

魏伟(1960-),男,博士,教授,博士生导师,研究方向:抗炎免疫药理学,通讯作者,E-mail:wwei@ahmu.edu.cn
类风湿关节炎(rheumatoid arthritis,RA)是一种全身性自身免疫病,主要累及关节滑膜,产生慢性滑膜炎症,最终导致关节破坏和功能丧失。该病的病理机制涉及多种类型细胞之间的相互作用,其中成纤维样滑膜细胞(fibroblast-like synoviocytes,FLS)起关键作用,表现出一种侵袭性表型,产生炎症介质导致滑膜炎症,造成软骨、骨损伤。随着代谢组学的发展,细胞代谢研究为RA的治疗提供了潜在新靶点。虽然,目前对于RA的治疗已经取得一定的进展,但缺乏直接针对FLS代谢异常的药物。越来越多的证据表明,干扰RA-FLS代谢途径中的某些分子,可以缓解RA疾病严重程度,一些关键的转运体和代谢酶成为潜在的药物治疗靶点。
1 FLS能量代谢异常介导了RA的病理机制和发生发展FLS是滑膜组织的主要组成细胞,具有分泌炎性细胞因子及趋化因子促进炎症反应、分泌基质金属蛋白酶(matrix metalloproteinase,MMP)降解软骨、刺激破骨细胞分化导致骨侵蚀等功能。此外,由于表面标记物(Tab 1)的表达差异,不同功能FLS表型的发现[1-2],进一步阐明了FLS在RA病理机制中的作用。
| Markers | Marked regions in synovial | |
| Type IV collagen | - | |
| Type V collagen | - | |
| Vimentin | - | |
| Integrins | - | |
| Intercellular adhesion molecule 1 | ICAM-1/CD54 | - |
| Hyaluronan receptor | CD44 | - |
| Leukocyte common antigen | CD45 | - |
| Cadherin 11 | DH11 | - |
| Uridine diphosphate glucuronate dehydrogenase | UDPGD | lining layer |
| Vascular cell adhesion molecule-1 | VCAM-1/CD106 | lining layer |
| Decay acceleration factor | DAF /CD55 | lining layer |
| Dodoplanin | PDPN/Gp38 | lining layer |
| Fibroblast activated protein-α | FAPα | lining layer |
| Endosialin | TEM-1/CD248 | sublining layer |
| Thymocyte antigen 1 | THY1/ CD90 | sublining layer |
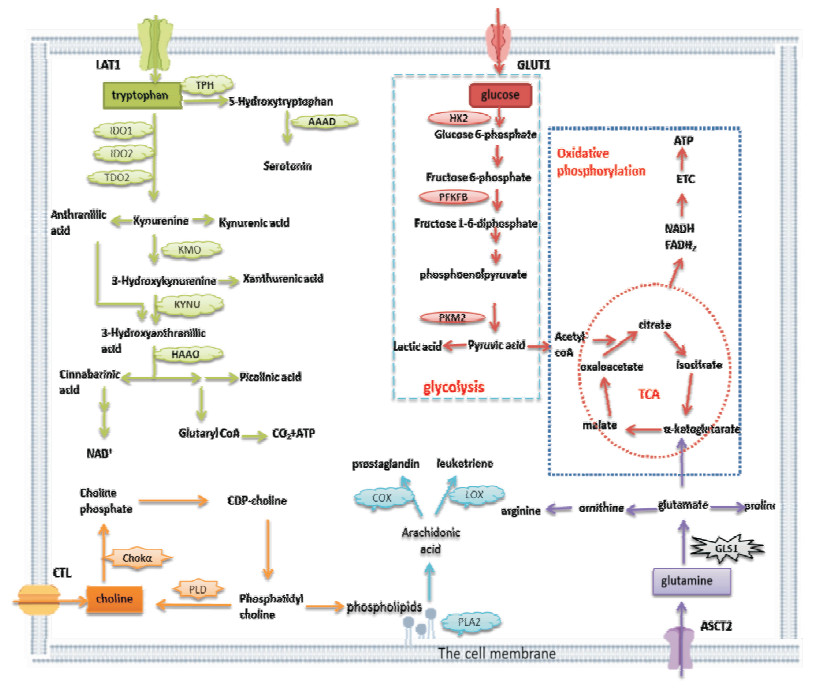
|
| Fig 1 The metabolic pathway of RA-FLS GLUT1, LAT1, CTL, ASCT2 and other transporters are highly activated in the activated RA-FLS, leading to a large amount of glucose, tryptophan, choline, glutamine and other substances transporting into the cells. The hypoxic environment in the inflammatory joint allows a lot of glucose to provide energy to the cells through the glycolysis pathway. Increased glycolytic activity leads to the conversion of pyruvate to lactic acid, which reduces the synthesis of acetyl-CoA and hinders TCA. As compensation, RA-FLS increases the catabolism of glutamine, which is metabolized to glutamate by GLS1. On the one hand, glutamate can provide nutrients for cells; on the other hand, it can be metabolized to produce α-ketoglutarate into TCA to provide energy to the cells. A few of tryptophan is used to synthesize proteins and neurotransmitters (such as serotonin), and more than 95% of free tryptophan is degraded by the kynurenine pathway. RA-FLS absorbs more choline, which is rapidly phosphorylated by Chokα to promote de novo synthesis of phosphatidylcholine. So that it can provide the phospholipids needed to maintain proper membrane fluidity and make up the cell membrane, as well as promote the production and release of cytokines.PLA2 releases arachidonic acid from cell membrane phospholipids and further metabolizes into prostaglandins and leukotrienes, promoting joint inflammation. |
近年来,对RA代谢途径的研究改变了对自身抗原识别引发自身免疫的早期观点。RA患者血清中糖酵解产物和氨基酸代谢产物增加,且脂质代谢产物与C-反应蛋白水平相关,提示RA患者能量代谢发生异常[3]。RA患者的原始T细胞代谢紊乱,戊糖磷酸途径活性增强,产生高水平的还原型烟酰胺腺嘌呤二核苷酸磷酸和生物合成前体,降低了细胞内活性氧水平,促进Th1和Th17的分化。葡萄糖转运体1(glucose transporter 1,GLUT1)表达增强,增加葡萄糖摄取及糖酵解通量,提供细胞所需能量,增强M1巨噬细胞及B细胞活性;线粒体脂质氧化途径可以刺激M2巨噬细胞及记忆B细胞功能[4]。RA-FLS异常活化具有侵袭性,介导炎症和关节破坏,代谢需求高。研究表明RA-FLS中糖酵解途径高度激活,脂质(如胆碱、花生四烯酸)及氨基酸(如色氨酸、谷氨酰胺)的代谢发生改变,这些代谢变化参与了FLS的异常活化和滑膜炎症。
临床上用于治疗RA的药物可以通过影响代谢发挥治疗作用。甲氨蝶呤(methotrexate,MTX)作为治疗RA患者的一线药物,影响叶酸代谢,抑制DNA的生物合成及FLS增殖。并且,MTX能显著降低RA-FLS中己糖激酶II(hexokinases 2,HK2)的表达,调控FLS糖酵解活性。甾体抗炎药糖皮质激素能调节许多与糖酵解和雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)通路相关的代谢酶的基因转录,从而发挥治疗RA的作用[5]。以上结果提示,调控RA异常代谢恢复其正常免疫稳态可能是治疗RA的潜在新策略。
2 FLS能量代谢途径及异常关键分子葡萄糖、脂质、氨基酸代谢途径中异常活化的转运体及代谢酶,导致FLS能量代谢异常,可能是靶向能量代谢异常细胞的关键分子(Tab 2)。抑制FLS代谢途径中异常关键分子的活化,具有潜在的治疗关节炎的作用,对RA药物的开发具有重要作用。
| Metabolic pathway | Key molecule | Inhibitor |
| Glycometabolism | GLUT1 | CG-5, WZB-117, STF31 |
| HK2 | BrPa, 2-DG, PA | |
| PFKFB3 | 3PO, PFK15, PFK158 | |
| PKM2 | TLN-232, CAP-232, VK 3, VK 5 | |
| Lipid metabolism | CTL1 | HC3 |
| PLTP | H00005360-M01 | |
| Chokα | MN58b, TCD-717, EB-3D, V-11-0711 | |
| PLD | CAY10593, CAY10594, FIPI, VU0155069, 5W0, EVJ | |
| Amino acid metabolism | LAT1 | BCH, JPH203, GPNA, SKN103, Triiodoadenosine |
| IDO1 | 1-MT, D/L-1MT, epacadostat, navoximod, BMS-986205 | |
| IDO2 | 1-MT, D -1MT | |
| TDO2 | 680C91, LM10, Aminoisoxazoles | |
| GLS1 | BPTES, CB839, compound 698 |
研究表明[6],RA-FLS中GLUT1表达增加,并且与HK2、MMP-3表达水平相关。将RA患者CD4+T细胞与RA-FLS体外共培养后,CD4+T细胞分泌促炎因子的能力增强,RA-FLS中GLUT1、GLUT3、MMP-3、MMP-9表达增加,导致RA-FLS侵袭性及糖酵解活性增强,糖酵解抑制剂2-DG可逆转RA-FLS上述反应。动物实验发现,K/BxN关节炎小鼠FLS中GLUT1表达增加并促进FLS迁移及分泌MMP-3的能力,糖酵解抑制剂BrPa降低GLUT1的表达,缓解小鼠关节炎症[7]。
2.1.2 糖酵解途径代谢酶糖酵解过程中有几种关键的限速酶参与了FLS侵袭性表型,第一种酶是HK2。与骨关节炎(osteoarthritis,OA)患者FLS相比,HK2在RA-FLS中表达增加,且脂多糖等炎症介质能促进RA-FLS表达HK2,导致其迁移和增殖能力明显增强,HK2基因沉默后,RA-FLS迁移和增殖能力降低。动物实验表明,向K/BxN关节炎小鼠关节腔内注射HK2可增强FLS增殖和迁移能力,HK2敲除小鼠关节炎症状能得到明显改善[8]。第二种酶是双功能6-磷酸果糖-2-激酶/果糖-2, 6-双磷酸酶3(6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase3,PFKFB3)。RA患者滑膜组织中PFKFB3表达高于OA,PFKFB3小分子抑制剂PFK15可降低葡萄糖摄取和利用,抑制糖酵解途径,显著降低RA-FLS迁移、侵袭及产生炎症介质的能力,减轻胶原诱导型关节炎小鼠关节炎的严重程度[9]。此外,RA患者CD4+T细胞PFKFB3缺乏活性,影响糖代谢途径,ATP产生不足,容易导致细胞凋亡。第三种酶是丙酮酸激酶2(pyruvate kinase2,PKM2)。与OA相比,RA患者滑膜中PKM2的表达明显增加,低氧环境下RA-FLS中PKM2表达升高,而通过PKM2抑制糖酵解途径,可以逆转RA-FLS侵袭、迁移及分泌功能[10]。
2.2 脂质代谢途径 2.2.1 脂质代谢途径转运体磷脂酰胆碱是磷脂的主要成分之一,由胆碱合成。Seki等[11]发现胆碱类转运体1(choline transporter-like 1,CTL1)(高亲和力)和CTL2(低亲和力)均在RA-FLS中高表达,与OA-FLS相比,RA-FLS胆碱摄取显著增加。氟西汀等阳离子药物可抑制胆碱摄取,显著降低RA-FLS活力、增加细胞凋亡蛋白-3/7活性,可促进RA-FLS凋亡。此外,胆碱代谢与巨噬细胞介导的炎症反应有关,M1巨噬细胞中CTL1表达增强,增加了胆碱摄取,进而促进磷脂酰胆碱生物合成,增加细胞因子分泌,促进巨噬细胞介导的炎症免疫反应[12]。
血浆磷脂转运蛋白(plasma phospholipid transfer protein,PLTP)是真核生物中普遍存在的转运蛋白,PLTP可以通过其活性形式转运脂质,也可以与ATP结合盒转运蛋白A1(ATP-binding cassette transporter A1,ABCA1)等受体结合产生作用。研究表明,PLTP和ABCA1在RA-FLS中表达程度高,且RA-FLS中PLTP活性明显高于OA,PLTP通过增加脂质转运及与ABCA1结合激活STAT3途径,促进了RA-FLS增殖及分泌白介素8(interleukin-8,IL-8)、IL-6、MMP-3和血管内皮生长因子的能力,参与RA发病机制[13]。
2.2.2 脂质代谢途径代谢酶磷脂酶A2(phospholipase A2,PLA2)可将花生四烯酸(arachidonic acid,AA)从细胞膜磷脂中游离出来,AA可通过环氧合酶(cyclooxygenase,COX)代谢成前列腺素,通过脂氧合酶(lipoxygenase,LOX)代谢成白三烯,这两种代谢物均在体内充当炎性介质,介导RA患者关节炎症的发生发展。PLA2、COX、LOX在RA患者滑膜中表达增高,提示AA代谢途径的激活[14]。研究表明,LOX抑制剂能减少肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)诱导RA-FLS释放炎症因子,缓解关节炎小鼠的足爪肿胀[15];抑制PLA2可显著降低TNF-α诱导FLS释放AA、PGE2、IL-8和MMP-3[16];抑制COX的非甾体抗炎药能有效地减轻RA患者临床症状和体征,消除关节局部炎症反应。
胆碱激酶α(choline kinase α,Chokα)是一种重要的磷脂酰胆碱合成酶,是细胞增殖的必需酶。研究表明,TNF-α和血小板源性生长因子可诱导RA-FLS中Chokα表达增加,使磷酸化胆碱水平升高。Chokα抑制剂MN58b缓解K/BxN关节炎小鼠炎症反应程度,体外可抑制RA-FLS迁移并促进其凋亡[17]。磷脂酶D(phospholipase D,PLD)为磷脂酰胆碱的特异性切割酶,PLD1基因干扰和PLD1、PLD2小分子抑制剂均能显著减少RA-FLS分泌IL-6、IL-8和趋化因子[18]。
2.3 氨基酸代谢途径 2.3.1 氨基酸代谢途径转运体L型氨基酸转运蛋白1(L-type amino acid transporter 1,LAT1)是普遍存在的L型氨基酸转运蛋白家族成员之一,亮氨酸、异亮氨酸、色氨酸等均通过细胞LAT1转运。研究发现LAT1在RA患者滑膜中表达增加,LAT1抑制剂BCH可降低mTOR及其下游靶基因4EBP1磷酸化、亮氨酸摄取和RA-FLS迁移[19]。Ozaki等[20]发现LAT1基因敲除的小鼠破骨细胞生成减少,体外研究发现LAT1通过mTOR复合体1通路调节破骨细胞形成,维持骨内环境稳定。
谷氨酰胺主要通过Na+依赖性丙氨酸-丝氨酸-半胱氨酸转运载体2(alanine-serine-cysteine transporter 2,ASCT2)进行转运,该转运体参与T细胞与巨噬细胞活化,抑制ASCT2可以减少RA等自身免疫病中T细胞增殖和过度活化[21]。
2.3.2 氨基酸代谢途径代谢酶色氨酸是机体必需氨基酸之一,RA等自身免疫病患者的血清和尿液中色氨酸水平降低、其分解代谢物水平升高[22]。色氨酸主要通过犬尿氨酸途径进行分解代谢,吲哚胺-2, 3-双加氧酶1(indoleamine-2, 3-dioxygenase 1,IDO1)、吲哚胺-2, 3-双加氧酶2(indoleamine-2, 3-dioxygenase2,IDO2)、色氨酸-2, 3-双加氧酶(tryptophan-2, 3-dioxygenase 2,TDO2)是犬尿氨酸代谢途径关键的限速酶。IDO1抑制剂1-MT,可降低早期K/BxN关节炎小鼠B细胞产生细胞因子和抗体的水平,从而减轻关节炎小鼠足爪肿胀程度。1-MT和MTX联合给药,能更有效地缓解K/BxN小鼠关节炎症[23]。IDO2是IDO1的同工酶,两者具有43%的基因同源性,但IDO2酶解效率明显低于IDO1。近年来研究发现,IDO2可能通过调控B细胞和T细胞介导的免疫反应,也参与了自身免疫性关节炎的发生发展[22]。TDO2参与多种疾病发病,其在多种肿瘤组织中表达程度较高,且参与肿瘤的免疫反应。研究发现[24],神经胶质瘤患者中TDO2表达程度较高,而在TDO2表达高的部位中浸润的CD8+T细胞水平大大降低;胶质瘤小鼠的T细胞TDO2表达增强,导致分泌的INF-γ水平明显降低。此外,敲除TDO2基因的实验性自身免疫性脑脊髓炎(experimental autoimmune encephalomyelitis,EAE)小鼠,缓解脊髓神经元的损伤,提示抑制TDO2可能成为多发性硬化症等自身免疫病的新策略[25]。
Takahashi等[26]在2017年首次报道了谷氨酰胺在RA发病中的作用。谷氨酰胺酶1(glutaminase1,GLS1)是谷氨酰胺代谢途径关键分子。研究发现,GLS1在RA-FLS中高表达,GLS1抑制剂化合物968可抑制RA-FLS生长,化合物968给药后可减少自发性关节炎小鼠(SKG小鼠)关节炎评分及FLS数量,提示GLS 1在调节RA-FLS增殖中起重要作用。此外,BPTES能改善系统性红斑狼疮和EAE模型小鼠病理状况,减少Th17细胞中低氧诱导因子1α表达,从而影响糖酵解途径[27]。
3 小结目前还没有较好的治疗RA的药物,传统药物起效慢,副作用大,新型生物制剂也存在胃肠道反应、骨髓抑制等诸多不良反应[28]。对RA代谢内容的不断认识与深入研究,不仅有助于理解该病的病理机制,更有望为RA临床诊断提供新指标、为RA的治疗提供新靶点和新方向。葡萄糖、脂质、氨基酸代谢途径中转运体及代谢酶等分子异常活化,导致RA-FLS代谢异常,参与RA发生发展。通过调节异常活化分子的活性,可改善能量代谢异常细胞的功能,从而达到缓解关节炎症的效果。靶向FLS能量代谢异常关键分子,只针对功能异常细胞,使过度活化的细胞功能恢复至正常水平,在发挥治疗作用的同时尽可能的降低不良反应,即达到“炎症免疫反应软调节”[29]目的,对未来RA药物的开发具有重要意义。因此,全面、系统地阐述FLS代谢的变化,将为RA病理机制研究和药物新靶点的发现提供重要理论依据和实验依据。
| [1] |
Croft A P, Campos J, Jansen K, et al. Distinct fibroblast subsets drive inflammation and damage in arthritis[J]. Nature, 2019, 570(7760): 246-51. doi:10.1038/s41586-019-1263-7 |
| [2] |
Mizoguchi F, Slowikowski K, Wei K, et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis[J]. Nat Commun, 2018, 9(1): 789-90. |
| [3] |
Okano T, Saegusa J, Takahashi S, et al. Immunometabolism in rheumatoid arthritis[J]. Immunol Med, 2018, 41(3): 89-97. doi:10.1080/25785826.2018.1531186 |
| [4] |
Rhoads J P, Major A S, Rathmell J C. Fine tuning of immunometabolism for the treatment of rheumatic diseases[J]. Nat Rev Rheumatol, 2017, 13(5): 313-20. doi:10.1038/nrrheum.2017.54 |
| [5] |
Fearon U, Hanlon M M, Wade S M, et al. Altered metabolic pathways regulate synovial inflammation in rheumatoid arthritis[J]. Clin Exp Immunol, 2019, 197(2): 170-80. |
| [6] |
Petrasca A, Phelan J J, Ansboro S, et al. Targeting bioenergetics prevents CD4 T cell-mediated activation of synovial fibroblasts in rheumatoid arthritis[J]. Rheumatology (Oxford), 2020, 59(2): 1-13. |
| [7] |
Garcia-Carbonell R, Divakaruni A S, Lodi A, et al. Critical role of glucose metabolism in rheumatoid arthritis fibroblast-like synoviocytes[J]. Arthritis Rheumatol, 2016, 68(7): 1614-26. doi:10.1002/art.39608 |
| [8] |
Bustamante M F, Oliveira P G, Garcia-Carbonell R, et al. Hexokinase 2 as a novel selective metabolic target for rheumatoid arthritis[J]. Ann Rheum Dis, 2018, 77(11): 1636-43. doi:10.1136/annrheumdis-2018-213103 |
| [9] |
Zou Y, Zeng S, Huang M, et al. Inhibition of 6-phosphofructo-2-kinase suppresses fibroblast-like synoviocytes-mediated synovial inflammation and joint destruction in rheumatoid arthritis[J]. Br J Pharmacol, 2017, 174(9): 893-908. |
| [10] |
Filer A. The fibroblast as a therapeutic target in rheumatoid arthritis[J]. Curr Opin Pharmacol, 2013, 13(3): 413-9. doi:10.1016/j.coph.2013.02.006 |
| [11] |
Seki M, Kawai Y, Ishii C, et al. Functional analysis of choline transporters in rheumatoid arthritis synovial fibroblasts[J]. Mod Rheumatol, 2017, 27(6): 995-1003. doi:10.1080/14397595.2017.1280118 |
| [12] |
Sanchez-Lopez E, Zhong Z, Stubelius A, et al. Choline uptake and metabolism modulate macrophage IL-1beta and IL-18 production[J]. Cell Metab, 2019, 29(6): 1350-62. doi:10.1016/j.cmet.2019.03.011 |
| [13] |
Audo R, Deckert V, Daien C I, et al. PhosphoLipid transfer protein (PLTP) exerts a direct pro-inflammatory effect on rheumatoid arthritis (RA) fibroblasts-like-synoviocytes (FLS) independently of its lipid transfer activity[J]. PLoS One, 2018, 13(3): e0193815-39. doi:10.1371/journal.pone.0193815 |
| [14] |
Brouwers H, Von Hegedus J, Toes R, et al. Lipid mediators of inflammation in rheumatoid arthritis and osteoarthritis[J]. Best Pract Res Clin Rheumatol, 2015, 29(6): 741-55. doi:10.1016/j.berh.2016.02.003 |
| [15] |
Lin H C, Lin T H, Wu M Y, et al. 5-Lipoxygenase inhibitors attenuate TNF-alpha-induced inflammation in human synovial fibroblasts[J]. PLoS One, 2014, 9(9): e107890-901. doi:10.1371/journal.pone.0107890 |
| [16] |
Sommerfelt R M, Feuerherm A J, Jones K, et al. Cytosolic phospholipase A2 regulates TNF-induced production of joint destructive effectors in synoviocytes[J]. PLoS One, 2013, 8(12): e83555-63. doi:10.1371/journal.pone.0083555 |
| [17] |
Guma M, Sanchez-Lopez E, Lodi A, et al. Choline kinase inhibition in rheumatoid arthritis[J]. Ann Rheum Dis, 2015, 74(7): 1399-407. doi:10.1136/annrheumdis-2014-205696 |
| [18] |
Friday S C, Fox D A. Phospholipase D enzymes facilitate IL-17- and TNFalpha-induced expression of proinflammatory genes in rheumatoid arthritis synovial fibroblasts (RASF)[J]. Immunol Lett, 2016, 174(4): 9-18. |
| [19] |
Yu Z, Lin W, Rui Z, et al. Fibroblast-like synoviocyte migration is enhanced by IL-17-mediated overexpression of L-type amino acid transporter 1 (LAT1) via the mTOR/4E-BP1 pathway[J]. Amino Acids, 2018, 50(2): 331-40. |
| [20] |
Ozaki K, Yamada T, Horie T, et al. The L-type amino acid transporter LAT1 inhibits osteoclastogenesis and maintains bone homeostasis through the mTORC1 pathway[J]. Sci Signal, 2019, 12(589): 3921-35. |
| [21] |
Song W, Li D, Tao L, et al. Solute carrier transporters: the metabolic gatekeepers of immune cells[J]. Acta Pharm Sin B, 2020, 10(1): 61-78. |
| [22] |
Merlo L M F, Pigott E, Duhadaway J B, et al. IDO2 Is a Critical mediator of autoantibody production and inflammatory pathogenesis in a mouse model of autoimmune arthritis[J]. J Immunol, 2014, 192(5): 2082-90. |
| [23] |
Pigott E, Duhadaway J B, Muller A J, et al. 1-Methyl-tryptophan synergizes with methotrexate to alleviate arthritis in a mouse model of arthritis[J]. Autoimmunity, 2014, 47(6): 409-18. |
| [24] |
Opitz C A, Litzenburger U M, Sahm F, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor[J]. Nature, 2011, 478(7368): 197-203. |
| [25] |
Lanz T V, Williams S K, Stojic A, et al. Tryptophan-2, 3-Dioxygenase (TDO) deficiency is associated with subclinical neuroprotection in a mouse model of multiple sclerosis[J]. Sci Rep, 2017, 7(1): 41271-84. |
| [26] |
Takahashi S, Saegusa J, Sendo S, et al. Glutaminase 1 plays a key role in the cell growth of fibroblast-like synoviocytes in rheumatoid arthritis[J]. Arthritis Res Ther, 2017, 19(1): 76-86. |
| [27] |
Kono M, Yoshida N, Maeda K, et al. Glutaminase 1 inhibition reduces glycolysis and ameliorates lupus-like disease in MRL/lpr mice and experimental autoimmune encephalomyelitis[J]. Arthritis Rheumatol, 2019, 71(11): 1869-78. |
| [28] |
张玲玲, 魏伟. 治疗自身免疫病药物研究进展[J]. 中国药理学通报, 2019, 35(2): 149-56. Zhang L L, Wei W. Research progress of drugs for treating autoimmune diseases[J]. ChinPharmacol Bull, 2019, 35(2): 149-56. |
| [29] |
魏伟. 炎症免疫反应软调节[J]. 中国药理学通报, 2016, 32(2): 297-303. Wei W. Soft regulation of inflammatory immune responses[J]. Chin Pharmacol Bull, 2016, 32(2): 297-303. |

