


大肠杆菌不耐热肠毒素由产毒型大肠埃希菌(ETEC)产生,因其不耐热,在60℃处理30 min即被破坏,故称作不耐热肠毒素(LT)[1-2]。LT是一类六聚体大分子,由1个A亚基(LTA)和5个B亚基(LTB)组成,结构和功能与霍乱毒素相似[3]。LTB作为LT的非毒性亚基,能刺激机体产生强烈的黏膜和系统免疫[4]。LTB的免疫调节作用包括促进Th1和Th2型细胞反应的细胞因子的表达,CD8+T细胞的凋亡和CD4+T细胞的增殖;使B细胞活化的MHCⅡ、ICAM-1、CD25、CD40分子表达增加,影响巨噬细胞[5-6]和B细胞的抗原处理和呈递过程[7, 8],以及DC的发育和成熟,使DC细胞更好的发挥抗原递呈的作用[9],从而达到增强机体对抗原的免疫应答[10-12]。然而对于LTB引起这些免疫调节变化的机制尚不清楚,因而本实验选用小鼠单核细胞巨噬细胞白血病细胞株RAW 264.7[13]作为研究对象,研究LTB介导的免疫应答的分子机制。
1 材料与方法 1.1 材料LTB质粒由本实验室构建并保存;E.coli BL21(DE3)由本教研室保存;原核表达载体pET-32a购自Invitrogen公司;RAW 264.7细胞源于本实验室免疫学课题组陈全教授馈赠;胎牛血清、DMEM培养基购自Hyclon公司;Protein Interaction Kit购自Thermo Fisher公司;兔源GM130抗体、兔源Vimentin抗体、兔源Hspd1抗体和兔源β-actin抗体均购自武汉博士德生物技术有限公司,鼠源抗His标签抗体、羊抗兔DY488绿色荧光抗体和羊抗鼠DY549红色荧光抗体购自Santa Cruz公司;Toxin Eraser购自金斯瑞生物科技有限公司;蛋白纯化磁珠购自北京鼎国生物技术有限公司。
1.2 方法 1.2.1 LTB融合蛋白的纯化鉴定透析袋预处理:依次用10 mmol·L-1 Na2CO3溶液和10 mmol·L-1 EDTA-Na溶液各煮沸15 min,蒸馏水彻底洗净备用;诱导表达LTB融合蛋白,收集菌体,提取并纯化LTB蛋白,纯化后蛋白经透析复性并浓缩后,用Toxin Eraser Kit去除内毒素,用BCA法测定其浓度,SDS-PAGE检测纯化蛋白纯度及其分子质量大小。
1.2.2 细胞培养及Pull-down实验RAW 264.7细胞用含10%胎牛血清的DMEM培养, 待细胞密度达到60%~70%,用无血清DMEM处理8 h使细胞同步化,实验组加入100 mg·L-1 LTB蛋白,对照组加等体积生理盐水,处理12 h后收集细胞,提取细胞蛋白,测蛋白浓度。取等量的纯化的LTB融合蛋白和提取的两组细胞蛋白(保证LTB融合蛋白过量),用Protein Interaction Kit做pull-down实验,分离与LTB有相互作用的蛋白分子,SDS-PAGE检测分离得到蛋白的差异,并将分离得到的相互作用蛋白进行质谱鉴定,对鉴定结果进行分析处理。
1.2.3 免疫荧光验证生理情况下与LTB存在相互作用的蛋白RAW 264.7细胞于24孔板中爬片,待细胞密度达到50%~60%,分为4个组,每组3个孔,第1组只加生理盐水,第2组和第3组加10 μg LTB融合蛋白,第4组加已用神经节苷脂(GM)封闭30 min的LTB融合蛋白(LTB :GM=1 :5),分别收集处理时间为5、15、30 min的爬片,快速洗涤后,马上用4%多聚甲醛固定,PBS洗5次,每次5 min;2% triton 100处理10 min,洗3次;加山羊血清于37℃孵箱,封闭30 min;一抗37℃孵育2 h,洗涤5次;二抗避光37℃孵育90 min,荧光显微镜检测结果。
1.2.4 Western blot检测相互作用蛋白Hspd1和β-actin表达水平收集LTB融合蛋白处理12 h的RAW 264.7细胞,提取细胞蛋白,采用BCA法测定蛋白浓度。按每孔50 μg蛋白量上样,经12% PAGE胶电泳分离后,将目的蛋白电转于PVDF膜上,5% BSA封闭2 h,一抗4℃孵育过夜,TBST洗3次,每次15 min,加二抗室温摇床孵育2 h,ECL发光检测结果。
1.2.5 统计学分析应用SPSS 17.0统计软件,数据以x±s表示。两组数据间的比较采用独立样本t检验,多组数据间的比较采用单因素方差分析。
2 结果 2.1 LTB蛋白纯化SDS-PAGE结果显示,纯化得到的蛋白分子量约为30 ku,与LTB蛋白实际分子量大小一致(Fig 1),表明获得了高纯度的LTB融合蛋白,可以用于后续实验。
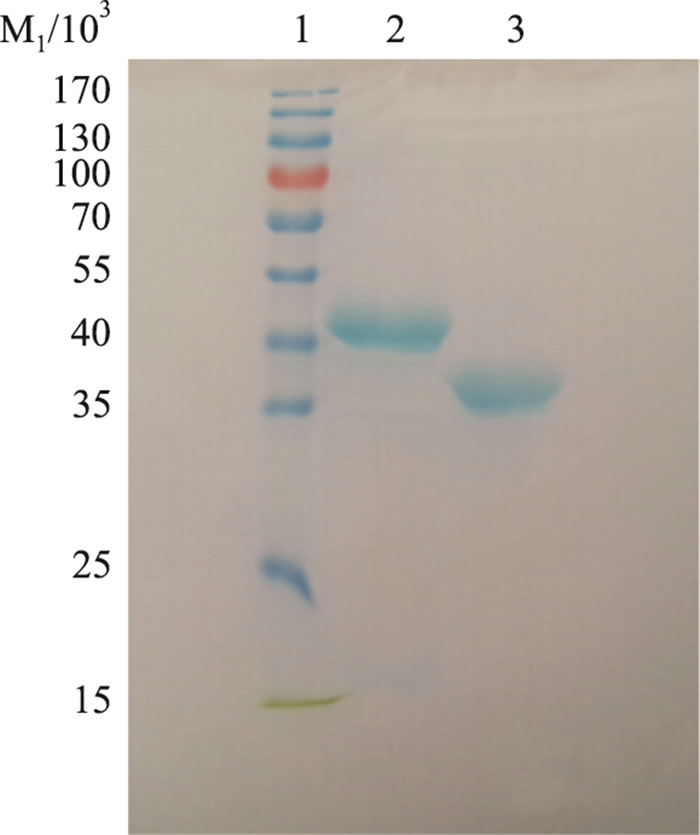
|
| Fig 1 SDS-PAGE analysis of purified VP8fusion protein and LTB fusion protein 1:Marker; 2:VP8 fusion protein; 3:LTB fusion protein |
SDS-PAGE结果显示,与对照组相比,LTB处理组有明显多于对照组的条带,差异有统计学意义(P < 0.05),表明通过pull down捕获到了与LTB有相互作用的蛋白分子(Fig 2)。
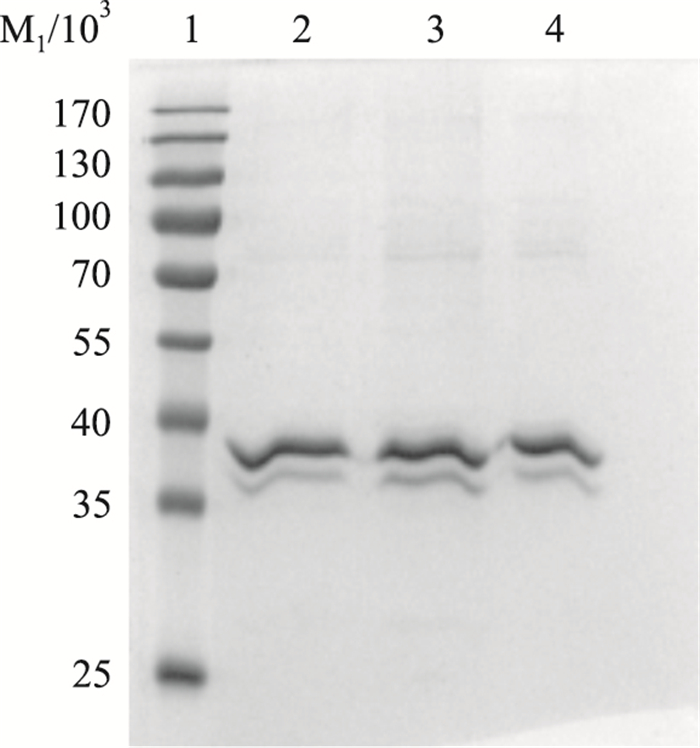
|
| Fig 2 SDS-PAGE analysis of proteins interaction with LTB 1:Marker; 2:Control group; 3:Experimenal group; 4:LTB fusion protein |
将得到的质谱数据根据筛选标准进行筛选,标准要求鉴定出的蛋白大于阈值可信肽段数大于3,Unused (置信度)值大于1.3,筛选得到的相互作用蛋白见Tab 2,共25个相互作用蛋白,其中有12个属于Keratin蛋白家族,可信肽段数小于10的蛋白有9个,可信肽段数在10~20之间的共9个,可信肽段数大于20的共7个,并对其进行功能性分析,它们的功能见Tab 3。
| Protein | Name | Unused | Peptides (95%) |
| Krt14 | Keratin-14 | 31.14 | 36 |
| Krt5 | Keratin 5 | 32.1 | 33 |
| Krt1 | Keratin-1 | 4.13 | 25 |
| Krt6a | Keratin-6A | 11.91 | 24 |
| Jup | Junction plakoglobin | 35.43 | 22 |
| Dsp | Desmoplakin | 44.45 | 21 |
| Krt77 | Keratin 77 | 8.5 | 21 |
| Krt2e | Keratin-2e | 6.21 | 20 |
| Krt42 | Keratin-42 | 4.4 | 17 |
| Krt17 | Keratin-17 | 9.61 | 15 |
| Krt78 | Keratin-78 | 4.25 | 14 |
| Krt79 | Keratin-79 | 2 | 14 |
| Pkm | Pyruvate kinase | 20.93 | 12 |
| Krt16 | Keratin-16 | 4.73 | 11 |
| Krt76 | Keratin-76 | 3.99 | 11 |
| Prss1 | Trypsinogen 16 | 2.15 | 11 |
| Ero1l | Ero1l protein | 14.62 | 8 |
| Vim | Vimentin | 13.83 | 8 |
| Hspd1 | Heat shock protein | 11.2 | 7 |
| Csp1 | Carbamoyl-phosphate synthetase I | 12.07 | 6 |
| Anxa2 | Annexin | 9.15 | 5 |
| Actb | A-X actin | 6.74 | 5 |
| Hnrnpl | Heterogeneous nuclear ribonucleoprotein L | 3.43 | 4 |
| Pkp1 | Plakophilin-1 | 6.41 | 3 |
| Ddx5 | DEAD box polypeptide 5 | 3.01 | 3 |
| Proteins | Function |
| Keratin 1 | Contributes to the formation of the cytoskeleton, cellular components, cytoskeletal intermediate fibers. |
| Keratin 2e | Contributes to the formation of the cytoskeleton, cellular components, cytoskeletal intermediate fibers. |
| Keratin 5 | Contributes to the formation of the cytoskeleton, cellular components, cytoskeletal intermediate fibers. |
| Keratin-6A | Contributes to the formation of the cytoskeleton, cellular components, cytoskeletal intermediate fibers. |
| Keratin 14 | Contributes to the formation of the cytoskeleton, cellular components, cytoskeletal intermediate fibers. |
| Keratin-16 | Contributes to the formation of the cytoskeleton, cellular components, cytoskeletal intermediate fibers |
| Keratin 17 | Contributes to the formation of the cytoskeleton, cellular components, cytoskeletal intermediate fibers. |
| Keratin 42 | Contributes to the formation of the cytoskeleton, cellular components, cytoskeletal intermediate fibers. |
| Keratin 76 | Contributes to the formation of the cytoskeleton, cellular components, cytoskeletal intermediate fibers. |
| Keratin 77 | Contributes to the formation of the cytoskeleton, cellular components, cytoskeletal intermediate fibers. |
| Proteins | Function |
| Keratin-78 | Contributes to the formation of the cytoskeleton, cellular components, cytoskeletal intermediate fibers |
| Keratin-79 | Contributes to the formation of the cytoskeleton, cellular components, cytoskeletal intermediate fibers. |
| Vimentin | class-Ⅲ intermediate filaments found in various non-epithelial cells, especially mesenchymal cells. Vimentin is attached to the nucleus, endoplasmic reticulum, and mitochondria, either laterally or terminally |
| Desmoplakin | Involves in the organization of the desmosomal cadherin-plakoglobin complexes into discrete plasma membrane domains and in the anchoring of intermediate filaments to the desmosomes. |
| A-X actin | Contributes to the formation of the cytoskeleton, cellular, cytoskeletal intermediate fibers. |
| Plakophilin-1 | Seems to play a role in junctional plaques. Contributes to epidermal morphogenesis. |
| Jup | Plays a central role in the structure and function of submembranous plaques. Acts as a substrate for VE-PTP and is required by it to stimulate VE-cadherin function in endothelial cells. Can replace beta-catenin in E-cadherin/catenin adhesion complexes which are proposed to couple cadherins to the actin cytoskeleton. |
| PKM | Pyruvate kinase, oxidative decomposition of glucose and generation ATP. |
| Prss1 | Serine like enzyme catalytic activity, protein hydrolysis. |
| Ero1l protein | Redox enzyme activity, electron transport chain, protein. folding, intracellular protein transport, endocytosis and efflux |
| Hspd1 | 60 ku heat shock protein. May facilitate the correct folding of imported proteins. |
| Carbamoyl-phosphate synthetase I | Glutamyl transferase activity, hydrolase activity, ligase activity, nitride Compound metabolism, synthesis of base compounds, biological synthesis of cellular amino acids. plays an important role in removing excess ammonia from the cell. |
| Annexin | Plays important roles in the innate immune response as effector of glucocorticoid-mediated responses and regulator of the inflammatory process. Has anti-inflammatory activity. Plays a role in glucocorticoid-mediated down-regulation of the early phase of the inflammatory response. |
| Heterogeneous nuclear ribonucleoprotein L | Likely to play a role in the nuclear metabolism of hnRNAs, particularly for pre-mRNAs that contain cytidine-rich sequences. Can also bind poly (C) single-stranded DNA. Plays an important role in p53/TP53 response to DNA damage, acting at the level of both transcription activation and repression. |
| Ddx5 | The translation initiation factor, translation regulation, RNA helicase. Involves in the alternative regulation of pre-mRNA splicing. |
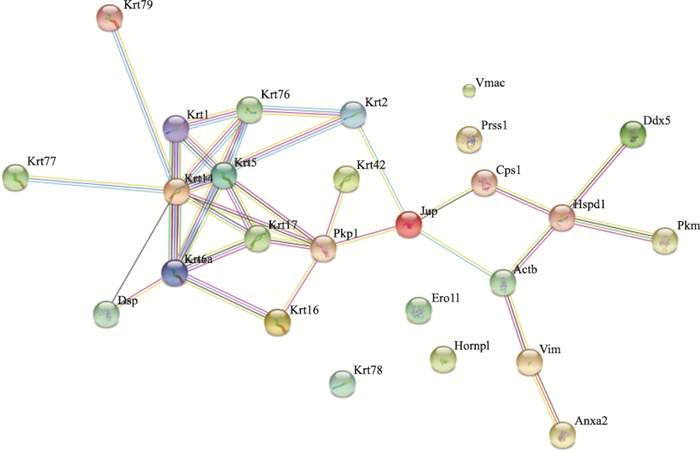
内容通过http://string-db.org/网站提供的分析工具对这些蛋白的相互作用进行了预测(Fig 3)。根据这些蛋白分子相互作用网络图,发现有4个蛋白分子与其他蛋白分子没有相互作用,因此推测这些蛋白分子与其他蛋白分子的相互作用可能还未被发现,或者是非特异性结合的蛋白分子。剩余的21种蛋白,以Jup为界限分为左右两半,左边的13种蛋白均为细胞骨架蛋白,11种属于Keratin家族,剩下的2个分别是Pkp1,并且发现这些分子间的相互作用都比较强。右边的蛋白以Jup为起始,以Ddx5、PKM、Anxa2 3个分子结束。
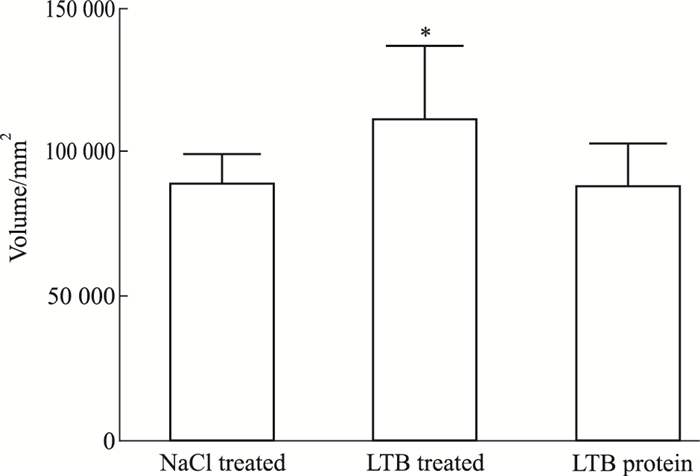
|
| Fig 3 Analysis for volumeof proteins captured by LTB fusion protein *P < 0.05 vs NaCl |
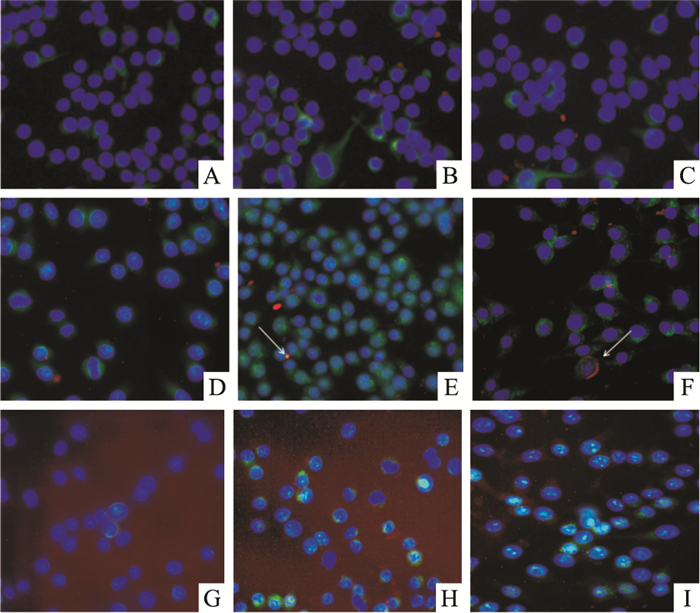
免疫荧光结果显示,对照组(NaCl组)未加LTB处理,所以5、15、30 min细胞内均未出现红色荧光信号,而GM130标记组和Vimentin标记组细胞内均有红色荧光信号;Vimentin标记组没有与LTB相互作用的阳性信号,表明LTB在细胞内与Vimentin蛋白不存在相互作用;而GM130标记组有与LTB相互作用的橙色阳性信号(箭头标记),而且随着时间增加阳性信号增强,表明LTB是能够进入巨噬细胞内,且随时间延长,进入细胞内的LTB的量也增加;GM130+GM组5、15、30 min细胞内均无红色荧光信号,表明神经节苷脂(GM)能够阻断LTB进入巨噬细胞内(Fig 5)。
|
| Fig 4 Map of protein-protein interaction |
|
| Fig 5 The immunofluorescence analysis of interactionsbetween LTB fusion protein and Vim or GM130 A:Vim 5 min; B:Vim 15 min; C:Vim 30 min; D:GM130 5 min; E:GM130 15 min; F:GM130 30 min; G:GM130+GM 5 min; H:GM130+GM 15 min; I:GM130+GM 30 min |
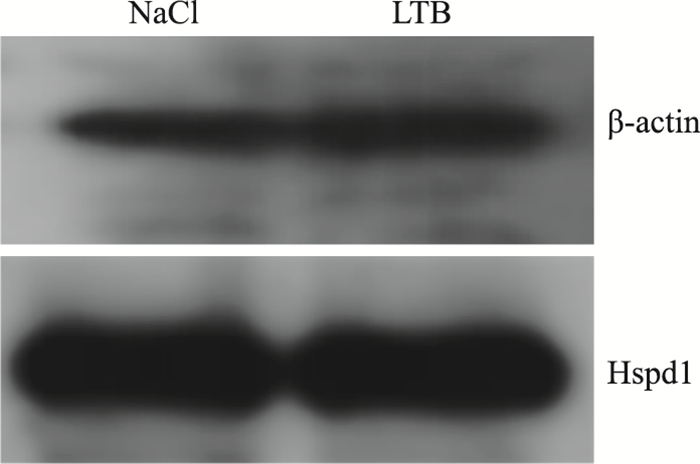
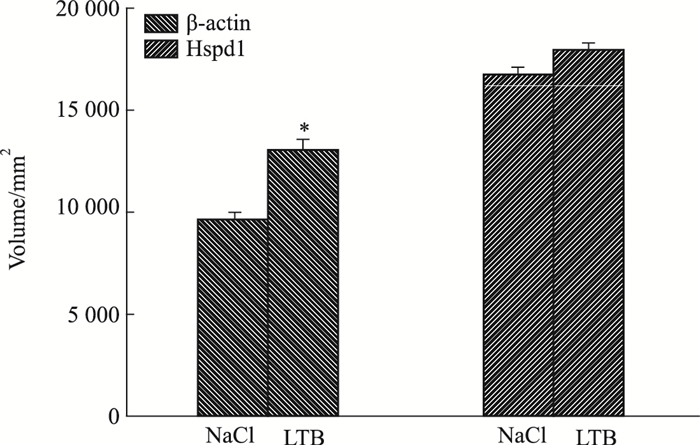
LTB处理RAW 264.7细胞12 h后,与对照组相比,β-actin的表达是上调的,且差异具有统计学意义(P < 0.05);Hspd1表达无变化,差异无统计学意义(Fig 6, Fig 7)。
|
| Fig 6 Expression of β-actin andHspd1 in RAW 264.7 induced by LTB |
|
| Fig 7 Analysis for volume of interacting proteins of LTB *P < 0.05 vs NaCl |
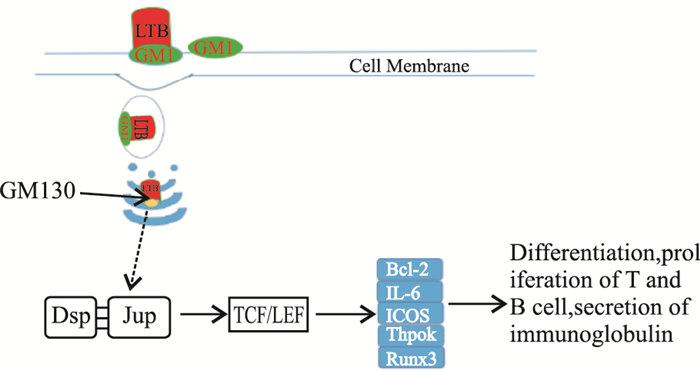
大肠杆菌不耐热肠毒素B亚单位(LTB)是一种非常有效的黏膜免疫佐剂,它能刺激机体产生强烈的黏膜和系统免疫应答。大量研究表明LTB与GM1的结合对于LT的佐剂活性是必需的[14],并且GM1是广泛表达于绝大多数细胞表面[15-16],这使得LTB的佐剂活性的应用范围极广。还有研究表明LT不但能与神经节苷脂结合,还能与缺乏唾液酸的GM1、GD1b以及乳糖神经酰胺等受体结合,然而,这些受体对于LTB的佐剂活性的作用尚不清楚[17]。还有研究表明LTB在某些反应中表现出比LT和CT更好的黏膜佐剂效应[18-20],且LTB几乎没有毒性。LTB通过与细胞表面的GM1结合,不仅能增强机体对抗原的免疫应答效应,还能够改变黏膜免疫应答的类型。一些研究表明LTB并不能促进DC细胞的成熟[9]。LTB对于T细胞的作用也不全是促进作用,LTB除能促进CD4+T细胞的增殖外,还能诱导CD8+T细胞的凋亡[6, 8]。因此对于LTB佐剂的活性机制的研究就显得十分必要。为此,本文通过pull-down实验结合质谱,鉴定了与LTB存在相互作用的25种蛋白分子,通过对这些蛋白分子的功能和信号通路查询,发现有4种蛋白未显示出与其他蛋白的相互作用,也未发现与之相关信号通路;11种Keratin家族蛋白主要构成细胞骨架,维持细胞形态功能的稳定,也没有找到它们与免疫调节相关的证据;余下的10种蛋白分子中Pkp1、Anxa2、Hspd1、Ddx5都没有与之相关的信号通路,除Ddx5能够调节Th17细胞的功能同时能促进细胞的增殖外,未见到他们参与免疫调节的报道,Actb参与白细胞的跨内皮细胞转移,它在上皮细胞的表达上调能够影响细胞骨架蛋白的重排,从而促进白细胞由血液透过上皮细胞向组织细胞的转移,但在巨噬细胞中表达增加作用尚不清楚;Vimentin参与人类疱疹病毒第四型的感染的信号通路,但通过免疫荧光实验表明LTB在细胞内与Vimentin不存在相互作用;Dsp能够通过与Jup作用激活TCF/LEF,达到促进cyclin1、c-Myc和PPARy基因的表达从而促进细胞的增殖与活化,研究表明TCF/LEF能够通过影响Bcl-2、IL-6、ICOS影响TFH细胞的活动,从而促进T细胞和B细胞的增殖与分化,以及影响IL-4, IL-21等细胞因子和免疫球蛋白的表达[21-23]。免疫荧光阻断实验验证了LTB内吞进入巨噬细胞内必须依赖于与细胞表面的GM1受体结合,内吞进入细胞内通过与GM130的结合,由高尔基体进行加工转运,LTB本身也是一种抗原,免疫细胞对其加工处理很可能与其他一般抗原加工处理途径是一致的。再与Jup等作用激活TCF/LEF从而引起一系列免疫调节反应,因此推测LTB发挥佐剂活性的信号通路(Fig 8)。
|
| Fig 8 T and B cell differentiation and proliferation: Immunoglobulin and cytokine secretion |
LTB作为佐剂的研究由来已久,LTB已被认为是最有潜力的黏膜免疫佐剂,本研究通过蛋白质相互作用与生物信息学预测,推测了LTB发挥佐剂活性可能的信号通路,为LTB的进一步深入研究和临床应用奠定了基础。
( 致谢: 本实验完成于重庆医科大学分子医学与肿瘤研究中心,在此感谢本实验室的老师及实验管理人员,同时也感谢我的指导老师马永平教授在实验过程的指导及帮助。 )
| [1] | Salimian J, Salmanian A, Khalesi R, et al. Antibody against recombinant heat labile enterotoxin B subunit (rLTB) could block LT binding to ganglioside M1 receptor[J]. Iran J Microbiol, 2010, 2 (3): 120-7. |
| [2] | De Haan L, Verweij W, Agsteribbe E, et al. The role of ADP-ribosylation and G (M1)-binding activity in the mucosal immunogenicity and adjuvanticity of the Escherichia coli heat-labile enterotoxin and Vibrio cholerae cholera toxin[J]. Immunol Cell Biol, 1998, 76 (3): 270-9. doi:10.1046/j.1440-1711.1998.00745.x |
| [3] | De Haan L, Verweij W R, Feil I K, et al. Role of GM1 binding in the mucosal immunogenicity and adjuvant activity of the Escherichia coli heat-labile enterotoxin and its B subunit[J]. Immunology, 1998, 94 (3): 424-30. doi:10.1046/j.1365-2567.1998.00535.x |
| [4] | Fraser S A, de Haan L, Hearn A R, et al. Mutant Escherichia coli heat-labile toxin B subunit that separates toxoid-mediated signaling and immunomodulatory action from trafficking and delivery functions[J]. Infect Immun, 2003, 71 (3): 1527-37. doi:10.1128/IAI.71.3.1527-1537.2003 |
| [5] | Fingerut E, Gutter B, Goldway M, et al. B subunit of E. coli enterotoxin as adjuvant and carrier in oral and skin vaccination[J]. Vet Immunol Immunop, 2006, 112 (3-4): 253-63. doi:10.1016/j.vetimm.2006.03.005 |
| [6] | Brereton C F, Sutton C E, Ross P J, et al. Escherichia coli heat-labile enterotoxin promotes protective Th17 responses against infection by driving innate IL-1 and IL-23 production[J]. J Immunol, 2011, 186 (10): 5896-906. doi:10.4049/jimmunol.1003789 |
| [7] | Millar D G, Hirst T R, Snider D P. Escherichia coli heat-labile enterotoxin B subunit is a more potent mucosal adjuvant than its vlosely related homologue, the B subunit of cholera toxin[J]. Infect Immun, 2001, 69 (5): 3476-82. doi:10.1128/IAI.69.5.3476-3482.2001 |
| [8] | Ji J, Griffiths K L, Milburn P J, et al. The B subunit of Escherichia coli heat-labile toxin alters the development and antigen-presenting capacity of dendritic cells[J]. J Cell Mol Med, 2015, 19 (8): 2019-31. doi:10.1111/jcmm.2015.19.issue-8 |
| [9] | Nashar T O, Williams N A, Hirst T R. Cross-linking of cell surface ganglioside GM1 induces the selective apoptosis of mature CD8+ T lymphocytes[J]. Int Immunol, 1996, 8 (5): 731-6. doi:10.1093/intimm/8.5.731 |
| [10] | Truitt R L, Hanke C, Radke J, et al. Glycosphingolipids as novel targets for T-cell suppression by the B subunit of recombinant heat-labile enterotoxin[J]. Infect Immun, 1998, 66 (4): 1299-308. |
| [11] | Yankelevich B, Soldatenkov V A, Hodgson J, et al. Differential induction of programmed cell death in CD8+ and CD4+ T cells by the B subunit of cholera toxin[J]. Cell Immunol, 1996, 168 (2): 229-34. doi:10.1006/cimm.1996.0070 |
| [12] | 袁琴, 袁丁, 周志勇, 等. 竹节参齐墩果烷皂苷对RAW 264.7巨噬细胞SIRT1活性影响及抗炎作用研究[J]. 中国药理学通报, 2016, 32 (3) : 349-54. Yuan Q, Yuan D, Zhou Z Y, et al. Anti-inflammatory effect of Chikusetsu oleanane saponin on RAW 264.7 cell through regulating SIRT1 activity[J]. Chin Pharmacol Bull, 2016, 32 (3): 349-54. |
| [13] | Francis M L, Ryan J, Jobling M G, et al. Cyclic AMP-independent effects of cholera toxin on B cell activation. II. Binding of ganglioside GM1 induces B cell activation[J]. J Immunol, 1992, 148 (7): 1999-2005. |
| [14] | Moreno-Altamirano M M, Aguilar-Carmona I, Sanchez-Garcia F J. Expression of GM1, a marker of lipid rafts, defines two subsets ofhuman monocytes with differential endocytic capacity and lipopolysaccharide responsiveness[J]. Immunology, 2007, 120 (4): 536-43. doi:10.1111/imm.2007.120.issue-4 |
| [15] | Spangler B D. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin[J]. Microbiol Rev, 1992, 56 (4): 622-47. |
| [16] | Bibolini M J, Julia Scerbo M, Peinetti N, et al. The hybrid between the ABC domains of synapsin and the B subunit of Escherichia coli heat-labile toxin ameliorates experimental autoimmune encephalomyelitis[J]. Cell Immunol, 2012, 280 (1): 50-60. doi:10.1016/j.cellimm.2012.11.012 |
| [17] | Grassmann A A, Felix S R, dos Santos C X, et al. Protection against lethal leptospirosis after vaccination with LipL32 coupled or coadministered with the B subunit of Escherichia coli heat-labile enterotoxin[J]. Clin Vaccine Immunol, 2012, 19 (5): 740-5. doi:10.1128/CVI.05720-11 |
| [18] | Williams N A. Immune modulation by the cholera-like enterotoxin B-subunits: from adjuvant to immunotherapeutic[J]. Int J Med Microbiol, 2000, 290 (4-5): 447-53. doi:10.1016/S1438-4221(00)80062-4 |
| [19] | Yang X, Mariuzza R A. Pre-T-cell receptor binds MHC: Implications for thymocyte signaling and selection[J]. Proc Natl Acad Sci USA, 2015, 112 (27): 8166-7. doi:10.1073/pnas.1510127112 |
| [20] | 陈艳芳, 刘培庆. PIP140/PGC-1α在AngⅡ调节心肌能量代谢中的作用研究[J]. 中国药理学通报, 2015, 31 (2) : 194-8. Chen Y F, Liu P Q. Role of RIP140 and PGC-1α in angiotensin Ⅱ mediated energy metabolism in cardiomyocytes[J]. Chin Pharmacol Bull, 2015, 31 (2): 194-8. |
| [21] | Steinke F C, Yu S, Zhou X, et al. TCF-1 and LEF-1 act upstream of Th-POK to promote the CD4+T cell fate and interact with Runx3 to silence CD4 in CD8+ T cells[J]. Nat Immunol, 2014, 15 (7): 646-56. doi:10.1038/ni.2897 |
| [22] | Mookerjee-Basu J, Kappes D J. New ingredients for brewing CD4+T (cells):TCF-1 and LEF-1[J]. Nat Immunol, 2014, 15 (7): 593-4. doi:10.1038/ni.2927 |
| [23] | Masato Kubo. TCF-1 and LEF-1 help launch the T (FH) program[J]. Nat Immunol, 2015, 16 (9): 900-1. doi:10.1038/ni.3254 |

