

2. 四川大学华西药学院,四川 成都 610041;
3. 成都市中西医结合医院,四川 成都 610041
2. West China School of Pharmacy, Sichuan University, Chengdu 610041, China;
3. Chengdu Integrated TCM & Western Medicine Hospital, Chengdu 610041, China
俄色为蔷薇科(Rosaceae)苹果属(Malus)陇东海棠系(M.kansuensis)植物变叶海棠Malus toringoides (Rehd)Hughes.和花叶海棠Malus transitoria(Batal)Schneid.干燥叶及叶芽[1],经考证藏族人民有用其叶制作饮品使用的习惯,藏医中的传统用法为:取适量“俄色”,和酥油同煮后口服,因其味酸,性温,具有消解培根的功效,可缓解“京尼萨库”症状和降低人体脂肪,用于治疗消化不良、高血压病,降血脂、降血糖等[2-8]。“京尼萨库”病属现代医学“糖脂代谢障碍疾病”范畴。糖尿病(diabetes mellitus,DM)及代谢综合征(metabolic syndrome, MS)都是以胰岛素抵抗(insulin resistance, IR)为共同病理基础,糖调节受损造成的全身性进行性疾病。本文通过研究俄色提取物对自发性2型糖尿病模型KK-Ay小鼠的血糖、胰岛素耐量、血清胰岛素水平、胰高血糖素样肽-1(glucagon-like peptide-1, GLP-1)、病理、胰腺组织蛋白含量等的影响,探讨其对2型糖尿病胰岛素抵抗的作用及机制。
1 材料与方法 1.1 药物俄色是蔷薇科苹果属植物变叶海棠Malus toringoides (Rehd)Hughes.的叶,本研究所用的药材由四川省中医药科学院中药资源与种植研究所提供,并由周先建副研究员鉴定。俄色提取物,由四川省中医药转化医学中心制备,批号:20200527。制备工艺:取适量俄色药材,称定质量,置于1 000 mL圆底烧瓶中,加入10倍量水,加热微沸提取1 h,过滤,滤液保存备用,滤渣加入10倍量水后再次加热微沸提取1 h,过滤,合并两次滤液,浓缩滤液,得到俄色水提物浸膏(以生药材计,每g浸膏含生药3.23 g)。取适量俄色提取物浸膏,加适量蒸馏水溶解后定容至相应浓度备用。爱能(罗格列酮片),批号:200512,规格:4 mg/片,由成都恒瑞制药有限公司生产。取罗格列酮片4 mg置于研钵中研细,加入适量蒸馏水研磨均匀后定容至相应浓度备用。
1.2 动物8周龄SPF级,♂,KK-Ay小鼠70只,8周龄SPF级雄性C57BL/6J小鼠10只,均购于北京华阜康生物科技股份有限公司,许可证编号:SCXK(京)2019-0008。实验动物环境符合国家兔、大鼠、豚鼠、小鼠屏障系统使用设施标准,通过四川省实验动物管理委员会伦理审查,许可证号SYXK(川)2018-100。饲养于标准环境:温度(20±1)℃,相对湿度60%±5%,明暗周期12 h,KK-Ay小鼠高脂饲料(蛋白质17.5%、碳水化合物48.5%、脂肪17.9%,北京华阜康生物科技股份有限公司)喂养,C57小鼠标准饲料喂养。
1.3 试剂Mouse INS ELISA KIT(货号:ZC-38920),上海茁彩生物公司;GLP-1兔单克隆抗体(批号ab133329),abcam公司;山羊抗兔工作液(批号:SP-9001),正常山羊血清(批号:ZLI-9021),浓缩型DAB试剂盒(批号:K135925C),北京中杉金桥生物有限公司;罗氏(Roche)公司TUNEL试剂盒(批号:11684795910),瑞士罗氏公司(Roche Group)。
1.4 仪器罗氏卓越型血糖仪及配套血糖试纸(德国罗氏诊断有限公司,批号: 478100);InfiniteM200酶标仪(Tecan公司);转轮式切片机(徕卡-2016);TSJ-Ⅱ型全自动封闭式组织脱水机、PHY-Ⅲ型病理组织漂烘仪(常州市中威电子仪器有限公司);BMJ-Ⅲ型包埋机(常州郊区中威电子仪器厂);数码三目摄像显微镜(BA400Digital,麦克奥迪实业集团有限公司);图像分析软件Image-Pro Plus6.0(Media Cybernetics),Pannoramic 250型全景数字幻灯片扫描仪(3Dhisech);SpectraMAX Plus384酶标仪(美谷分子仪器有限公司);分析天平(最小分度值d=0.000 1 g,博特勒-托利多仪器有限公司)。
1.5 方法 1.5.1 分组与给药70只8周龄♂ SPF级KK-Ay小鼠,体质量(18~20) g,高脂饲料喂养14 d后,上午十点开始禁食,禁食不禁水4 h,测定小鼠空腹血糖,血糖值大于11.1 mol·L-1的小鼠视为模型诱导成功。10只C57BL/6J小鼠作为正常对照组。按照空腹血糖值将50只成模的KK-Ay小鼠随机分为模型对照组、阳性对照组(罗格列酮片2.67 mg·kg-1)和俄色提取物组(0.75、1.50、3.00 g生药·kg-1),共5组,每组10只。各组分别灌胃给予相应药物(正常对照组和模型对照组给予),给药体积为10 mL·kg-1,每日定时给药1次,连续给药35 d。
1.5.2 观察指标 1.5.2.1 体质量于实验期间每周固定时间测定1次。
1.5.2.2 血糖于实验期间每周测定小鼠空腹血糖值1次。所有小鼠自由饮水,禁食4 h后剪去尾端取血,用血糖仪及试纸测定各组小鼠空腹血糖值。
1.5.2.3 胰岛素耐量水平测定于给药d 32测定小鼠胰岛素耐量水平。所有小鼠禁食不禁水4 h后剪尾取血测定空腹血糖值(0 min),测定后腹腔注射胰岛素,浓度为0.5 U·kg-1,测量第15、30、60和120 min血糖水平。
1.5.2.4 血清胰岛素测定末次给药后,所有小鼠禁食不禁水4 h,摘眼球取血,4 000 r·min-1离心10 min,收集血清,测定血清胰岛素水平。
1.5.2.5 胰腺组织病理学观察试验结束时,取胰腺组织固定,脱水包埋,再脱水后,用苏木精进行染色,返蓝后再用伊红染色,接着进行梯度酒精脱水和二甲苯透明,最后用中性树胶封固和完成切片的图像采集,按下表分级评分标准进行胰腺组织病理评分。
| The lesion type | Degree of lesion | Lesion classification |
| increased islets in volume/increased islet cells | none | 0 |
| mild(5%~20%) | 1 | |
| moderate(21%~50%) | 2 | |
| severe(>50%) | 3 | |
| vacuolar degeneration of islets | none | 0 |
| 1 to 4 cells, at 400 times field | 1 | |
| 5 to 10 cells, at 400 times field | 2 | |
| 11 to 15 cells, at 400 times field | 3 | |
| more than 16 cells, at 400 times field | 4 | |
| islet cells are necrotic | none | 0 |
| 1 to 4 dead cells, at 400 times field | 1 | |
| 5 to 10 dead cells, at 400 times field | 2 | |
| 11 to 15 dead cells, at 400 times field | 3 | |
| more than 16 dead cells, at 400 times field | 4 | |
| acinar cells are denaturated and necrotic | none | 0 |
| vacuolar degeneration of cells around the catheter(< 5%) | 1 | |
| focal acinar epithelial cell vacuolation degeneration(5%~20%) | 2 | |
| vacuolar degeneration of diffuse acinar epithelial cells(21%~50%) | 3 | |
| a large area of acinar epithelial cells is vacuolated(>50%) | 4 |
10%的中性甲醛固定的小鼠胰腺组织,切片,脱蜡,修复,荧光TUNEL孵育液孵育,PBS冲洗,加DAPI染核15 min,PBS冲洗,甘油明胶封片,荧光显微镜下镜检。Pannoramic 250全景数字幻灯片扫描仪扫描,采集400倍图像3张,读片,镜下统计凋亡细胞数和总细胞数,计算凋亡率,进行凋亡细胞分析。
1.5.2.7 胰腺组织GLP-1蛋白含量将固定于4%多聚甲醛溶液中的胰腺组织取出进行石蜡包埋,切片,脱蜡,用柠檬酸盐缓冲液进行抗原修复,用山羊血清封闭,加入一抗(GLP-1)4 ℃过夜,再加入生物素化二抗,在37 ℃温度下孵育30 min,之后用PBS进行水洗和DAB显色,显色后再用苏木精轻度复染,最后脱水并用中性树胶封片。BA200Digital数码三目显微摄像系统进行图像采集。
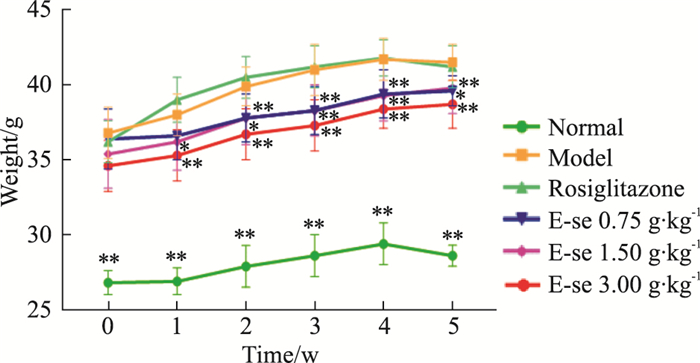
2 结果 2.1 俄色提取物对KK-Ay小鼠体质量的影响如Tab 2、Fig 1所示,模型对照组体质量均明显升高,与正常对照组比较,差异有统计学意义(P < 0.01);俄色3.00、1.50 g生药·kg-1剂量组体质量均明显减轻,俄色0.75 g生药·kg-1剂量组在给药第2、3、4、5周体质量降低,差异有统计学意义(P < 0.01或P < 0.05)。
| Group | Dose/ g·kg-1 |
Weight/g | |||||
| 0 w | 1 w | 2 w | 3 w | 4 w | 5 w | ||
| Normal | - | 26.8±0.8 | 26.9±0.9 | 27.9±1.4 | 28.6±1.4 | 29.4±1.4 | 28.6±0.7 |
| Model | - | 36.8±1.7## | 38.0±1.4## | 39.9±1.3## | 41.0±1.7## | 41.7±1.4## | 41.5±1.2## |
| Rosiglitazone | 2.67 | 36.2±1.4 | 39.0±1.5 | 40.5±1.4 | 41.2±1.4 | 41.8±1.2 | 41.2±1.4 |
| E-se | 0.75 | 36.4±2.0 | 36.6±1.6 | 37.8±1.6** | 38.3±1.6** | 39.4±1.6** | 39.6±1.0** |
| E-se | 1.50 | 35.4±2.3 | 36.2±1.9* | 37.8±1.8* | 38.3±1.7** | 39.3±1.7** | 39.8±1.7* |
| E-se | 3.00 | 34.6±1.7 | 35.3±1.7** | 36.7±1.7** | 37.3±1.7** | 38.4±1.3** | 38.7±1.6** |
| ##P < 0.01 vs normal;*P < 0.05,**P<0.01 vs model. | |||||||
|
| Fig 1 Effect of E-se on body weight of KK-Ay mice *P < 0.05, **P < 0.01 vs Normal |
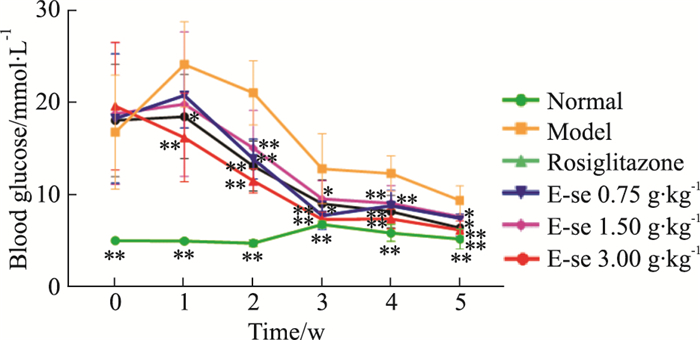
如Tab 3、Fig 2所示,模型对照组空腹血糖均高于正常对照组,差异有统计学意义(P < 0.01);俄色3.00 g生药·kg-1g剂量组空腹血糖值均降低,1.50、0.75g生药·kg-1剂量组在给药第2、3、4、5周空腹血糖值下降,差异有统计学意义(P < 0.01或P < 0.05)。
| Group | Dose/ g·kg-1 |
Blood glucose level/ mmol·L-1 | |||||
| 0 w | 1 w | 2 w | 3 w | 4 w | 5 w | ||
| Normal | - | 5.03±0.20 | 4.99±0.21 | 4.75±0.36 | 6.79±0.55 | 5.87±0.91 | 5.20±1.08 |
| Model | - | 16.78±6.17## | 24.11±4.62## | 21.03±3.47## | 12.82±3.78## | 12.30±1.92## | 9.39±1.56## |
| Rosiglitazone | 2.67 | 19.60±6.91 | 16.19±4.79** | 11.50±1.30** | 7.34±0.79** | 7.38±1.07** | 6.18±0.98** |
| E-se | 0.75 | 18.25±7.00 | 20.76±3.52 | 13.88±2.16** | 7.77±1.51** | 8.80±1.67** | 7.45±1.95* |
| E-se | 1.50 | 18.79±7.67 | 19.81±7.83 | 15.07±4.07** | 9.55±1.97* | 9.10±1.88** | 7.61±1.39* |
| E-se | 3.00 | 18.05±6.10 | 18.47±4.56* | 13.11±2.70** | 9.03±2.59* | 8.19±1.76** | 6.41±1.17** |
| ##P < 0.01 vs normal;*P < 0.05,**P<0.01 vs model. | |||||||
|
| Fig 2 Effect of E-se on fasting blood glucose in KK-Ay mice *P < 0.05, **P < 0.01 vs Normal |
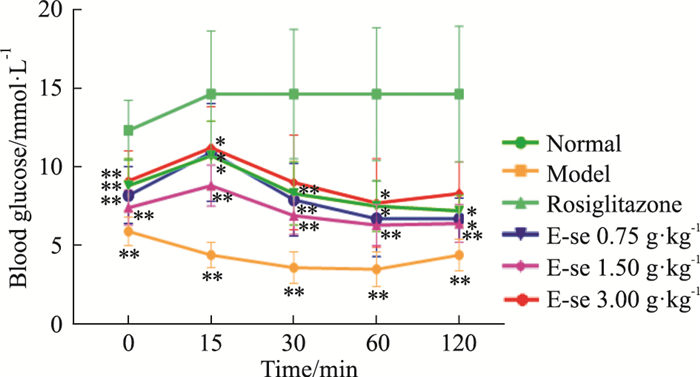
如Tab 4、Fig 3所示,模型对照组在实验期间的空腹血糖值均高于正常对照组,差异有统计学意义(P < 0.01);俄色3.00 g、0.75 g生药·kg-1剂量组在实验期间血糖值均降低,1.50 g生药·kg-1组在第0、15 min血糖值降低,差异有统计学意义(P < 0.01或P < 0.05)。
| Group | Dose/ g·kg-1 |
Blood glucose level/ mmol·L-1 | ||||
| 0 min | 15 min | 30 min | 60 min | 120 min | ||
| Normal | - | 5.9±0.9 | 4.4±0.8 | 3.6±1.0 | 3.5±1.1 | 4.4±1.0 |
| Model | - | 12.3±1.9## | 14.6±4.0## | 14.6±4.1## | 14.6±4.2## | 14.6±4.3## |
| Rosiglitazone | 2.67 mg·kg-1 | 7.4±1.1** | 8.8±1.3** | 6.9±1.2** | 6.3±1.3** | 6.4±1.2** |
| E-se | 0.75 | 8.8±1.7** | 10.7±2.2* | 8.3±2.0** | 7.5±1.6* | 7.2±1.0* |
| E-se | 1.50 | 9.1±1.9** | 11.2±2.6* | 9.0±3.0 | 7.7±2.8 | 8.3±2.0 |
| E-se | 3.00 | 8.2±1.8** | 10.9±3.1* | 7.9±2.3** | 6.7±2.4* | 6.7±1.3* |
| ##P < 0.01 vs normal;*P < 0.05,**P<0.01 vs model. | ||||||
|
| Fig 3 Effect of E-se on insulin tolerance in KK-Ay mice *P < 0.05, **P < 0.01 vs Normal |
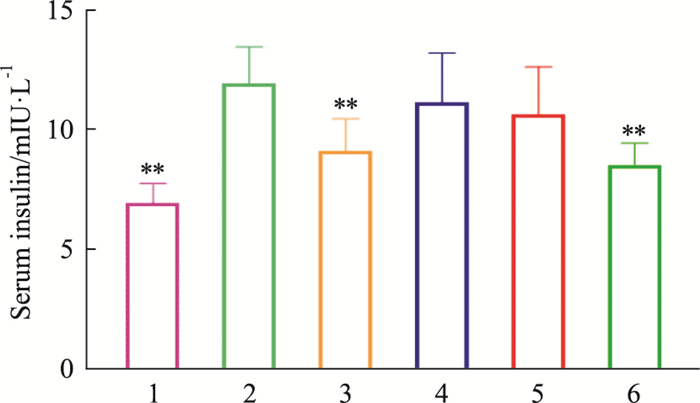
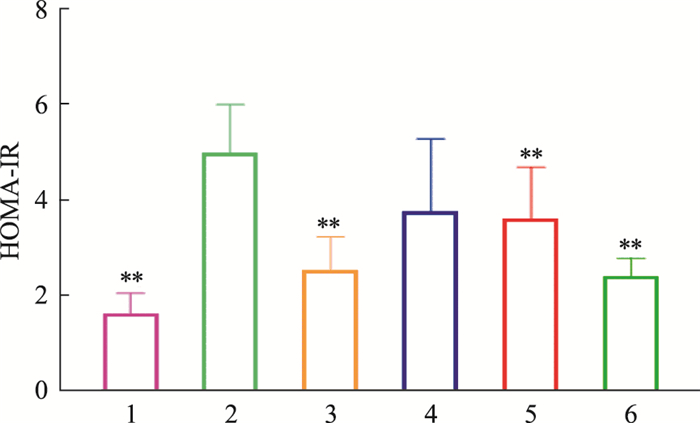
如Tab 5、Fig 4-6所示,模型对照组小鼠血清中胰岛素浓度高于正常对照组,差异有统计学意义(P < 0.01);俄色3.00 g生药·kg-1剂量组小鼠血清胰岛素浓度降低(P < 0.01)。模型对照组小鼠胰岛素抵抗指数(homeostasis model assessment for insulin resistance index, HOMA-IR)高于正常对照组(P < 0.01);俄色3.00 g、1.50 g生药·kg-1剂量组小鼠HOMA-IR降低(P < 0.01)。模型对照组小鼠胰岛素敏感指数(insulin sensitivity index, ISI)低于正常对照组,差异有统计学意义(P < 0.01);俄色各剂量组小鼠ISI升高(P < 0.01或P < 0.05)。
| Group | Dose/g·kg-1 | Concentration of INS/mIU·L-1 | HOMA-IR | ISI |
| Normal | - | 6.9±0.8 | 1.6±0.4 | 0.029 0±0.006 1 |
| Model | - | 12.0±1.5## | 5.0±1.0## | 0.009 2±0.001 6## |
| Rosiglitazone | 2.67 mg·kg-1 | 9.1±1.4** | 2.5±0.7** | 0.0187 ±0.004 6** |
| E-se | 0.75 | 11.2±2.0 | 3.8±1.5 | 0.013 3±0.004 2* |
| E-se | 1.50 | 10.6±2.0 | 3.6±1.1** | 0.013 1±0.003 3** |
| E-se | 3.00 | 8.5±0.9** | 2.4±0.4** | 0.018 9±0.003 1** |
| ##P < 0.01 vs normal;*P < 0.05,**P<0.01 vs model. | ||||
|
| Fig 4 Effects of E-se on insulin (INS) concentration in serum of KK-Ay mice 1:Normal; 2:Model; 3:Rosiglitazone; 4:E-se 0.75 g·kg-1; 5:E-se 1.50 g·kg-1; 6:E-se 3.00 g·kg-1. *P < 0.05, **P < 0.01 vs Normal |
|
| Fig 5 Effects of E-se on insulin resistance index(HOMA-IR) of KK-Ay mice 1:Normal; 2:Model; 3:Rosiglitazone; 4:E-se 0.75 crude drug·kg-1; 5:E-se 1.50 crude drug·kg-1; 6:E-se 3.00 crude drug·kg-1. **P < 0.01 vs Normal |

|
| Fig 6 Effects of E-se on insulin sensitivity index (ISI) of KK-Ay mice 1:Normal; 2:Model; 3:Rosiglitazone; 4:E-se 0.75 crude drug·kg-1; 5:E-se 1.50 crude drug·kg-1; 6:E-se 3.00 crude drug·kg-1. *P < 0.05, **P < 0.01 vs Normal |
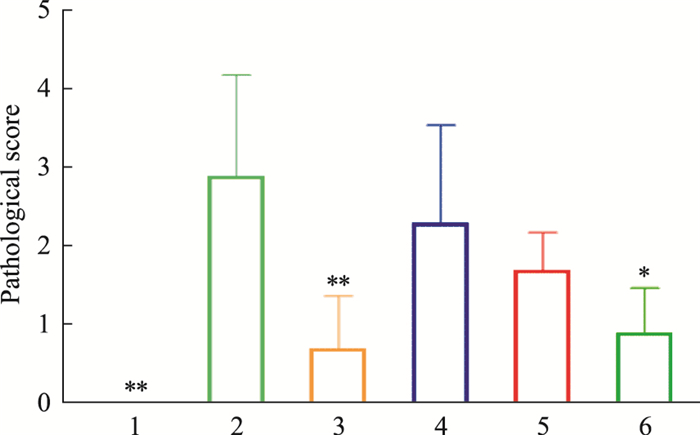
由Tab 6、Fig 7-8可见,模型对照组小鼠胰腺组织病变分级评分高于正常对照组,差异有统计意义(P < 0.01);俄色3.00 g生药·kg-1剂量组小鼠胰腺组织病变分级评分降低,与模型对照组比较差异有统计学意义(P < 0.05)。
| Group | Dose/ g·kg-1 | A |
| Normal | - | 0.0±0.0 |
| Model | - | 2.9±1.3## |
| Rosiglitazone | 2.67 mg·kg-1 | 0.7±0.7** |
| E-se | 0.75 | 2.3±1.2 |
| E-se | 1.50 | 1.7±0.5 |
| E-se | 3.00 | 0.9±0.6* |
| ##P < 0.01 vs normal;*P < 0.05,**P<0.01 vs model. | ||
|
| Fig 7 Effects of E-se extract on pathological changes of pancreatic tissues in KK-Ay mice 1:Normal; 2:Model; 3:Rosiglitazone; 4:E-se 0.75 g·kg-1; 5:E-se 1.50 g·kg-1; 6:E-se 3.00 g·kg-1. *P < 0.05, **P < 0.01 vs Normal |
|
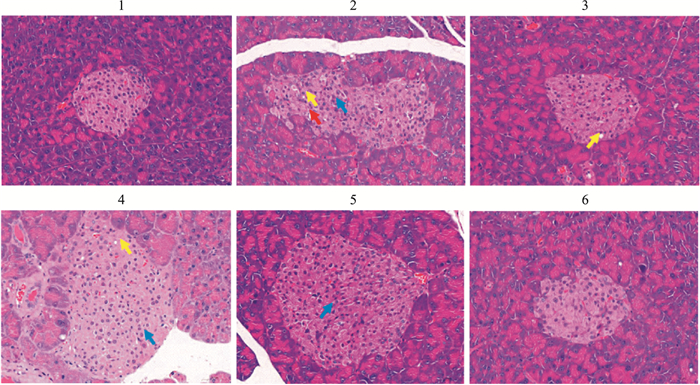
Fig 8 Effects of E-se extract on pathological changes of pancreatic tissues in KK-Ay mice(HE×400)
 Vacuolar degeneration of islets, Vacuolar degeneration of islets,  Increased islet cells, Increased islet cells,  Islet cells were necrotic.1: Normal control; 2: Model control; 3: Rosiglitazone; 4. E-se 0.75g·kg-1; 5. E-se 1.50 g·kg-1; 6. E-se 3.00 g·kg-1 Islet cells were necrotic.1: Normal control; 2: Model control; 3: Rosiglitazone; 4. E-se 0.75g·kg-1; 5. E-se 1.50 g·kg-1; 6. E-se 3.00 g·kg-1
|
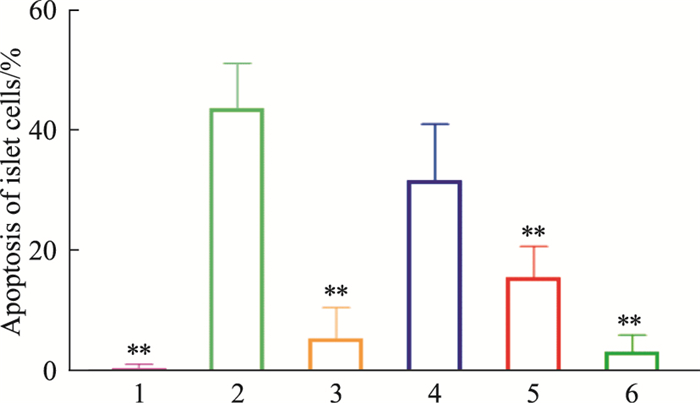
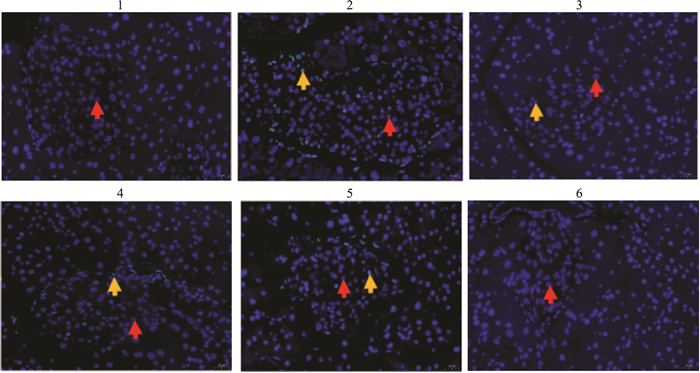
由Tab 7、Fig 9-10可见,模型对照组胰腺组织中胰岛细胞凋亡百分比高于正常对照组(P < 0.01);俄色3.00 g、1.50 g生药·kg-1剂量组胰岛细胞凋亡百分比降低,与模型对照组比较差异有统计学意义(P < 0.01)。
| Group | Dose/ g·kg-1 | A |
| Normal | - | 0.4±0.8 |
| Model | - | 43.8±7.5## |
| Rosiglitazone | 2.67 | 5.3±5.2** |
| E-se | 0.75 | 31.8±9.4 |
| E-se | 1.50 | 15.6±5.2** |
| E-se | 3.00 | 3.2±2.8** |
| ##P < 0.01 vs normal;*P < 0.05,**P<0.01 vs model. | ||
|
| Fig 9 Effect of E-se extract on islet cell apoptosis in KK-Ay mice 1:Normal; 2:Model; 3:Rosiglitazone; 4:E-se 0.75 g·kg-1; 5:E-se 1.50 g·kg-1; 6:E-se 3.00 g·kg-1. *P < 0.05, **P < 0.01 vs Normal |
|
Fig 10 Effect of E-se extract on islet cell apoptosis in KK-Ay mice(HE, ×400)
normal cells  , apoptotic cells , apoptotic cells  . 1: Normal control; 2. Model control; 3: Rosiglitazone; 4. E-se 0.75g·kg-1; 5. E-se 1.50 g·kg-1; 6. E-se 3.00 g·kg-1 . 1: Normal control; 2. Model control; 3: Rosiglitazone; 4. E-se 0.75g·kg-1; 5. E-se 1.50 g·kg-1; 6. E-se 3.00 g·kg-1
|
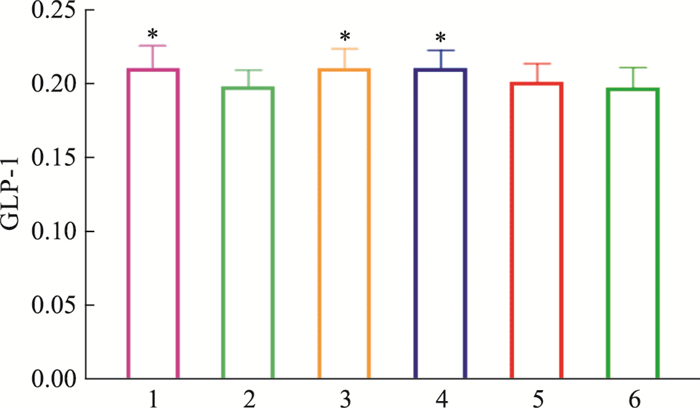
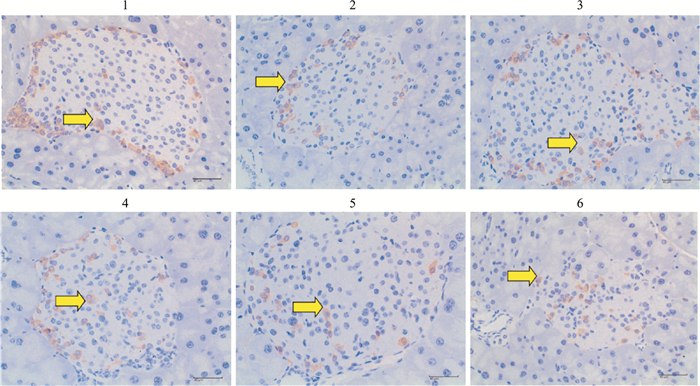
由Tab 8、Fig 11-12可见,模型对照组小鼠胰腺组织GLP-1蛋白含量低于正常对照组,差异有统计学意义(P < 0.05);俄色0.75 g生药·kg-1剂量组小鼠胰腺组织GLP-1蛋白含量升高,与模型对照组比较差异有统计学意义(P < 0.05);俄色3.00 g、1.50 g生药·kg-1剂量组小鼠胰腺组织GLP-1蛋白含量升高,与模型对照组比较差异无统计学意义(P>0.05)。
| Group | Dose/g·kg-1 | GLP-1 |
| Normal | - | 0.210±0.016 |
| Model | - | 0.198±0.011# |
| Rosiglitazone | 2.67 | 0.210±0.013* |
| E-se | 0.75 | 0.211±0.012* |
| E-se | 1.50 | 0.202±0.012 |
| E-se | 3.00 | 0.197±0.014 |
| #P < 0.05 vs normal;*P < 0.05 vs model. | ||
|
| Fig 11 Effect of E-se extract on glucagon-like peptide-1 (GLP-1) concentration in pancreatic tissues of KK-Ay mice 1:Normal; 2:Model; 3:Rosiglitazone; 4:E-se 0.75 g·kg-1; 5:E-se 1.50 g·kg-1; 6:E-se 3.00 g·kg-1. *P < 0.05, **P < 0.01 vs Normal |
|
| Fig 12 Effect of E-se extract on glucagon-like peptide-1 (GLP-1) concentration in pancreatic tissues of KK-Ay mice(immunohistochemistry, ×400) 1: Normal control; 2: Model control; 3: Rosiglitazone; 4: E-se 0.75g·kg-1; 5: E-se 1.50 g·kg-1; 6: E-se 3.00 g·kg-1 |
京尼萨库病为藏医很早就认识的一种因饮食起居不当,使“培根”和过盛的脂肪不能转化成人体需要的精华,混入尿液并随着尿液排出体外所造成的全身性疾病。藏医认为,“京尼萨库”病与现代医学的由糖脂代谢紊乱引起的疾病有相同的临床特点。胰岛素调控血糖代谢功能的受损,导致了代偿性胰岛素分泌的增多,故常伴有高胰岛素血症。胰岛素抵抗不仅是糖尿病的核心发病机制,也是贯穿代谢综合征、高血脂、肥胖、动脉粥样硬化、冠心病等多种代谢相关疾病的主线;既是连接它们的纽带,也是它们共同的病理基础。
课题组前期研究表明,俄色水提物可降低链脲佐菌素所致糖尿病小鼠的空腹血糖和血清胰岛素水平[9-10]。KK/upj-Ay小鼠是KK自发糖尿病小鼠的同源突变近交系,具有过量饮食、中度肥胖、高血糖、胰岛素抵抗等代谢异常综合征等生理特点,常用于研究非胰岛素依赖型糖尿病的模型动物[11-12]。
本实验在前期研究基础上,以俄色为研究对象,采用自发性2型糖尿病KK-Ay小鼠模型,观察俄色对2型糖尿病模型KK-Ay小鼠胰岛素抵抗的改善作用。结果显示,俄色各剂量组均能降低小鼠空腹血糖;胰岛素耐量试验研究中,俄色3.00 g、0.75 g生药·kg-1剂量组各时间点血糖值均降低;此外,俄色3.00 g生药·kg-1剂量组可降低小鼠血清胰岛素水平,提示其在一定程度上可改善KK-Ay小鼠胰岛素抵抗,增加机体对胰岛素的敏感性。研究表明,高血糖和高胰岛素血症的长期存在会加重靶组织的损伤,促进胰岛素抵抗的发生发展[13]。本研究观察俄色对胰腺组织病理和胰岛细胞凋亡的影响,模型对照组小鼠胰腺组织病变分级评分显著升高,可见胰岛细胞增多、胰岛细胞空泡变性、胰岛细胞坏死和胰岛细胞凋亡百分比增高,俄色可降低胰腺组织病变分级评分和胰岛细胞凋亡百分比,提示俄色对胰腺组织和胰岛细胞具有一定的保护作用。胰高血糖素样肽是一种由肠道L细胞分泌的肠促胰岛素激素,可参与糖代谢调节[14],为进一步证实俄色对胰岛素分泌的作用,结果提示俄色可增加胰腺组织中胰高血糖素样肽水平。综上,俄色提取物可通过降低2型糖尿病KK-Ay小鼠血糖值、降低血清胰岛素水平、减少胰岛细胞凋亡和增加机体对胰岛素的敏感性,从而改善胰岛素抵抗。
| [1] |
宇妥·元丹贡布. 四部医典(藏文版)[M]. 拉萨: 西藏人民出版社, 1982: 330. Yutuo Yuandangongbu. Medical Canon in Four Sections (Tibetan Edition)[M]. Lhasa: Tibet People′s Publishing House, 1982: 330. |
| [2] |
王道清, 李敏, 石万银. 藏药"俄色"的资源调查及生药学研究[J]. 中药与临床, 2011, 2(3): 14-6. Wang D Q, Li M, Shi W Y. Resource survey and pharmacognosy reasearch of Zang medicine "ESe"[J]. Pharm Clin Chin MaterMed, 2011, 2(3): 14-6. |
| [3] |
毛羽, 吴永康, 冯豫川, 等. 苹果属花楸苹果组植物的新用途[P]. 中国: 200810170831.8, 2008-10-17. Mao Y, Wu Y K, Feng Y C, et al. New Uses of Malus (Malus) [P]. China: 200810170831.8, 2008-10-17. |
| [4] |
李燕, 谢晓芳, 李丹, 等. 俄色总黄酮对糖尿病的药理作用研究[J]. 中外医疗, 2013, 14: 25. Li Y, Xie X F, Li D, et al. Pharmacological effect of total flavonoids from Ese on diabetes mellitus[J]. Chin Foreign Med Treatt, 2013, 14: 25. |
| [5] |
炉霍县林业开发总公司. 一种预防高原反应的胶囊剂的制备方法及用途[P]. 中国: 200810166068.1, 2008-10-17. Luhuo County Forestry Development Corporation. Preparation method and application of a capsule for preventing altitude sickness [P]. China: 200810166068.1, 2008-10-17. |
| [6] |
赵军宁, 华桦, 杨安东, 等. 蔷薇科苹果属植物或其提取物的新用途[P]. 中国: 201310216177.0, 2013-06-04. Zhao J N, Hua H, Yang A D, et al. New Uses of Malus and its extracts in Rosaceae [P]. China: 201310216177.0, 2013-06-04. |
| [7] |
赵军宁, 华桦, 杨安东, 等. 一种变叶海棠提取物及其制备方法和其用途[P]. 中国: ZL201410259005.6, 2016-08-17. Zhao J N, Hua H, Yang A D, et al. The preparation and application of extracts from Malus toringoides (Rehd)Hughes [P]. China: ZL201410259005.6, 2016-08-17. |
| [8] |
李丹, 彭成, 谢晓芳, 等. 藏药俄色不同提取物降血糖作用比较[J]. 中国实验方剂学杂志, 2013, 19(21): 199-203. Li D, Peng C, Xie X F, et al. Effects of three different extracts from Malus toringoides in experimental diabetic mice[J]. Chin J Exp Tradit Med Formulae, 2013, 19(21): 199-203. |
| [9] |
华桦, 廖利, 朱宁, 等. 变叶海棠对自发性2型糖尿病小鼠(KK-Ay)小鼠的作用[J]. 中药药理与临床, 2014, 30(2): 96-8. Hua H, Liao L, Zhu N, et al. Effect of Malus toringoides (Rehd.) Hughes. on spontaneous type 2 diabetic mice (KK-Ay mice)[J]. Pharmacol Clin Chin Mater Med, 2014, 30(2): 96-8. |
| [10] |
罗园, 华桦, 曾安琪, 等. 变叶海棠提取物对链脲佐菌素所致糖尿病小鼠的作用研究[J]. 中药药理与临床, 2020, 36(1): 115-20. Lu oY, Hua H, Zeng A Q, et al. Effect of Malus toringoides (Rehd.) Hughes. aqueous extract on streptozotocin-induced diabetic mice[J]. Pharmacol Clin Chin Mater Med, 2020, 36(1): 115-20. |
| [11] |
heng H F, Hayashi K, Matsumura Y, et al. Anti-inflammatory and antioxidative properties of isoflavones provide renal protective effects distinct from those of dietary soy proteins against diabetic nephropathy[J]. Mol Nutr Food Res, 2020, 64(10): 2000015. |
| [12] |
Zhang J, Yin J M, Wang Y J, et al. Apelin impairs myogenic response to induce diabetic nephropathy in mice[J]. Faseb J, 2018, 32(8): 4315-27. |
| [13] |
Sasaki N, Ozono R, Higashi Y, et al. Association of insulin resistance, plasma glucose level, and serum insulin level with hypertension in a population with different stages of impaired glucose metabolism[J]. J Am Heart Associat, 2020, 9(7): e015546. |
| [14] |
帅青云, 涂强, 黄小川, 等. Exendin-4对Ⅰ型糖尿病小鼠内皮祖细胞功能及AKT/eNOS信号通路的影响[J]. 中国药理学通报, 2021, 37(5): 693-8. Shuai Q Y, Tu Q, Huang X C, et al. Effects of Exendin-4 on endothelial progenitor cell function and AKT/eNOS signaling pathway in mouse model of type 1 diabetes[J]. Chin Pharmacol Bull, 2021, 37(5): 693-8. |

