

2. 包头医学院神经科学研究所,内蒙古 包头 014040;
3. 山西大学中医药现代研究中心,山西 太原 030006;
4. 广东省人民医院麻醉科,广东省 广州市 510080;
5. 内蒙古监狱管理局第一医院麻醉科,内蒙古 呼和浩特市 010000;
6. 包头医学院麻醉学院,内蒙古 包头 014040;
7. 中国医学科学院、北京协和医学院药物研究所,北京市药物靶标研究与药物筛选重点实验室,北京 100050
石瑞丽(1971-),女,博士,教授,硕士生导师,研究方向:中蒙药药理,通讯作者;E-mail:ruilishi@sina.com
 ,
GAO Li3,
LYU Hong, HOU Qiu-li4,
CHEN Jin-shuang, KONG Ling-lei5,
SHI Rui-li6,
KONG Ling-lei7
,
GAO Li3,
LYU Hong, HOU Qiu-li4,
CHEN Jin-shuang, KONG Ling-lei5,
SHI Rui-li6,
KONG Ling-lei7
 ,
SHI Rui-li1,2
,
SHI Rui-li1,2

2. Institute of Neuroscience, Baotou Medical College, Baotou, Inner Mongolia 014040, China;
3. Modern Research Center of Traditional Chinese Medicine, Shanxi University, Taiyuan 030006, China;
4. Dept of Anesthesia, Guangdong General Hospital, Guang dong Academy of Medidal Science, Guangzhou 510080, China;
5. Dept of Anesthesiology, First Hospital of Inner Mongolia Prison Administration, Huhhot 010000, China;
6. School of Anesthesia, Baotou Medical College, Baotou, Inner Mongolia 014040, China;
7. Beijing Key Lab of Drug Targets Identification and Drug Screening, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100050, China
类风湿关节炎(rheumatoid arthritis,RA)是病因尚未明确的、以侵蚀关节为主的全身性免疫疾病。临床表现为对称性多关节炎及关节外病理性改变,逐渐出现骨组织的破坏与侵蚀,导致关节不同程度的畸形。患者需要长期服药,药物的副作用及经济负担严重影响患者的生活质量。
蒙药复方忠伦-5,别名合日呼-5汤、忠伦阿汤等,由苦参(Sophora Flavescens Ait,SFA)、栀子(Gardenia jasminoides Ellis,GJE)、川楝子(Fructus Toosendan,FT)、诃子(Terminalia chebula Retz,TCR)、肋柱花(Lomatogonium rotatum,LR)组成,出自蒙医著作《观者之喜》,具有清热凉血、舒筋止痛功效,是蒙医治疗热性黄水病、游痛证、痛风、陈旧热等疾病的常用方剂,传统蒙医临床应用本方治疗RA[1],能够明显改善RA患者的生活质量,疗效明确[2]。动物试验中,忠伦-5对小鼠急性、亚急性和慢性炎症,均有减轻炎性细胞因子对关节的刺激作用[3]。
作为“非物质文化遗产”的蒙医学,经过长时间的发展,形成了一个全面而独特的医学体系。然而,从分子水平来理解蒙药方剂仍然是一大挑战。随着生物信息学、系统生物学和多向药理学的飞速发展,“网络药理学”概念被提出,基于网络的药物研发成为一种新的高效可行的方法。通过网络桥接药物和疾病,多方向、多角度地阐述药物治疗疾病的机制。已有研究证实了该方法的可行性[4-5]。为全面阐释蒙药忠伦-5的作用机制,本课题拟应用网络药理学方法全面地解析忠伦-5治疗RA的机制,为传统蒙药的研发提供新的线索。
1 材料与方法 1.1 忠伦-5成分信息及作用靶点的获取登录TCMSP数据库[6](http://lsp.nwu.edu.cn/tcmsp.php,Version:2.3)和TCMID数据库[7](http://www.megabionet.org/tcmid/,Version:2.0)获得苦参、诃子、栀子、川楝子、肋柱花化合物信息,并以“经口服生物利用度(OB)≥30%,类药性(DL)≥0.18”为条件筛选化合物。将化合物的2D分子结构式输入到Swisstarget prediction数据库[8](http://www.swisstargetprediction.ch)中,预测化合物的作用靶点,保存靶点uniprot ID和名称等信息。
1.2 RA相关靶点的获得在DISGENET数据库[9](http://www.disgenet.org/,version 5.0)中,以“Rheumatoid arthritis”为关键词,检索与RA相关的靶点。删除重复靶点并剔除相关性得分小于0.3的靶点,保留剩余靶点,与上述的忠伦-5靶点相匹配,得到忠伦-5治疗RA的作用靶点数据集。
1.3 VENNY图的制作登录Draw Venn Diagram网站(http://bioinformatics.psb.ugent.be/webtools/Venn/)将各单味药及靶点输入在表格中,得到VENNY图,找到5种成分共同作用的靶点。
1.4 蛋白-蛋白相关作用网路构建分析将数据集的靶点信息导入STRING数据库[10](https://string-db.org/,Version 10.5),种属选择“Homo sapiens”,获得靶点间的蛋白-蛋白相互作用网络,下载相关数据并导入Cytoscape(Version:3.2.1)软件中,构建网络、拓扑分析,根据自由度设置节点颜色及大小,构建为蛋白-蛋白相互作用网络。
1.5 生物功能分析及参与通路获得将忠伦-5治疗类风湿关节炎作用的靶点uniprot ID导入Cytoscape软件的GO插件(Version:2.3.5)中,设置物种“Homo sapiens”选择“GO分析、参与生物过程分析及KEGG通路分析”设置P值<0.05,提交获得相关结果。
1.6 Hub节点选择及分子对接验证应用Cytoscape软件的cytoHubba插件,cytoHubba应用Maximal Clique Centrality(MCC)方法,综合11个拓扑分析方法(Degree,Edge Percolated Component,Maximum Neighborhood Component,Densityof Maximum Neighborhood Component,Maximal Clique Centrality)和6个中心性方法(Bottleneck,EcCentricity,Closeness,Radiality,Betweenness,and Stress),选择关键靶点,找到作用于关键靶点的活性成分,将成分及应对的关键靶点输入Systems Dock Web Site (http://systemsdock.unit.oist.jp,Version 2.0)进行分子对接,保存结果,根据对接得分分析活性成分与各靶点间的结合活性。
2 结果 2.1 靶点预测通过TCMSP和TCMID数据集得到忠伦-5的潜在靶点,并将这些靶点与DISGENET数据库中RA的靶点进行对比,交集为治疗RA的靶点,通过用uniprot ID获得靶点全称及简写,按“uniprot ID”及DisGeNET数据库给出的“Probability”数值排序,得到忠伦-5治疗RA的潜在靶点90个(Tab 1)。
| No. | Uniprot | Target | Gene symbol | Probability |
| 1 | O15438 | Canalicular multispecific organic anion transporter 2 | ABCC3 | 0.96 |
| 2 | O60218 | Aldo-keto reductase family 1 member B10 | AKR1B10 | 0.95 |
| 3 | O60882 | Matrix metalloproteinase-20 | MMP20 | 0.89 |
| 4 | O75469 | Nuclear receptor subfamily 1 group I member 2 | NR1I2 | 0.97 |
| 5 | O94788 | Retinal dehydrogenase 2 | ALDH1A2 | 0.91 |
| 6 | P00352 | Retinal dehydrogenase 1 | ALDH1A1 | 0.91 |
| 7 | P00533 | Epidermal growth factor receptor | EGFR | 0.95 |
| 8 | P00915 | Carbonic anhydrase 1 | CA1 | 0.95 |
| 9 | P00918 | Carbonic anhydrase 2 | CA2 | 0.95 |
| 10 | P01130 | Low-density lipoprotein receptor | LDLR | 0.88 |
| 11 | P03372 | Estrogen receptor | ESR1 | 0.94 |
| 12 | P03956 | 22 kDa interstitial collagenase | MMP1 | 0.95 |
| 13 | P04035 | 3-hydroxy-3-methylglutaryl-coenzyme A reductase | HMGCR | 0.92 |
| 14 | P04054 | Phospholipase A2 | PLA2G1B | 0.95 |
| 15 | P04626 | Receptor tyrosine-protein kinase erbB-2 | ERBB2 | 0.95 |
| 16 | P05093 | Steroid 17-alpha-hydroxylase/17 | CYP17A1 | 0.87 |
| 17 | P05113 | Interleukin-5 | IL5 | 0.68 |
| 18 | P05164 | Myeloperoxidase | MPO | 0.95 |
| 19 | P05177 | Cytochrome P450 1A2 | CYP1A2 | 1 |
| 20 | P05413 | Fatty acid-binding protein | FABP3 | 0.76 |
| 21 | P06493 | Cyclin-dependent kinase 1 | CDK1 | 0.95 |
| 22 | P07333 | Macrophage colony-stimulating factor 1 receptor | CSF1R | 0.48 |
| 23 | P07451 | Carbonic anhydrase 3 | CA3 | 0.95 |
| 24 | P07711 | Cathepsin L1 light chain | CTSL1 | 0.51 |
| 25 | P08183 | Multidrug resistance protein 1 | ABCB1 | 1 |
| 26 | P08253 | PEX | MMP2 | 0.95 |
| 27 | P08254 | Stromelysin-1 | MMP3 | 0.95 |
| 28 | P09238 | Stromelysin-2 | MMP10 | 0.95 |
| 29 | P09917 | Arachidonate 5-lipoxygenase | ALOX5 | 1 |
| 30 | P10275 | Androgen receptor | AR | 0.94 |
| 31 | P10415 | Apoptosis regulator Bcl-2 | BCL2 | 0.55 |
| 32 | P10635 | Cytochrome P450 2D6 | CYP2D6 | 0.65 |
| 33 | P11511 | Cytochrome P450 19A1 | CYP19A1 | 0.96 |
| 34 | P12931 | Proto-oncogene tyrosine-protein kinase Src | SRC | 0.44 |
| 35 | P13631 | Retinoic acid receptor gamma | RARG | 0.21 |
| 36 | P14061 | Estradiol 17-beta-dehydrogenase 1 | HSD17B1 | 1 |
| 37 | P14780 | 67 kDa matrix metalloproteinase-9 | MMP9 | 0.95 |
| 38 | P16050 | Arachidonate 15-lipoxygenase | ALOX15 | 1 |
| 39 | P17706 | Tyrosine-protein phosphatase non-receptor type 2 | PTPN2 | 0.96 |
| 40 | P17948 | Vascular endothelial growth factor receptor 1 | FLT1 | 0.38 |
| 41 | P18054 | Arachidonate 12-lipoxygenase | ALOX12 | 1 |
| 42 | P18089 | Alpha-2B adrenergic receptor | ADRA2B | 0.61 |
| 43 | P21554 | Cannabinoid receptor 1 | CNR1 | 0.57 |
| 44 | P21860 | Receptor tyrosine-protein kinase erbB-3 | ERBB3 | 0.84 |
| 45 | P22001 | Potassium voltage-gated channel subfamily A member 3 | KCNA3 | 0.96 |
| 46 | P22894 | Neutrophil collagenase | MMP8 | 0.33 |
| 47 | P23219 | Prostaglandin G/H synthase 1 | PTGS1 | 0.83 |
| 48 | P24666 | Low molecular weight phosphotyrosine protein phosphatase | ACP1 | 0.93 |
| 49 | P25774 | Cathepsin S | CTSS | 0.51 |
| 50 | P28223 | 5-hydroxytryptamine receptor 2A | HTR2A | 0.61 |
| 51 | P28845 | Corticosteroid 11-beta-dehydrogenase isozyme 1 | HSD11B1 | 0.93 |
| 52 | P28907 | ADP-ribosyl cyclase 1 | CD38 | 0.95 |
| 53 | P29274 | Adenosine receptor A2a | ADORA2A | 0.87 |
| 54 | P31749 | RAC-alpha serine/threonine-protein kinase | AKT1 | 0.83 |
| 55 | P32246 | C-C chemokine receptor type 1 | CCR1 | 0.38 |
| 56 | P33527 | Multidrug resistance-associated protein 1 | ABCC1 | 1 |
| 57 | P34972 | Cannabinoid receptor 2 | CNR2 | 0.57 |
| 58 | P35348 | Alpha-1A adrenergic receptor | ADRA1A | 0.61 |
| 59 | P35354 | Prostaglandin G/H synthase 2 | PTGS2 | 0.83 |
| 60 | P35869 | Aryl hydrocarbon receptor | AHR | 1 |
| 61 | P35916 | Vascular endothelial growth factor receptor 3 | FLT4 | 0.38 |
| 62 | P35968 | Vascular endothelial growth factor receptor 2 | KDR | 0.38 |
| 63 | P37231 | Peroxisome proliferator-activated receptor gamma | PPARG | 0.65 |
| 64 | P39900 | Macrophage metalloelastase | MMP12 | 0.95 |
| 65 | P40763 | Signal transducer and activator of transcription 3 | STAT3 | 0.43 |
| 66 | P42224 | Signal transducer and activator of transcription 1-alpha/beta | STAT1 | 0.43 |
| 67 | P43235 | Cathepsin K | CTSK | 0.51 |
| 68 | P45452 | Collagenase 3 | MMP13 | 0.95 |
| 69 | P45983 | Mitogen-activated protein kinase 8 | MAPK8 | 0.46 |
| 70 | P45984 | Mitogen-activated protein kinase 9 | MAPK9 | 0.46 |
| 71 | P47895 | Aldehyde dehydrogenase family 1 member A3 | ALDH1A3 | 0.91 |
| 72 | P51580 | Thiopurine S-methyltransferase | TPMT | 0.47 |
| 73 | P80365 | Corticosteroid 11-beta-dehydrogenase isozyme 2 | HSD11B2 | 0.88 |
| 74 | Q02880 | DNA topoisomerase 2-beta | TOP2B | 0.95 |
| 75 | Q03181 | Peroxisome proliferator-activated receptor delta | PPARD | 0.65 |
| 76 | Q04759 | Protein kinase C theta type | PRKCQ | 0.56 |
| 77 | Q07817 | Bcl-2-like protein 1 | BCL2L1 | 0.55 |
| 78 | Q07820 | Induced myeloid leukemia cell differentiation protein Mcl-1 | MCL1 | 1 |
| 79 | Q07869 | Peroxisome proliferator-activated receptor alpha | PPARA | 0.65 |
| 80 | Q14765 | Signal transducer and activator of transcription 4 | STAT4 | 0.43 |
| 81 | Q16539 | Mitogen-activated protein kinase 14 | MAPK14 | 0.46 |
| 82 | Q16665 | Hypoxia-inducible factor 1-alpha | HIF1A | 0.31 |
| 83 | Q8N1Q1 | Carbonic anhydrase 13 | CA13 | 0.66 |
| 84 | Q8NER1 | Transient receptor potential cation channel subfamily V member 1 | TRPV1 | 0.46 |
| 85 | Q92731 | Estrogen receptor beta | ESR2 | 0.94 |
| 86 | Q92847 | Growth hormone secretagogue receptor type 1 | GHSR | 0.1 |
| 87 | Q92887 | Canalicular multispecific organic anion transporter 1 | ABCC2 | 0.96 |
| 88 | Q99814 | Endothelial PAS domain-containing protein 1 | EPAS1 | 0.31 |
| 89 | Q9NPH5 | NADPH oxidase 4 | NOX4 | 1 |
| 90 | Q9UNQ0 | ATP-binding cassette sub-family G member 2 | ABCG2 | 0.94 |
为获得5味药的共同作用靶点,通过VENNY图对结果进行可视化,将各味药及相应的靶点通过Venn diagram网站(http://bioinformatics.psb.ugent.be/webtools/Venn/)制作VENNY图,发现雌激素受体β(estrogen receptor beta,ESR2)、细胞色素P450 19A1(cytochrome P450 19A1,CYP19A1)、雄激素受体(Androgen receptor,AR)、雌激素受体(Estrogen receptor,ESR1)靶点为5种单味药共同作用的靶点,且较多作为共同靶点出现(Fig 1)。
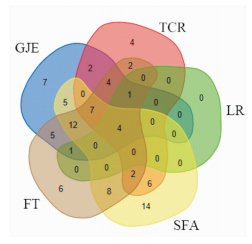
|
| Fig 1 Different colors represented different herbs. The number of overlapping parts represented the targets |
考虑到疾病靶点和其它蛋白的相互作用,为全面阐释靶点机制,将上述数据集的靶点及已有实验证明的靶点肿瘤坏死因子(tumor necrosis factor,TNF)及白介素6(interleukin-6,IL-6)通过STRING数据库获得蛋白-蛋白相互作用信息[3],下载数据导入Cytoscape软件进行可视化处理及拓扑分析,根据自由度设置节点颜色及大小,得到网络图(Fig 2)。图中蛋白节点92个,边754个。节点颜色由白到黄,颜色越深,节点自由度越高,节点大小由自由度决定,节点自由度越高节点越大。内圈为自由度排序为前30的节点。
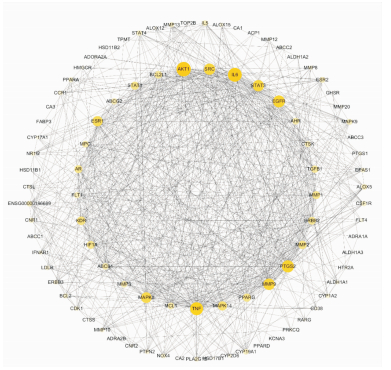
|
| Fig 2 Target protein interaction network of Zhonglun-5 The yellow node were the targets, and the inner circle was the top 30 targets of the degree of freedom. |
通过Cytoscape中的GO插件对数据集中的靶点进行参与生物过程分析及KEGG通路分析。生物过程分析结果表明,靶点主要富集于对活性氧的反应(response to reactive oxygen species)、破骨细胞分化(osteoclast differentiation)、糖皮质激素生物合成过程(glucocorticoid biosynthetic process)、凋亡信号通路的正调节(positive regulation of apoptotic signaling pathway)、等多种生物过程中(Fig 3),图中Y轴为生物过程名称,X轴为靶点在生物过程中的比例,各节点颜色代表P值,节点大小代表参与生物过程的靶点数;KEGG结果表明靶点参与的信号通路包括类固醇激素生物合成(steroid hormone biosynthesis)、花生四烯酸代谢(arachidonic acid metabolism)、氮代谢(nitrogen metabolism)、ABC转运器(ABC transporters)、PPAR信号通路(PPAR signaling pathway)等43条通路。将靶点参与通路信息导入到Cytoscape软件中得到“靶点-通路”图(Fig 4),图中以自由度设置节点大小,橙色通路,蓝色为靶点,节点109个,边341个,节点平均自由度为5.927。
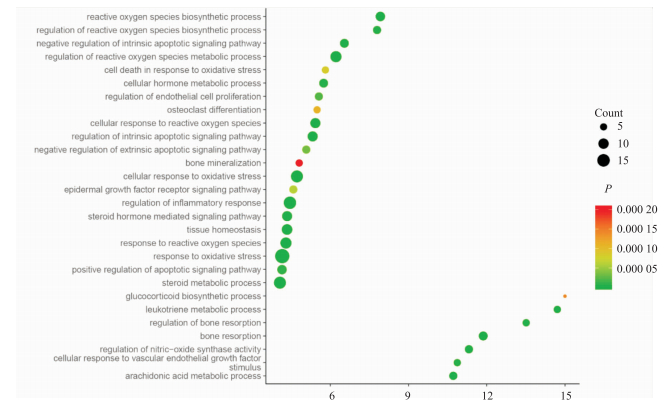
|
| Fig 3 Gene ontology terms for biological process of Zhonglun-5 The Y axis wasthe biological processes, the X axis was the proportion of the targets in the biological process. |
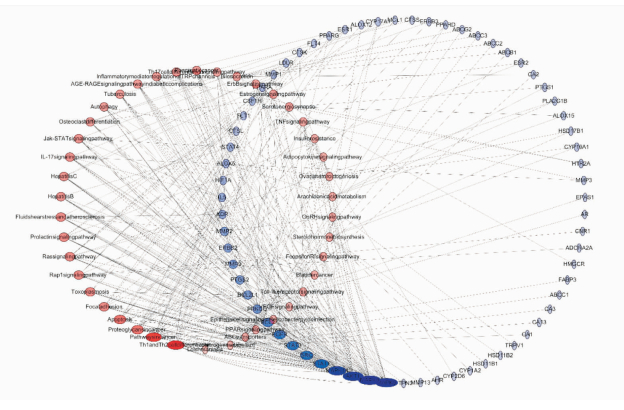
|
| Fig 4 Target-pathways The red nodes was the pathways, the blue nodes was the targets, and the nodes size represented the degree of freedom. |
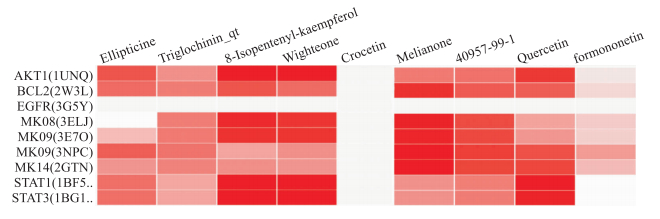
将上述建立的“靶点-通路”图导入Cytoscape软件中,运行cytoHubba插件,对这些节点进行排序,选取排名前8的关键靶点(得分大于10),分别为丝裂原活化蛋白激酶9(mitogen-activated protein kinase 9,MAPK9)、丝裂原活化蛋白激酶8(mitogen-activated protein kinase 8,MAPK8)、RAC-α丝氨酸/苏氨酸-蛋白激酶(RAC-alpha serine/threonine-protein kinase,Akt1)、丝裂原活化蛋白激酶14(mitogen-activated protein kinase 14,MAPK14)、信号转导和转录激活因子1(signal transducer and activator of transcription 1,STAT1)、信号转导和转录激活因子3(signal transducer and activator of transcription 3,STAT3)、表皮生长因子受体(epidermal growth factor receptor,EGFR)和凋亡调节因子Bcl-2。找到与靶点对应的活性成分包括番红花酸(crocetin)、玫瑰树碱(ellipticine)、水麦冬甙(triglochinin_qt)、8-异戊烯基-山奈酚(8-isopentenyl-kaempferol)、5, 7, 4'三羟基-6-异戊烯基异黄酮(wighteone)、刺芒柄花素(formononetin)、苦楝子酮(melianone)、槲皮素(quercetin)和皮树脂醇(40957-99-1),应用Systems Dock进行分子对接,结果如下(Fig 5),图中颜色越深得分越高(最高值为8.416)。
|
| Fig 5 Results of molecular docking The left side was the targets name, the upper side was the major ingredients, and the score was indicated by the color depth. |
首先通过数据库获得了90个蒙药忠伦-5抗RA的靶点,并通过STRING数据库,获得了这些靶点的相互作用关系,其中Akt1、IL-6、TNF、前列腺素G/H合成酶2(prostaglandin G/H synthase 2,PTGS2)、EGFR及基质金属蛋白酶9(matrix metalloproteinase-9,MMP9)等与多种蛋白存在联系,这些结果与前期研究结果一致,证明了本次研究的科学性和可靠性。蛋白-蛋白相互作用结果证明这些靶点并非单独起效,而是通过相关的作用关系、相互联系而产生效应。随后我们通过网络分析,发现忠伦-5主要参与氧化应激、内皮细胞增殖、糖皮质激素合成代谢、骨代谢、炎症及凋亡等生物过程作为主要途径作用于RA,反映出忠伦-5是多途径的作用特点。在KEGG通路分析中,发现靶点参与的ras信号通路、PPAR信号通路、erbb信号通路、自噬相关通路、JAK-STAT信号通路、肿瘤坏死因子信号通路、雌激素信号通路、IL-17信号通路均与RA密切相关。分析靶点-通路图,得到蒙药忠伦-5治疗RA的Hub节点:MAPK9、MAPK8、Akt1、MAPK14、STAT1、STAT3、EGFR和Bcl-2,找到的活性成分包括番红花酸、玫瑰树碱、水麦冬甙、8-异戊烯基-山奈酚、5, 7, 4'三羟基-6-异戊烯基异黄酮、刺芒柄花素、苦楝子酮、槲皮素和皮树脂醇,最后通过分子对接确定了活性成分与靶点的结合活性,发现活性成分中番红花酸不能与关键靶点结合,但番红花酸可以与其他RA相关靶点结合,且有较多研究证实其具有抗炎作用,因此番红花酸可作为活性成分存在。靶点中的EGFR与活性成分的结合能力较低,其它靶点均可与活性成分结合。
Hub节点中MAPK8、MAPK9、MAPK14为MAPK家族成员,MAPK作为重要的信号转导通路,参与细胞的多种生物过程,且已有以MAPK作为治疗靶点的成功案例。MAPK14是p38的α亚型,其参与的p38-MAPK通路与RA发病机制中的炎症密切相关,活化的p38通过激活下游蛋白,间接影响基因表达。有实验证实[11],在RA滑膜组织中,炎性因子等因素刺激使T细胞的p38异常活化,抑制肿瘤坏死因子受体超家族成员6(tumor necrosis factor receptor superfamily member 6,Fas)诱导的细胞凋亡,使组织中的炎症持续存在;p38还能导致炎性因子、趋化因子的基因过度表达,进一步促进RA的滑膜细胞炎症的发展。MAPK8和MAPK9是JNK的1/2亚型,JNK通路被认为与RA相关,当JNK和JNK上游的蛋白磷酸化被抑制时,基质金属蛋白酶1(matrix metalloproteinase-1,MMP1)、基质金属蛋白酶13(matrix metalloproteinase-13,MMP13)表达都被降低[12],而MMP-1和MMP-13是介导软骨破坏的关键蛋白。Akt1是蛋白激酶B,现有研究中,Akt主要通过PI3 Kinase-Akt经典信号途径参与RA的发病,PI3 K/Akt通路通过激活各种下游靶蛋白,产生不同的生物效应[13],且PI3 K/Akt/mTOR通路能够调节自噬,为RA的研究热点。STAT1和STAT3为信号转导子和转录激活子,在RA纤维样滑膜细胞增殖及炎症反应中均有影响[14],此外JAK/STAT信号通路通过诱导基质金属蛋白酶(matrix metalloproteinase,MMPs)基因的表达[15],参与软骨的破坏中。有关忠伦-5与MMPs表达的研究已有证实,但对上游的STAT的影响,还需进一步研究。Bcl-2是与凋亡密切相关的基因,具有抗凋亡和延长细胞生命的作用。上述很多靶点及通路都作用于Bcl-2,产生相应的生物学效应。本研究发现,Bcl-2作为诃子和川楝子共同的靶点,参与多种生物过程及信号通路,并且可能以抗凋亡的作用干预疾病的发展,具有一定的研究价值。
最终我们确定了忠伦-5治疗类风湿关节炎的主要靶点MAPK9、MAPK8、Akt1、MAPK14、STAT1、STAT3、EGFR和Bcl-2,活性成分有玫瑰树碱、水麦冬甙、8-异戊烯基-山奈酚、5, 7, 4'三羟基-6-异戊烯基异黄酮、刺芒柄花素、苦楝子酮、槲皮素和皮树脂醇,本研究发现蒙药忠伦-5主要通过MAPK通路、P13 K/Akt通路、JAK-STAT通路以及一些炎性因子发挥治疗RA的作用。有关忠伦-5在RA中的抗炎作用既往已有一些报道,反映了本研究结果的可靠性,其它靶点及通路可作为后续研究的理论基础。本研究较为全面地阐述了经典蒙药复方忠伦-5治疗RA的靶点及其信号转导通路,为临床应用该方提供了更多的理论依据,也为今后深入地研究忠伦-5的药理作用机制提供了新的线索。
| [1] |
王秀兰, 包冬梅, 图雅. 蒙药忠伦-5汤研究进展[J]. 时珍国医国药, 2002(3): 172-3. Wang X L, Bao D M, TU Y. The headway-making of the study of Mongolian drug Zhonglun-5 decoction[J]. Lishizhen Med Mater Med Res, 2002(3): 172-3. doi:10.3969/j.issn.1008-0805.2002.03.035 |
| [2] |
阿拉探巴干, 塔娜.甲氨蝶呤联合蒙药忠伦阿汤对类风湿关节炎患者的治疗效果分析[J].亚太传统医药, 2017, 13(19): 14-6. A L T, Ta N. Therapeutic effect analysis on the combination of methotrexate with Mongolian Medicine Zhong Lun Ah Tang on patients with rheumatoid arthritis[J]. Asia-Pacific Tradit, 2017, 13(19): 14-6. |
| [3] |
高自立, 董秋梅, 王滨, 等. 蒙药忠伦阿汤对胶原诱导性关节炎大鼠血清炎性细胞因子的影响[J]. 中医杂志, 2012, 53(7): 599-602. Gao Z L, Dong Q M, Wang B, et al. Effect of Zhonglun'e decoction on the expression of inflammatory cytokines in rats with collagen induced arthritis[J]. J Tradit Chin Med, 2012, 53(07): 599-602. |
| [4] |
吕燕妮, 付龙生, 周健, 等. 参麦注射液主要成分与卒中关联的网络药理作用机制和实验验证[J]. 中国药理学通报, 2017, 33(2): 293-4. Lyu Y N, Fu L S, Zhou J, et al. Network pharmacological mechanism of main components of Shenmai injection related with stroke and experimental verification[J]. Chin Parmacol Bull, 2017, 33(2): 293-4. doi:10.3969/j.issn.1001-1978.2017.02.029 |
| [5] |
李刚, 许波, 梁学振, 等. 基于网络药理学研究淫羊藿抗骨质疏松的分子机制[J]. 中国药理学通报, 2018, 34(2): 267-73. Li G, Xu B, Liang X Z, et al. Study on the molecular mechanism of osteoporosis treated by Epimedium based on network pharmacology[J]. Chin Parmacol Bull, 2018, 34(2): 267-73. doi:10.3969/j.issn.1001-1978.2018.02.023 |
| [6] |
Ru J L, Li P, Wang J N, et al. TCMSP:a database of systems pharmacology for drug discovery from herbal medicines[J]. J Cheminform, 2014, 6: 13. doi:10.1186/1758-2946-6-13 |
| [7] |
Huang L, Xie D L, Yu Y R, et al. TCMID 2.0:a comprehensive resource for TCM[J]. Nucleic Acids Res, 2018, 46(D1): D111-20. |
| [8] |
Gfeller D, Grosdidier A, Wirth M, et al. Swiss Target Prediction:a web server for target prediction of bioactive small molecules[J]. Nucleic Acids Res, 2014, 42(Web Server issue): W32-8. |
| [9] |
Pinero J, Bravo A, Queralt-Rosinach N, et al. DisGeNET:a comprehensive platform integrating information on human disease-associated genes and variants[J]. Nucleic Acids Res, 2017, 45(D1): D833-9. doi:10.1093/nar/gkw943 |
| [10] |
Szklarczyk D, Morris J H, Cook H, et al. The STRING database in 2017:quality-controlled protein-protein association networks, made broadly accessible[J]. Nucleic Acids Res, 2017, 45(D1): D362-8. doi:10.1093/nar/gkw937 |
| [11] |
Lin Y P, Su C C, Huang J Y, et al. Aberrant integrin activation induces p38 MAPK phosphorylation resulting in suppressed Fas-mediated apoptosis in T cells:implications for rheumatoid arthritis[J]. Mol Immunol, 2009, 46(16): 3328-35. doi:10.1016/j.molimm.2009.07.021 |
| [12] |
Nishitani K, Ito H, Hiramitsu T, et al. PGE2 inhibits MMP expression by suppressing MKK4-JNK MAP kinase-c-JUN pathway via EP4 in human articular chondrocytes[J]. J Cell Biochem, 2010, 109(2): 425-33. |
| [13] |
Crociani O, Zanieri F, Pillozzi S, et al. hERG1 channels modulate integrin signaling to trigger angiogenesis and tumor progression in colorectal cancer[J]. Sci Rep, 2013, 3: 3308. doi:10.1038/srep03308 |
| [14] |
Kasperkovitz P V, Verbeet N L, Smeets T J, et al. Activation of the STAT1 pathway in rheumatoid arthritis[J]. Ann Rheum Dis, 2004, 63(3): 233-9. doi:10.1136/ard.2003.013276 |
| [15] |
Li W Q, Dehnade F, Zafarullah M. Oncostatin M-induced matrix metalloproteinase and tissue inhibitor of metalloproteinase-3 genes expression in chondrocytes requires Janus kinase/STAT signaling pathway[J]. J Immunol, 2001, 166(5): 3491-8. doi:10.4049/jimmunol.166.5.3491 |