


2. 潍坊医学院 病理学教研室,山东 潍坊 261053;
3. 潍坊医学院 护理学院,山东 潍坊 261053;
4. 潍坊医学院 医学研究实验中心,山东 潍坊 261053
2. Dept of Pathology, Weifang Medical University, Weifang Shandong 261053, China;
3. College of Nursing, Weifang Medical University, Weifang Shandong 261053, China;
4. Medical Research Center, Weifang Medical University, Weifang Shandong 261053, China
尽管近年来临床预防筛查和治疗乳腺癌的技术已获得长足发展,乳腺癌患者预后也有明显改善,然而,耐药性仍是乳腺癌治疗过程中不可避免的问题[1],如阿霉素耐药、顺铂耐药等。阿霉素是临床常见的化疗药之一,且越来越多的研究表明,阿霉素耐药与微小RNA(microRNAs,miRNAs)的异常表达密切相关[2]。miRNAs作为一类调控序列,可结合mRNA序列的3′非翻译区(3′UTR),以此调控癌细胞的耐药增殖,如过表达miR-210抑制胰腺癌的增殖,且可通过靶基因ABCC5逆转吉西他滨的耐药[3]。miR-101通过抑制胶质母细胞瘤糖原合成酶激酶3β(glycogen synthase kinase 3β,GSK-3β)表达,逆转替莫唑胺的耐药[4]。但是关于miR-186-5p在乳腺癌细胞阿霉素耐药性中的作用机制研究尚未见报道。本文对阿霉素耐药株MCF-7/ADR及MCF-7细胞进行共转染,检测miR-186-5p、RAB2A对耐药增殖的影响,进一步经LY294002处理后,检测p-Akt308、p-Akt473及Akt的变化,以此阐述miR-186-5p、RAB2A在乳腺癌细胞阿霉素耐药性中的作用机制。
1 材料与方法 1.1 材料 1.1.1 细胞株人乳腺癌细胞株MCF-7购自ATCC。
1.1.2 药物与试剂阿霉素由潍坊医学院附属医院提供(海正辉瑞制药有限公司,批准文号:国药准字H33021980);Mir-XTM miRNA qRT-PCR SYBR® Kit、SYBR®Green Ⅰ染料,均购自TaKaRa公司;RIPA裂解液、CCK-8,购自索莱宝公司;PI3K抑制剂LY294002,购自北京碧云天公司;鼠抗人β-actin单克隆抗体、兔抗人RAB2A多克隆抗体、兔抗人p-Akt473多克隆抗体、兔抗人p-Akt308多克隆抗体,均购自Abcam公司;胎牛血清和MEM培养基购自美国Hyclone。
1.1.3 仪器ABI7500荧光定量PCR仪(美国ABI公司);电泳仪、转膜仪(美国Bio-Rad公司);CO2培养箱(Thermo公司),酶标仪(Bio-Tek公司)。
1.2 方法 1.2.1 细胞培养及转染按照文献[5, 6],MCF-7、MCF-7/ADR细胞均在含10% FBS的MEM培养基、5% CO2、37℃培养箱培养,其中MCF-7/ADR细胞另外添加1.0 mg·L-1的阿霉素以维持其耐药性,实验前48 h不再添加阿霉素。细胞转染采用Lipofectamine 2000,具体步骤参照操作说明书。参照文献[9],细胞转染及共转染分组:① MCF-7组:常规培养,不做处理;② anti-NC/MCF-7组:转入敲除miR-186-5p的对照质粒;③ anti-miR-186-5P/MCF-7组:转入敲除miR-186-5p质粒;④ MCF-7/ADR组:MCF-7/ADR细胞常规培养,不做处理;⑤ NC组:将过表达miR-186-5p的对照质粒转入MCF-7/ADR细胞;⑥ miR-186-5p组:将过表达miR-186-5p质粒转入MCF-7/ADR细胞;⑦ miR-186-5p+Con组:MCF-7/ADR中同时转入过表达miR-186-5p质粒和过表达RAB2A质粒的对照质粒Con;⑧ miR-186-5p+RAB2A组:MCF-7/ADR中同时转入过表达miR-186-5p质粒和过表达RAB2A质粒;⑨ Scr+anti-miR-186-5p组:MCF-7中转入敲除RAB2A的对照质粒和敲除miR-186-5p的质粒;⑩ SiRAB2A+anti-miR-186-5p组:MCF-7中转入敲除RAB2A质粒和敲除miR-186-5p质粒。
1.2.2 双荧光素酶实验将RAB2A的3′-UTR的野生型(pGL3-RAB2A-3′UTR)和突变型(pGL3-RAB2A- 3′UTR-mut)分别与对照质粒NC、过表达miR-186-5p质粒、对照质粒anti-NC、anti-miR-186-5p质粒进行共转染处理。具体实验步骤参照文献[7]。
1.2.3 qRT-PCR用TRIzol法提取各组乳腺癌细胞的总RNA。用茎环法和普通逆转录法分别对miR-186-5p和RAB2A进行逆转录,获得第一链cDNA后,分别用Mir-XTM miRNA qRT-PCR SYBR® Kit和SYBR®Green Ⅰ染料进行qRT-PCR,miR-186-5p以U6为内参,RAB2A以β-actin为内参。反应条件及miR-186-5p的茎环序列、引物序列均参照文献[8]。RAB2A上游引物:5′-ACATCATAATCGGCGACACAGGTG-3′,下游引物:5′ -CATTCGAGCACCGAACTCTACACC-3′。
1.2.4 Western blotMCF-7、MCF-7/ADR细胞及转染后的细胞,用RIPA裂解液裂解后进行蛋白抽提,接着进行分离胶浓度为12%的SDS-PAGE电泳,转膜,5%脱脂奶粉封闭,一抗4℃孵育过夜,d 2 TBST洗膜,孵育二抗,再次洗膜后,加ECL曝光。一抗稀释度:RAB2A(1 :1 000)、p-Akt308(1 :500)、p-Akt473(1 :500)、Akt(1 :1 000)、β-actin(1 :1 000)。
1.2.5 CCK-8细胞增殖实验取各组细胞2×103个,接种于96孔板中。贴壁培养24 h后,向每孔加入10 μL CCK-8溶液。将培养板在培养箱内孵育4 h,用酶标仪测定在450 nm的吸光度值。以此分别测定MCF、MCF-7/ADR细胞在转染以及共转染后24、48、72、96 h细胞增殖能力的变化。
1.2.6 统计学分析数据用x±s表示,组间比较用F检验,两两比较用LSD法。
2 结果 2.1 RAB2A与miR-186-5p靶向结合通过Targetscan和miRNAMap预测软件,得出RAB2A与miR-186-5p存在靶向结合位点,我们采用双荧光素酶实验,检测RAB2A与miR-186-5p在乳腺癌阿霉素耐药株MCF-7/ADR中是否存在结合及调控相互关系。Fig 1结果显示,miR-186-5p可特异性地结合于RAB2A的3′UTR。
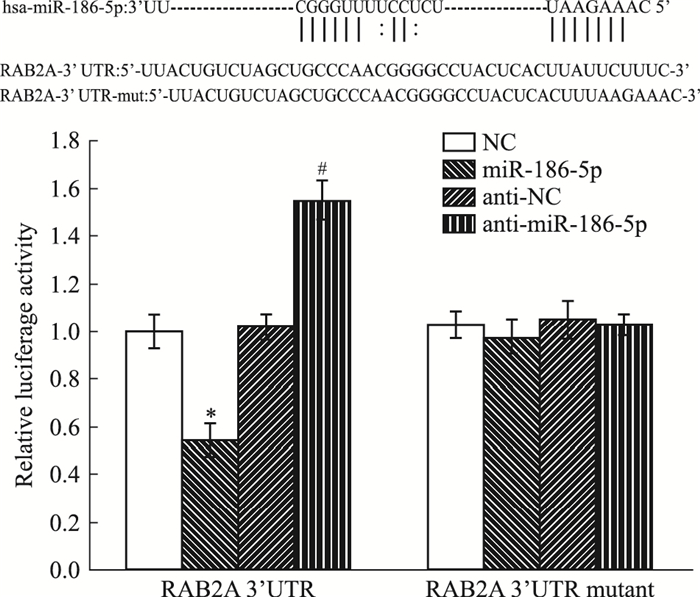
|
| Fig 1 RAB2A, a direct target of miR-186-5p(x±s, n=3) Targetscan and miRNAMap were used to predict the binding site between miR-186-5p and RAB2A. The dual luciferase assay was employed to detect the relationship between miR-186-5p and RAB2A.*P < 0.05 vs NC; #P < 0.05 vs anti-NC |
采用qRT-PCR分别检测miR-186-5p、RAB2A在乳腺癌细胞株MCF-7、MCF-7/ADR中mRNA的表达情况,同时,通过Western blot检测RAB2A的蛋白表达水平。Fig 2结果显示,与MCF-7细胞相比,MCF-7/ADR中miR-186-5p的表达水平明显降低(P < 0.05),RAB2A的mRNA表达没有明显变化(P>0.05),而RAB2A的蛋白表达水平明显升高(P < 0.05)。

|
| Fig 2 Expression levels of miR-186-5p and RAB2A(x±s, n=3) A: The mRNA levels of miR-186-5p and RAB2A were detected by qRT-PCR; B: The protein levels of RAB2A were detected by Western blot.*P < 0.05 vs MCF-7. U6 and β-actin were used respectively as a loading control |
为检测miR-186-5p对乳腺癌细胞耐药和增殖的影响,我们通过转染使MCF-7/ADR中的miR-186-5p高表达,敲低MCF-7中的miR-186-5p,CCK-8法检测细胞增殖能力的变化。Fig 3结果显示,转染72 h后,与MCF-7/ADR及NC组细胞相比,miR-186-5p组细胞的增殖能力明显降低(P < 0.05);与MCF-7及anti-NC组细胞相比,anti-miR-186-5p组细胞的增殖能力明显增加(P < 0.05)。
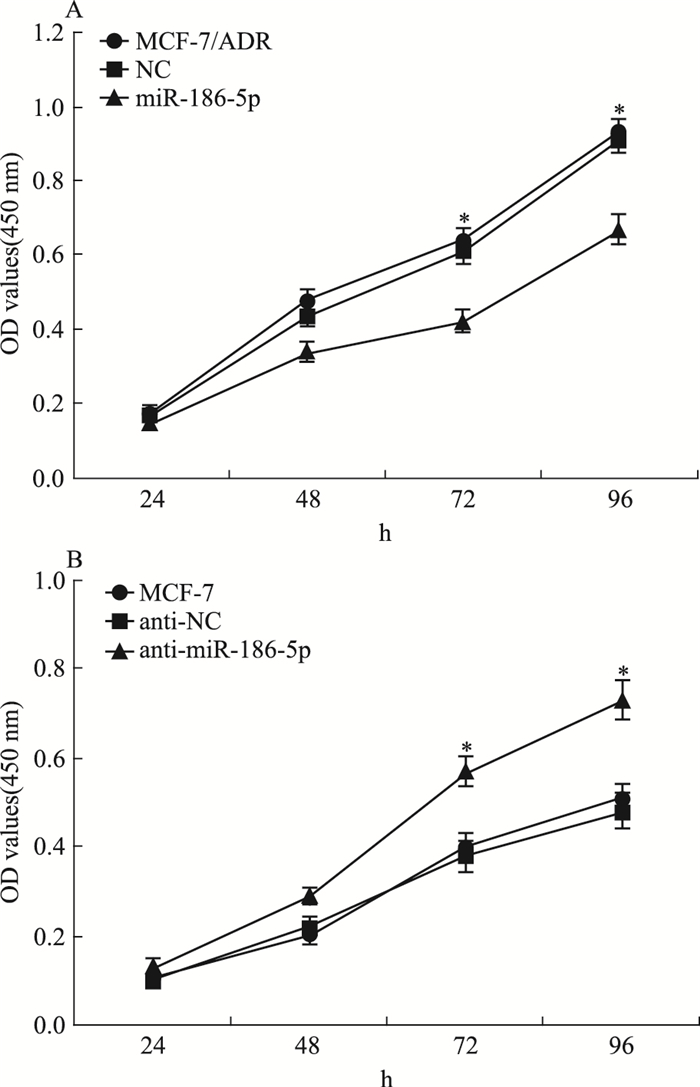
|
| 图 3 Influence of miR-186-5p on cell proliferation inMCF-7/ADR and MCF-7 cells(x±s, n=4) A:CCK-8 was employed to assess the cell proliferation in breast cancer cells after over-expression of miR-186-5p in MCF-7/ADR cells.*P < 0.05 vs NC. B: CCK-8 was employed to assess the cell proliferation in breast cancer cells after suppression of miR-186-5p in MCF-7cells.*P < 0.05 vs anti-NC |
Western blot检测miR-186-5p对乳腺癌细胞中RAB2A的影响。如Fig 4所示,过表达MCF-7/ADR中的miR-186-5p及敲低MCF-7中的miR-186-5p后,与NC组细胞相比,miR-186-5p组细胞中RAB2A的表达明显降低;与anti-NC组细胞相比,anti-miR-186-5p组细胞中RAB2A的表达明显增加(P < 0.05)。
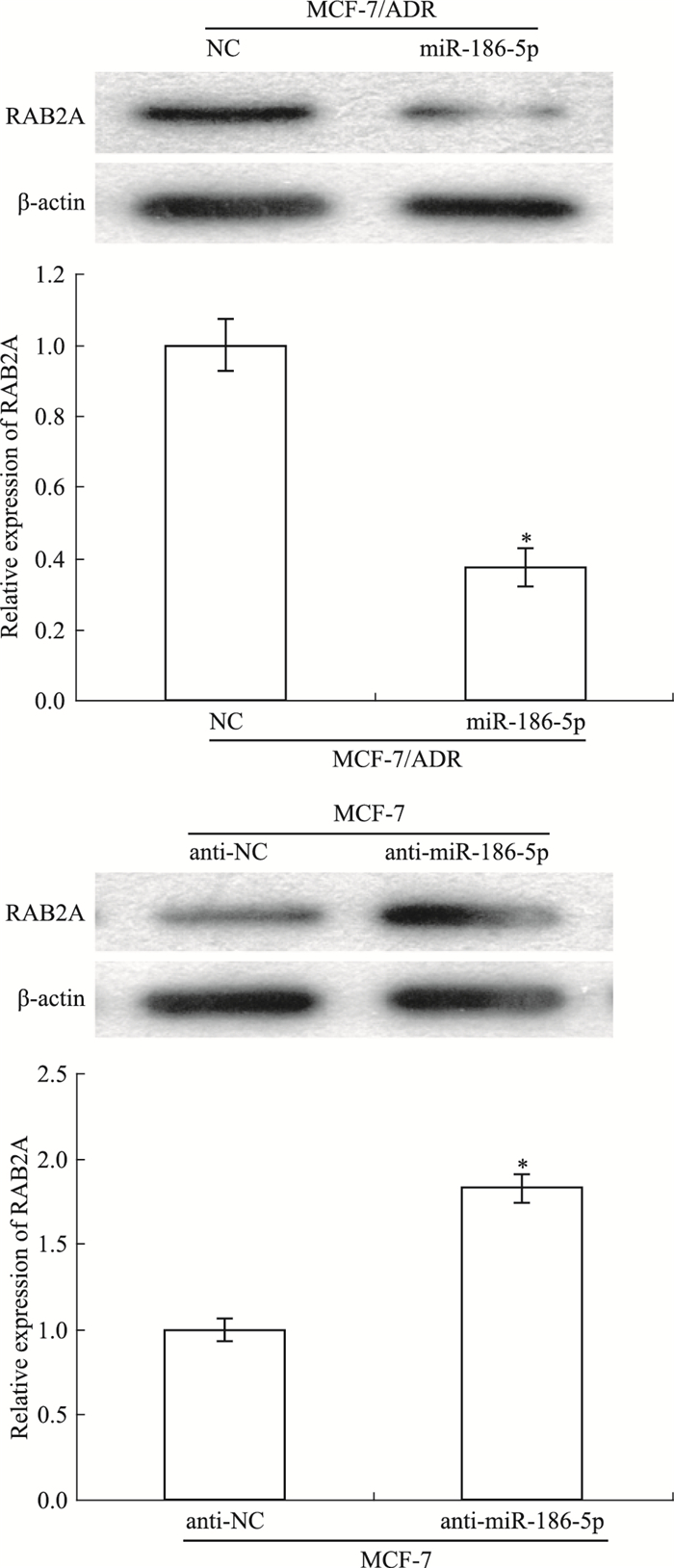
|
| 图 4 RAB2A modulated by miR-186-5p(x±s, n=3) Western blot was employed to assess the expression of RAB2A in breast cancer cells after over-expression of miR-186-5p in MCF-7/ADR cells and suppression of miR-186-5p in MCF-7cells.*P < 0.05 vs NC or anti-NC |
为进一步检测miR-186-5p是否通过作用于RAB2A,影响乳腺癌细胞的增殖,采用CCK-8检测共转染后RAB2A、miR-186-5p对乳腺癌细胞增殖能力的影响。如Fig 5所示,共转染72 h后,与miR-186-5p+Con组细胞相比,miR-186-5p+RAB2A组细胞的增殖能力明显增加(P < 0.05);与miR-186-5p+Scr组细胞相比,miR-186-5p+SiRAB2A组细胞的增殖能力明显降低(P < 0.05)。
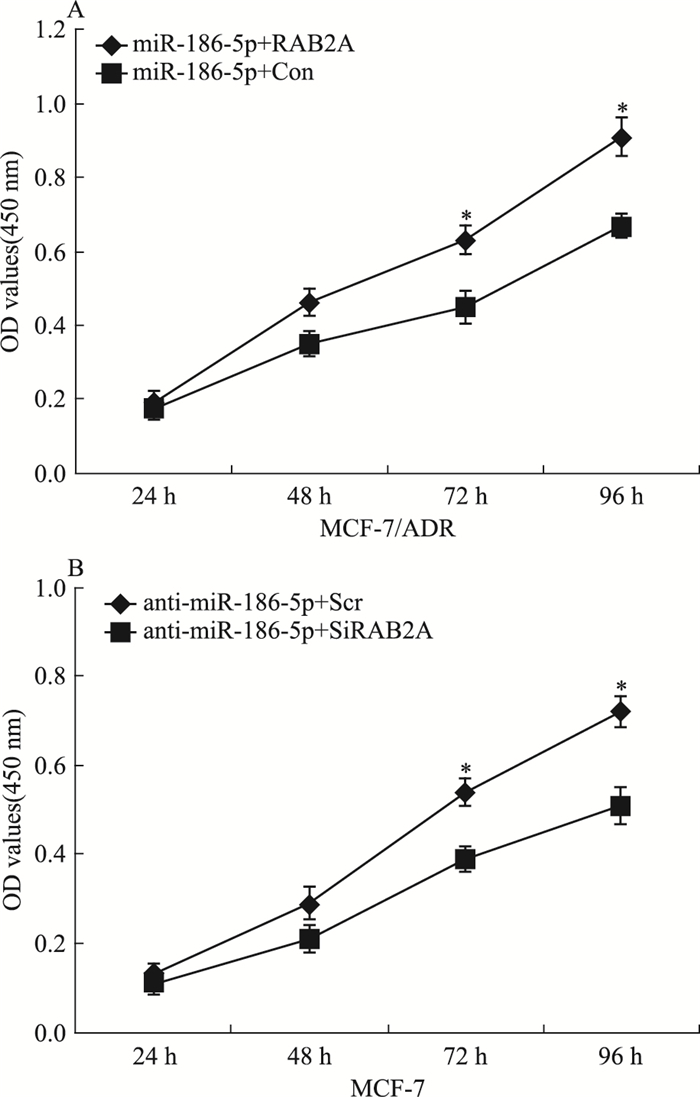
|
| Fig 5 Over-expressed RAB2A restored reduction ofcell proliferation by miR-186-5p(x±s, n=4) A:CCK-8 was employed to assess the cell proliferation in miR-186-5p+Con, miR-186-5p+RAB2A group cells.*P < 0.05 vs miR-186-5p+Con; B:CCK-8 was employed to assess the cell proliferation in anti-miR-186-5p+Scr, anti-miR-186-5p+SiRAB2A group cells.*P < 0.05 vs anti-miR-186-5p+Scr |
为检测miR-186-5p是否通过负向调控RAB2A,经PI3K/Akt信号通路逆转乳腺癌细胞的阿霉素耐药和增殖,终浓度为20 μmol·L-1的PI3K抑制剂LY294002添加到共转染后的各组细胞,Western blot检测p-Akt308、p-Akt473的表达水平。如Fig 6所示,在LY294002处理下,与miR-186-5p+Con组细胞相比,miR-186-5p+RAB2A组细胞的p-Akt308、p-Akt473的表达水平明显增加(P < 0.05),Akt的表达水平没有明显变化;与miR-186-5p+Scr组细胞相比,miR-186-5p+SiRAB2A组细胞的p-Akt308、p-Akt473表达水平明显降低(P < 0.05),Akt的表达没有明显变化。
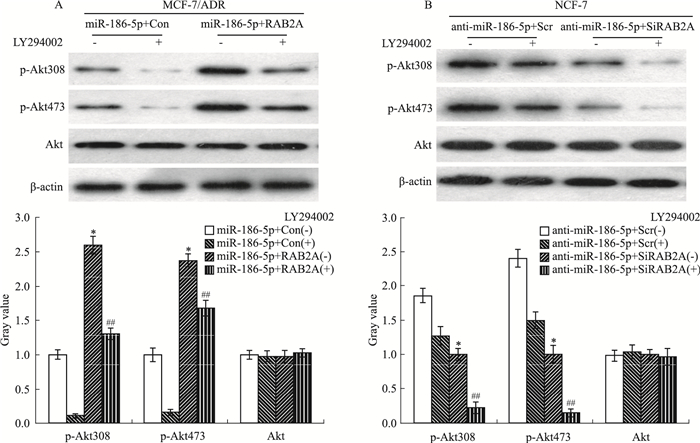
|
| Fig 6 Over-expressed RAB2A restored effects of p-Akt308, p-Akt 473 by miR-186-5p(x±s, n=3) A:The levels of p-Akt308, p-Akt 473 and Akt were detected in miR-186-5p+Con, miR-186-5p+RAB2A group cells treated with or without LY294002.*P < 0.05 vs miR-186-5p+Con(-); ##P < 0.01 vs miR-186-5p+Con(+); B:The levels of p-Akt308, p-Akt473 and Akt were detected in anti-miR-186-5p+Scr, anti-miR-186-5p+SiRAB2A group cells treated with or without LY294002.*P < 0.05 vs anti-miR-186-5p+Scr(-); ##P < 0.01 vs anti-miR-186-5p+Scr(+) |
乳腺癌是女性常见的恶性肿瘤,且化疗是治疗乳腺癌的常规手段。然而,多药耐药是导致化疗失败的主要障碍[9]。因此,研究乳腺癌的多药耐药机制将为临床治疗乳腺癌提供新的治疗靶点和途径,以期提高患者的预后。
RAB2A是小GTPase家族中的一员,参与囊泡转运、内质网的回收及运动。研究发现,RAB2A与Rab27a通过双效应物Noc2,协同调控成熟颗粒向胞外的运输。Dey等[10]发现,S100A7可激活RAB2A介导的MAPK信号通路,影响口腔鳞状细胞癌细胞的运动、侵袭。此外,Luo等[11]发现,RAB2A激活ERK信号通路,促进乳腺癌干细胞和肿瘤的发生。本实验通过双荧光素酶和Western blot实验,首次证实miR-186-5p在阿霉素耐药株MCF-7/ADR中存在RAB2A的结合位点,且RAB2A表达水平受miR-186-5p调控。
近年来,大量研究表明miRNAs的失调与多药耐药存在密切关系。先前文献表明,miR-186可靶向结合ABCB1和调节GST-π的表达,诱导卵巢癌细胞对紫杉醇、顺铂的耐药性[12]。同时,miR-186经对Twist1的调控,逆转卵巢癌细胞的顺铂耐药[13]。Ye等[14]发现,miR-186调控微管相关蛋白tau(microtubule-associated proteins tau, MAPT)的表达,进而调节非小细胞肺癌的化学耐药性。近来发现,TUG1经miR-186/CPEB2介导结直肠癌的甲氨蝶呤耐药[15]。本课题组先前研究发现,miR-186-5p靶向调控PTTG1,抑制非小细胞肺癌的侵袭、转移[8]。但是,关于miR-186-5p在乳腺癌阿霉素耐药和增殖中的作用尚未见报道。本实验发现,阿霉素耐药株MCF-7/ADR中miR-186-5p低表达,过表达miR-186-5p后明显抑制增殖。过表达RAB2A后明显逆转了miR-186-5p对增殖的影响,同时,经LY294002处理后,Western blot结果显示,RAB2A明显逆转了miR-186-5p对p-Akt308、p-Akt473的影响。此外,我们在MCF-7细胞中获得相同的结论。
综上所述,miR-186-5p负向调控RAB2A,经PI3K/Akt信号通路逆转乳腺癌细胞的阿霉素耐药和增殖,过表达RAB2A逆转了miR-186-5p对耐药和增殖的影响。为临床治疗阿霉素耐药的乳腺癌患者提供新的治疗靶点和理论支撑,以便提出更有效的治疗策略。
| [1] |
Shen H, Li L, Yang S, et al. MicroRNA-29a contributes to drug-resistance of breast cancer cells to adriamycin through PTEN/AKT/GSK3β signaling pathway[J]. Gene, 2016, 593(1): 84-90. |
| [2] |
Xu P, Wang L, Huang L, et al. Identification and characterization of microRNAs expressed in human breast cancer chemo-resistant MCF-7/Adr cells by Solexa deep-sequencing technology[J]. Biomed Pharmacother, 2015, 75: 173-8. doi:10.1016/j.biopha.2015.07.019 |
| [3] |
Amponsah P S, Fan P, Bauer N, et al. microRNA-210 overexpression inhibits tumor growth and potentially reverses gemcitabine resistance in pancreatic cancer[J]. Cancer Lett, 2017, 388: 107-17. doi:10.1016/j.canlet.2016.11.035 |
| [4] |
Tian T, Mingyi M, Qiu X, et al. MicroRNA-101 reverses temozolomide resistance by inhibition of GSK3β in glioblastoma[J]. Oncotarget, 2016, 7(48): 79584-95. |
| [5] |
李文静.人乳腺癌耐药细胞株的建立及其耐药机制研究[D].南京: 南京医科大学, 2012. Li W J. Establishment of human breast carcinoma drug-resistant cell lines and the study of drug-resistant mechanism[D]. Nanjing: Nanjing Medical University, 2012. |
| [6] |
孙志亮, 李洪利, 尹崇高. TGF-β1通过F-actin聚合促进乳腺癌细胞MCF-7发生上皮-间质转化[J]. 中国药理学通报, 2017, 33(5): 735-6. Sun Z L, Li H L, Yin C G. TGF-β1 promotes epithelial-mesenchymal transition in breast cancer cell line MCF-7 through F-actin polymerization[J]. Chin Pharmacol Bull, 2017, 33(5): 735-6. doi:10.3969/j.issn.1001-1978.2017.05.028 |
| [7] |
Yin C, Mou Q, Pan X, et al. MiR-577 suppresses epithelial-mesenchymal transition and metastasis of breast cancer by targeting Rab25[J]. Thorac Cancer, 2018, 9(4): 472-9. doi:10.1111/tca.2018.9.issue-4 |
| [8] |
Li H, Yin C, Zhang B, et al. PTTG1 promotes migration and invasion of human non-small cell lung cancer cells and is modulated by miR-186[J]. Carcinogenesis, 2013, 34(9): 2145-55. doi:10.1093/carcin/bgt158 |
| [9] |
An X, Sarmiento C, Tan T, et al. Regulation of multidrug resistance by microRNAs in anti-cancer therapy[J]. Acta Pharm Sin B, 2017, 7(1): 38-51. doi:10.1016/j.apsb.2016.09.002 |
| [10] |
Dey K K, Bharti R, Dey G, et al. S100A7 has an oncogenic role in oral squamous cell carcinoma by activating p38/MAPK and RAB2A signaling pathway[J]. Cancer Gene Ther, 2016, 23(11): 382-91. doi:10.1038/cgt.2016.43 |
| [11] |
Luo M L, Gong C, Chen C H, et al. The RAB2A GTPase promotes breast cancer stem cells and tumorigenesis via ERK signaling activation[J]. Cell Rep, 2015, 11(1): 111-24. doi:10.1016/j.celrep.2015.03.002 |
| [12] |
Sun K X, Jiao J W, Chen S, et al. MicroRNA-186 induces sensitivity of ovarian cancer cells to paclitaxel and cisplatin by targeting ABCB1[J]. J Ovarian Res, 2015, 8: 80. doi:10.1186/s13048-015-0207-6 |
| [13] |
Zhu X, Shen H, Yin X, et al. miR-186 regulation of Twist1 and ovarian cancer sensitivity to cisplatin[J]. Oncogene, 2016, 35(3): 323-32. doi:10.1038/onc.2015.84 |
| [14] |
Ye J, Zhang Z, Sun L, et al. miR-186 regulates chemo-sensitivity to paclitaxel via targeting MAPT in non-small cell lung cancer(NSCLC)[J]. Mol Biosyst, 2016, 12(11): 3417-24. doi:10.1039/C6MB00576D |
| [15] |
Li C, Gao Y, Li Y, et al. TUG1 mediates methotrexate resistance in colorectal cancer via miR-186/CPEB2 axis[J]. Biochem Biophys Res Commun, 2017, 491(2): 552-7. doi:10.1016/j.bbrc.2017.03.042 |

