRNA转录,以正反馈回环的调节方式使基因持续表达。近年来,CYTOR已被证明是多种致癌转录因子的激活剂,在癌症发生发展中具有重要作用(Fig 2)。
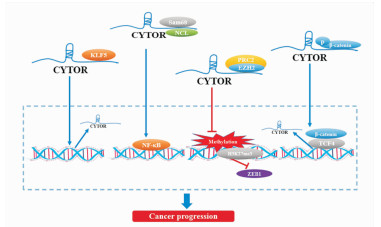
|
| Fig 2 CYTOR regulated activity of transcription factors and promoted oncogene transcription |
CYTOR具有调节转录因子的活性促进乳腺癌发生的作用,其作用机制在于CYTOR通过结合KLF5激活其转录活性,而KLF5亦可通过正反馈回路与CYTOR启动子结合促进CYTOR转录,二者共同介导体内外乳腺癌细胞的增殖以及致瘤性[6]。CYTOR还能调节转录因子的活性促进癌细胞侵袭及迁移。研究表明,结直肠癌中的CYTOR外显子1与RNA结合蛋白NCL(nucleolin)、Sam68 (Src-associated in mitosis 68 kD)具有共同结合位点,且CYTOR、NCL以及Sam68形成的异源三聚体复合物参与了NF-κB的激活,促进细胞上皮间充质转化(epithelial-mesenchymal transition,EMT)[21]。同样,CYTOR还能与细胞质中β-catenin结合并使其稳定避免被磷酸化,使β-catenin积累并入核诱导下游c-myc、cyclin D1以及EMT相关基因的转录,反过来,β-catenin/TCF复合物又增强CYTOR的转录活性,并以正反馈回路的形式促进结直肠癌细胞的增殖、侵袭与迁移[22]。此外,食管癌中的CYTOR可通过与多梳抑制复合物(polycomb repressive complex 2, PRC2)核心成员zeste同源物2增强子(enhancer of zeste homologue 2,EZH2)结合,抑制下游H3K27me3对锌指转录因子ZEB1的沉默,并促进ZEB1的转录活性,启动EMT进程; 同时,CYTOR降低了聚ADP核糖聚合酶(poly ADP-ribose polymerase,PARP)和Caspase 3的表达,使食管癌细胞对奥沙利铂产生耐药性[23]。
2.3 激活癌症相关信号通路CYTOR可作为上游信号分子参与了多条癌症相关信号通路的激活,是介导癌细胞增殖、侵袭及迁移的重要参与者(Fig 3)。表皮生长因子受体(epidermal growth factor receptor,EGFR)是一种存在于细胞表面的跨膜糖蛋白,作为一种信号传导受体,它可通过激活PI3K-AKT、MEK/ERK以及STAT3等下游信号通路来调控细胞增殖、分化、凋亡等多种重要的生物学过程。在食管癌中,CYTOR已被证实是通过激活EGFR/ PI3K/AKT信号通路,抑制P21的表达,介导细胞的增殖[24]。Wnt信号通路是哺乳动物进化过程中相对保守的信号传导途径,细胞质中的β-catenin积累并向核内转移是该通路激活的标志,活化的Wnt/β-catenin常与其它信号通路协同作用调控癌细胞的增殖、凋亡、侵袭及迁移。结肠癌中的CYTOR能够与细胞质中β-catenin结合使其避免磷酸化,从而使β-catenin积累并转移到细胞核内诱导下游靶基因c-myc、cyclin D1以及EMT相关基因的转录,促进结肠癌细胞增殖、侵袭及迁移[23]。同样CYTOR促进膀胱癌细胞的增殖和转移也是通过稳定β-catenin并诱导Wnt/β-catenin信号通路的异常激活来实现的[25]。MEK/ERK传导途径是在丝裂原、神经递质、细胞因子等因素作用下被激活,继而将胞外信号向内传导,进而调控细胞增殖、分化、凋亡和转移等多种生物过程。最近研究表明,CYTOR在胃癌中参与激活MEK/ERK途径及下游分子c-fos的活化,促进胃癌细胞的增殖、迁移和侵袭,并抑制细胞凋亡[2]。
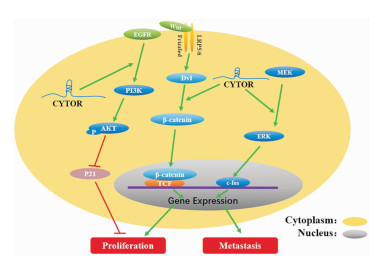
|
| Fig 3 Cancer related signaling pathways activated by CYTOR |
由于lncRNAs具有不同的结构域,能够结合不同的效应分子,这使得lncRNAs可作为支架分子来调控分子之间相互作用以及信号事件从而影响各种生物学过程。而lncRNAs作为支架分子所产生的生物学效应主要是通过与蛋白质结合形成RNA-蛋白复合物来实现的。研究表明,CYTOR能够与Bcl蛋白家族成员结合维持其稳定性,发挥促癌作用。具体而言,CYTOR可通过与卵巢癌组织及细胞中BCL-6蛋白的Ser333和Ser343位点结合使其免受泛素化而维持其蛋白质稳定性,从而促进卵巢癌细胞的增殖、侵袭及迁移[26]。同样,胃癌中的CYTOR通过与Bcl-2结合并保持其稳定性,抑制凋亡信号活化,介导细胞的增殖、侵袭及迁移[27]。
2.5 其他机制尽管大多数研究证实CYTOR主要通过竞争性结合miRNA、调节致癌转录因子的活性、激活癌症相关信号通路、维持蛋白质稳定性这四种方式来调控人类癌症多种生物学行为,但CYTOR调控癌症进展的机制似乎远不止这些。CYTOR亦可在表观遗传学、蛋白质翻译后修饰层面上介导癌症发生发展。在表观遗传学层面,CYTOR可通过促进DNA甲基转移酶的表达介导DNA甲基化,使肿瘤抑制因子乳腺癌易感基因1(breast cancer gene 1,BRCA1)、磷酸酶及张力蛋白同源物(phosphatase and tensin homolog,PTEN)的表达缺失,从而介导乳腺癌发生[28]; 在蛋白质翻译后修饰层面,CYTOR通过上调NEDD4-1(一种催化肿瘤抑制因子PTEN降解的E3泛素蛋白连接酶)的表达,使PTEN蛋白泛素化降解进而介导乳腺癌细胞的增殖和侵袭[29]。
3 CYTOR在人类癌症中异常表达与临床特征显著相关基于在线数据库分析以及临床样本的资料显示,CYTOR高表达与肺癌、乳腺癌、胃癌、结直肠癌等多种癌症组织学分级、TNM分期(tumor node metastasis classification)、淋巴结转移、不良预后以及生存期较短存在显著相关性(Tab 2),它可能是用于临床癌症早期诊断、分子靶向治疗以及评估预后的新生标志物。例如,CYTOR在非小细胞肺癌患者血浆中的水平与传统的非小细胞肺癌标志物癌胚抗原(CEA)呈正相关,CYTOR和癌胚抗原联合检测显示出比CYTOR或癌胚抗原单独检测具有更准确的诊断能力[5]。同样,CYTOR用于诊断胃癌的敏感性和特异性高达0.85和0.975 (AUC:0.95; 95% CI:0.9~1.0) [2]。在食管癌中,CYTOR的表达与TNM分期、淋巴结转移及预后不良呈正相关,与总体生存期呈负相关,被认为是食管癌患者独立危险因素[10]。另有资料显示,CYTOR的表达与卵巢癌组织学分级、临床分期、淋巴结转移及不良预后呈正相关,被发现是判断卵巢癌预后的独立预测因子[8, 27]。然而,在膀胱癌中,CYTOR高表达与组织学分级和淋巴结转移呈正相关,但未发现与癌症临床分期存在相关性,仍需要更多的样本数据去证实[25]。
| Cancer type | Expression | Clinical features | Reference |
| Gastric cancer | Upregulated | It was positively correlated with tumor volume, clinical stage and lymph node metastasis, and negatively correlated with the overall survival. | [2] |
| Colorectal cancer | Upregulated | It was positively correlated with pathological grade, TNM stage, lymph node metastasis and poor prognosis. | [4] |
| Breast cancer | Upregulated | It was positively correlated with TNM stage, lymphatic infiltration and short overall survival. | [29] |
| Esophageal cancer | Upregulated | It was positively correlated with TNM stage, lymph node metastasis and poor prognosis, and negatively correlated with overall survival. | [10] |
| Ovarian cancer | Upregulated | It was positively correlated with tumor volume, histological grade, clinical stage, lymph node metastasis and poor prognosis. | [8, 17] |
| Lung cancer | Upregulated | It was positively correlated with tumor volume and lymph node metastasis, and negatively correlated with survival. | [5] |
| Bladder cancer | Upregulated | It was positively correlated with histological grade and lymph node metastasis. | [25] |
| Cervical cancer | Upregulated | It was positively correlated with histological grade and poor prognosis. | [11] |
LncRNAs已被证实在包括癌症在内的许多疾病中特异性表达,发挥着至关重要的生物学效应[30]。近年研究资料显示,CYTOR作为一种致癌性lncRNA, 在肺癌、肝癌、乳腺癌、结肠癌等多种类型癌症中表达均升高,密切参与调控癌症发生、癌细胞增殖、凋亡、侵袭、迁移等生物学行为。相反,当CYTOR的表达受到抑制时,癌细胞增殖、侵袭、迁移数量减少,凋亡数量增多,放化疗敏感性升高。对CYTOR在人类癌症中的调控机制进行梳理表明,它所发挥的促癌作用是通过竞争性结合miRNA、调节转录因子的活性、激活癌症相关信号通路、维持蛋白质结合稳定性等多种方式来实现的。更为重要的是,目前已有的临床样本数据及在线数据库信息研究分析显示,CYTOR高表达与多种癌症病理分级、临床分期、淋巴结转移、患者总体生存期较短呈显著相关性。这使得lncRNA CYTOR可能被作为未来治疗癌症的新分子靶标及癌症早期诊断、评估预后的有效生物标志物,具有极其重要的临床应用价值。然而,目前关于lncRNA CYTOR与癌症的研究数据大多数源于基础实验,今后需更多地与临床研究紧密结合,进一步来阐明CYOTR在人类癌症中的生物学效应及其分子调控机制,系统完善其在人类癌症中的调控网络,使其尽早服务于癌症的诊疗。
| [1] |
Sung H, Ferlay J, Siegel R L, et al. Global cancer statistics 2020:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA CancerK J Clin, 2021, 71(3): 209-49. doi:10.3322/caac.21660 |
| [2] |
Shi Y, Sun H. Down-regulation of lncRNA LINC00152 suppresses gastric cancer cell migration and invasion through inhibition of the ERK/MAPK signaling pathway[J]. Oncol Targets Ther, 2020, 13(1): 2115-24. |
| [3] |
Li S Q, Chen Q, Qin H X, et al. Long intergenic nonprotein coding RNA 0152 promotes hepatocellular carcinoma progression by regulating phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin signaling pathway through miR-139/PIK3CA[J]. Am J Pathol, 2020, 190(5): 1095-107. doi:10.1016/j.ajpath.2019.11.010 |
| [4] |
Chen Z P, Wei J C, Wang Q, et al. Long non-coding RNA 00152 functions as a competing endogenous RNA to regulate NRP1 expression by sponging with miRNA-206 in colorectal cancer[J]. Int J Oncol, 2018, 53(3): 1227-36. |
| [5] |
Zhang J, Li W. Long noncoding RNA CYTOR sponges miR-195 to modulate proliferation, migration, invasion and radiosensitivity in nonsmall cell lung cancer cells[J]. Biosci Rep, 2018, 38(6): 1-12. |
| [6] |
Li Q, Wang X, Zhou L, et al. A positive feedback loop of long noncoding RNA LINC00152 and KLF5 facilitates breast cancer growth[J]. Front Oncol, 2021, 11(1): 619915-26. |
| [7] |
Hanahan D. Hallmarks of cancer: New dimensions[J]. Cancer Discov, 2022, 12(1): 31-46. doi:10.1158/2159-8290.CD-21-1059 |
| [8] |
Chen P, Fang X, Xia B, et al. Long noncoding RNA LINC00152 promotes cell proliferation through competitively binding endogenous miR-125b with MCL-1 by regulating mitochondrial apoptosis pathways in ovarian cancer[J]. Cancer Med, 2018, 7(9): 4530-41. doi:10.1002/cam4.1547 |
| [9] |
Huang Y, Luo H, Li F, et al. LINC00152 down-regulated miR-193a-3p to enhance MCL1 expression and promote gastric cancer cells proliferation[J]. Biosci Rep, 2018, 38(3): 1-14. |
| [10] |
Liu D, Gao M, Wu K, et al. LINC00152 facilitates tumorigenesis in esophageal squamous cell carcinoma via miR-153-3p/FYN axis[J]. Biomed Pharmacother, 2019, 112(3): 108654-61. |
| [11] |
Zheng J J, Du X J, Wang H P, et al. Long non-coding RNA 00152 promotes cell proliferation in cervical cancer via regulating miR-216b-5p/HOXA1 axis[J]. Eur Rev Med Pharmacol Sci, 2019, 23(9): 3654-63. |
| [12] |
Wang J, Zhang Y, Lu L, et al. Insight into the molecular mechanism of LINC00152/miR-215/CDK13 axis in hepatocellular carcinoma progression[J]. J Cell Biochem, 2019, 120(11): 18816-25. doi:10.1002/jcb.29197 |
| [13] |
Ma P, Wang H, Sun J, et al. LINC00152 promotes cell cycle progression in hepatocellular carcinoma via miR-193a/b-3p/CCND1 axis[J]. Cell Cycle, 2018, 17(8): 974-84. doi:10.1080/15384101.2018.1464834 |
| [14] |
Sun Z, Guo X, Zang M, et al. Long non-coding RNA LINC00152 promotes cell growth and invasion of papillary thyroid carcinoma by regulating the miR-497/BDNF axis[J]. J Cell Physiol, 2019, 234(2): 1336-45. doi:10.1002/jcp.26928 |
| [15] |
Chen W, Du M, Hu X, et al. Long noncoding RNA cytoskeleton regulator RNA promotes cell invasion and metastasis by titrating miR-613 to regulate ANXA2 in nasopharyngeal carcinoma[J]. Cancer Med, 2020, 9(3): 1209-19. doi:10.1002/cam4.2778 |
| [16] |
Ou C, Sun Z, He X, et al. Targeting YAP1/LINC00152/FSCN1 signaling axis prevents the progression of colorectal cancer[J]. Adv Sci, 2020, 7(3): 1901380-96. doi:10.1002/advs.201901380 |
| [17] |
Sun K, Hu P, Xu F. LINC00152/miR-139-5p regulates gastric cancer cell aerobic glycolysis by targeting PRKAA1[J]. Biomed Pharmacother, 2018, 97(1): 1296-302. |
| [18] |
Liu Y, Li M, Yu H, et al. lncRNA CYTOR promotes tamoxifen resistance in breast cancer cells via sponging miR-125a-5p[J]. Int J Mol Med, 2020, 45(2): 497-509. |
| [19] |
Yang J, Ma Q, Zhang M, et al. LncRNA CYTOR drives L-OHP resistance and facilitates the epithelial-mesenchymal transition of colon carcinoma cells via modulating miR-378a-5p/SERPINE1[J]. Cell Cycle, 2021, 20(14): 1415-30. doi:10.1080/15384101.2021.1934626 |
| [20] |
Chen S, Yang M, Wang C, et al. Forkhead box D1 promotes EMT and chemoresistance by upregulating lncRNA CYTOR in oral squamous cell carcinoma[J]. Cancer Lett, 2021, 503(1): 43-53. |
| [21] |
Wang X, Yu H, Sun W, et al. The long non-coding RNA CYTOR drives colorectal cancer progression by interacting with NCL and Sam68[J]. Mol Cancer, 2018, 17(1): 1-16. doi:10.1186/s12943-017-0753-1 |
| [22] |
Yue B, Liu C, Sun H, et al. A positive feed-forward loop between lncRNA-CYTOR and Wnt/β-Catenin signaling promotes metastasis of colon cancer[J]. Mol Ther, 2018, 26(5): 1287-98. doi:10.1016/j.ymthe.2018.02.024 |
| [23] |
Zhang S, Liao W, Wu Q, et al. LINC00152 upregulates ZEB1 expression and enhances epithelial-mesenchymal transition and oxaliplatin resistance in esophageal cancer by interacting with EZH2[J]. Cancer Cell Int, 2020, 20(1): 1-14. doi:10.1186/s12935-019-1086-5 |
| [24] |
Ding Y, Guo H, Zhu L, et al. LINC00152 knock-down suppresses ssophageal cancer by EGFR signaling pathway[J]. Open Med, 2020, 15(1): 126-33. doi:10.1515/med-2020-0019 |
| [25] |
Xian-Li T, Hong L, Hong Z, et al. Higher expression of Linc00152 promotes bladder cancer proliferation and metastasis by activating the Wnt/β-catenin signaling pathway[J]. Med Sci Monit, 2019, 25(1): 3221-30. |
| [26] |
Wang S, Weng W, Chen T, et al. LINC00152 promotes tumor progression and predicts poor prognosis by stabilizing BCL6 from degradation in the spithelial ovarian cancer[J]. Front Oncol, 2020, 10(1): 555132-48. |
| [27] |
Mao Y, Tie Y, Du J, et al. LINC00152 promotes the proliferation of gastric cancer cells by regulating B-cell lymphoma-2[J]. J Cell Biochem, 2019, 120(3): 3747-56. |
| [28] |
Wu J, Shuang Z, Zhao J, et al. Linc00152 promotes tumorigenesis by regulating DNMTs in triple-negative breast cancer[J]. Biomed Pharmacother, 2018, 97(1): 1275-81. |
| [29] |
Shen X, Zhong J, Yu P, et al. YY1-regulated LINC00152 promotes triple negative breast cancer progression by affecting on stability of PTEN protein[J]. Biochem Biophys Res Commun, 2019, 509(2): 448-54. |
| [30] |
王念, 刘骏, 王忠. 长链非编码RNA在冠心病发生发展过程中的研究进展[J]. 中国药理学通报, 2020, 36(10): 1358-61. Wang N, Liu J, Wang Z. Research progress of long chain noncoding RNA in the occurrence and development of coronary heart disease[J]. Chin Pharmacol Bull, 2020, 36(10): 1358-61. |

