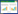文章信息
- 刘双双, 朱正帅, 杨子林, 段东奎, 符克优, 沈索姣
- LIU Shuangshuang, ZHU Zhengshuai, YANG Zilin, DUAN Dongkui, FU Keyou, SHEN Suojiao
- 积雪草苷调节HIF-1α/VEGF信号通路对食管癌细胞上皮-间质转化和放疗敏感性的影响
- Effect of asiaticoside on epithelial-mesenchymal transition and radiotherapy sensitivity of esophageal cancer cells via regulation of the HIF-1α/VEGF signaling pathway
- 中国医科大学学报, 2025, 54(2): 150-155
- Journal of China Medical University, 2025, 54(2): 150-155
-
文章历史
- 收稿日期:2023-12-19
- 网络出版时间:2025-01-15 17:44:52
食管癌(esophageal cancer,EC) 是一种具有强侵袭性的消化道恶性肿瘤,2020年全球新发病例超过60万,死亡人数超过54万,且中国的发病率最高,占全球新病例的50% [1]。放疗是晚期或转移性EC的主要治疗手段,放射耐药的EC患者预后较差[2]。研究[3]显示,DNA损伤修复、上皮-间质转化(epithelial-mesenchymal transition,EMT) 和细胞程序性死亡的异常调控等均与EC的放射耐药相关。积雪草苷(asiaticoside,AS) 是一种五环三萜皂苷,具有抗炎、抗肿瘤等多种药理作用[4]。AS可抑制电离辐射诱导的肺癌细胞迁移和侵袭,增强非小细胞肺癌患者的放疗效果[5]。AS对胃癌[6]、胰腺癌[7]等消化道肿瘤有抑制作用,因此推测AS对EC细胞进展也具有抑制作用。缺氧诱导因子-1α (hypoxia inducible factor-1α,HIF-1α) /血管内皮生长因子(vascular endothelial growth factor,VEGF) 可以介导血管生成,促进结直肠癌细胞侵袭和转移[8]。HIF-1α/VEGF信号通路的激活能够降低子宫内膜癌细胞放疗敏感性[9]。AS调节HIF-1α/VEGF信号通路对EC细胞EMT和放疗敏感性的影响尚不清楚。本研究旨在探讨AS对EC细胞系EC9706细胞EMT和放疗敏感性的影响。
1 材料与方法 1.1 细胞来源EC9706细胞购自美国ATCC。
1.2 主要试剂与仪器AS (纯度≥98%),南京道斯夫生物科技有限公司;四甲基偶氮唑蓝(methyl thiazolyl tetrazolium,MTT) 细胞增殖检测试剂盒,上海白益生物科技有限公司;HIF-1α/VEGF通路激活剂二甲基草酰甘氨酸(dimethyloxallyl glycine,DMOG),杭州昊鑫生物科技股份有限公司;实时定量PCR试剂盒,北京达科为生物技术有限公司;膜联蛋白V异硫氰酸荧光素/碘化丙啶(Annexin V fluorescein isothiocyanate/propy-liodide,Annexin V FITC/PI) 检测试剂盒,上海晶风生物科技有限公司;兔源基质金属蛋白酶(matrix me-talloproteinase,MMP) -2、波形蛋白(vimentin)、E-钙黏蛋白(E-cadherin)、Bcl-2相关X蛋白(Bcl-2 associated X protein,Bax) 及HIF-1α、VEGF、GAPDH一抗及HRP标记的羊抗兔二抗,英国abcam公司;Multiskan FC酶标仪、A24858流式细胞仪,美国赛默飞世尔科技公司;Turbo 16P实时荧光定量PCR仪,南京诺唯赞生物科技股份有限公司;TE2000-U荧光显微镜,日本Nikon公司。
1.3 实验方法 1.3.1 MTT法检测EC9706细胞增殖活性将EC9706以1×104/孔接种到96孔板中培养过夜。加入含0、0.5、1、2、4、8 μmol/L AS [10]的培养液24 h或用0、2、4、6、8 Gy照射1~2 min后用完全培养液培养24 h,加入20 μL MTT溶液,弃上清液,用二甲基亚砜溶解甲臜,酶标仪检测490 nm吸光度,计算细胞增殖抑制率和半数抑制浓度(half maximal inhibitory concentration,IC50)。
1.3.2 平板克隆实验将EC9706细胞分为control组、放射组(单纯X射线照射)、AS组、联合组(AS+X射线照射)、激活剂组(AS+X射线照射+HIF-1α/VEGF通路激活剂DMOG),AS组、联合组、激活剂组加入含有4 μmol/L的AS培养液,激活剂组加入10 μmol/L
DMOG[11],培养48 h,放射组、联合组、激活剂组用6 Gy照射。孵育10~14 d后,甲醇固定、结晶紫染色、水洗、干燥。在光学显微镜下计数,并计算细胞存活分数。
1.3.3 细胞迁移与侵袭实验细胞以1.0×105/mL悬浮于无血清培养基中。将带有或不带有Matrigel预包被的Transwell小室插入24孔板中,在上室加入200 μL悬液,在下室加入500 μL完全培养基。48 h后用5%多聚甲醛固定,0.1%结晶紫染色。随机选取5个视野进行计数。侵袭实验的Transwell小室用Matrigel预先包被。
1.3.4 细胞凋亡检测取对数生长期各组EC9706细胞,用磷酸盐缓冲液洗涤。加入5 μL Annexin V-FITC和PI染液,遮光染色15 min,采用流式细胞仪检测细胞凋亡。
1.3.5 HIF-1α、VEGF mRNA表达检测实时定量PCR检测HIF-1α、VEGF mRNA表达。用TRIzol试剂提取细胞总RNA。用反转录试剂盒将RNA合成cDNA,按照实时定量PCR试剂盒配置反应体系。用实时定量PCR仪检测HIF-1α、VEGF mRNA表达。实时定量PCR引物序列见表 1。GAPDH为内参,采用2-ΔΔCt法计算相对表达量。
| Gene | Forward primer (5’-3’) | Reverse primer (5’-3’) |
| HIF-1α | GAAGTGTACCCTAACTAGCCG | TCACAAATCAGCACCAAGC |
| VEGF | TGCTTTCTCCGCTCTGA | ACTGAGGAGTCCAACAT |
| GAPDH | GAAGGTGAAGGTCGGAGTCA | AATGAAGGGGTCATTGATGG |
1.3.6 蛋白表达检测
用RIPA缓冲液从细胞中分离提取蛋白。二辛可宁酸法测定蛋白浓度。25 µg蛋白经6%十二烷基硫酸钠聚丙烯酰胺凝胶电泳分离后,转移至聚偏二氟乙烯膜上。用5%脱脂牛奶37 ℃封闭1 h后,与MMP-2、vimentin、E-cadherin、Bax、HIF-1α、VEGF、GAPDH蛋白一抗4 ℃孵育过夜,再与二抗37 ℃孵育1 h。以GAPDH为内参,使用化学发光检测试剂对蛋白质进行可视化,并通过软件Imagepro Plus 6.0定量蛋白表达。
1.4 统计学分析采用Graphpad Prism 7.0软件分析数据。计量资料以x±s表示。单因素方差分析用于多组间的比较,组间两两比较采用SNK-q检验。P < 0.05为差异有统计学意义。
2 结果 2.1 AS对EC9706细胞活力的影响与0 μmol/L AS干预(0.00%±0.00%) 比较,EC9706细胞增殖抑制率在0.5、1、2、4、8 μmol/L AS干预下以剂量依赖性的方式增加(6.02%±2.15%、13.78%±2.36%、31.46%±3.42%、46.19%±3.90%、63.21%±4.68%,P < 0.05),EC9706细胞IC50为4.60 μmol/L,故选取4 μmol/L AS进行后续实验。与0 Gy照射(0.00%±0.00%) 比较,在2、4、6、8 Gy照射后EC9706细胞增殖抑制率增加(8.49%±1.67%、19.83%±2.19%、38.52%±2.71%、65.31%±3.84%,P < 0.05),IC50为6.48 Gy,故选取6 Gy放疗剂量进行后续实验。
2.2 AS对EC9706细胞放疗敏感性的影响与control组(91.27%±4.76%) 相比,放射组(72.31%±3.68%) 和AS组(65.13%±3.41%) 细胞存活分数降低(P < 0.05);与放射组和AS组相比,联合组(34.65%±2.52%) 细胞存活分数降低(P < 0.05);与联合组相比,激活剂组(54.82%±4.72%) 细胞存活分数升高(P < 0.05)。见图 1。
|
| A, control group; B, radiology group; C, AS group; D, combined group; E, activator group. 图 1 AS对EC9706细胞放疗敏感性的影响×40 Fig.1 Effect of AS on the sensitivity of EC9706 cells to radiotherapy ×40 |
2.3 AS对EC9706细胞迁移与侵袭的影响
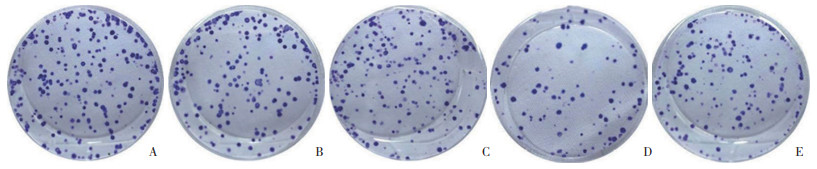
与control组(134.57±9.26,125.78±8.34) 相比,放射组(96.42±8.25,87.53±6.56) 和AS组(86.39±5.14,72.65±5.17) 迁移与侵袭细胞数降低(P < 0.05);与放射组和AS组相比,联合组(45.28±3.16,43.58±4.18) 迁移与侵袭细胞数降低(P < 0.05);与联合组相比,激活剂组(61.48±5.37,60.48±4.69) 迁移与侵袭细胞数升高(P < 0.05)。见图 2。
|
| 图 2 AS对EC9706细胞迁移与侵袭的影响 结晶紫染色×200 Fig.2 Effects of AS on the migration and invasion of EC9706 cells Crystal violet staining ×200 |
2.4 AS对EC9706细胞凋亡的影响
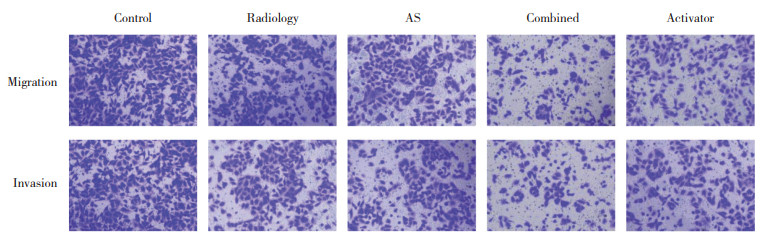
与control组(8.54%±2.16%) 相比,放射组(17.83%±3.09%) 和AS组(22.75%±3.28%) 凋亡率升高(P < 0.05);与放射组和AS组相比,联合组(45.68%±3.37%) 凋亡率升高(P < 0.05);与联合组相比,激活剂组(24.65%±2.61%) 凋亡率降低(P < 0.05)。见图 3。
|
| A, control group; B, radiology group; C, AS group; D, combined group; E, activator group. 图 3 AS对EC9706细胞凋亡的影响 Fig.3 Effect of AS on the apoptosis of EC9706 cells |
2.5 AS对EC9706细胞中HIF-1α、VEGF mRNA表达的影响
与control组(1.00±0.11,1.00±0.12) 相比,放射组(0.69±0.09,0.72±0.07) 和AS组(0.54±0.06,0.56±0.08) HIF-1α、VEGF mRNA表达降低(P < 0.05);与放射组和AS组相比,联合组(0.23±0.04,0.25±0.03) HIF-1α、VEGF mRNA表达降低(P < 0.05);与联合组相比,激活剂组(0.49±0.05,0.51±0.06) HIF-1α、VEGF mRNA表达升高(P < 0.05)。
2.6 AS对MMP-2、vimentin、E-cadherin、Bax、HIF-1α、VEGF蛋白表达的影响与control组相比,放射组和AS组MMP-2、vimentin、HIF-1α、VEGF表达降低,E-cadherin、Bax表达升高(P < 0.05);与放射组和AS组相比,联合组MMP-2、vimentin、HIF-1α、VEGF表达降低,E-cadherin、Bax表达升高(P < 0.05);与联合组相比,激活剂组MMP-2、vimentin、HIF-1α、VEGF表达升高,E-cadherin、Bax表达降低(P < 0.05)。见图 4、5。
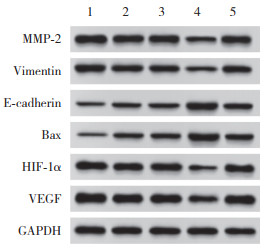
|
| 1, control group; 2, radiology group; 3, AS group; 4, combined group; 5, activator group. 图 4 EC9706细胞中MMP-2、vimentin、E-cadherin、Bax、HIF-1α、VEGF蛋白表达情况 Fig.4 Expressions of MMP-2, vimentin, E-cadherin, Bax, HIF-1α, and VEGF in EC9706 cells |
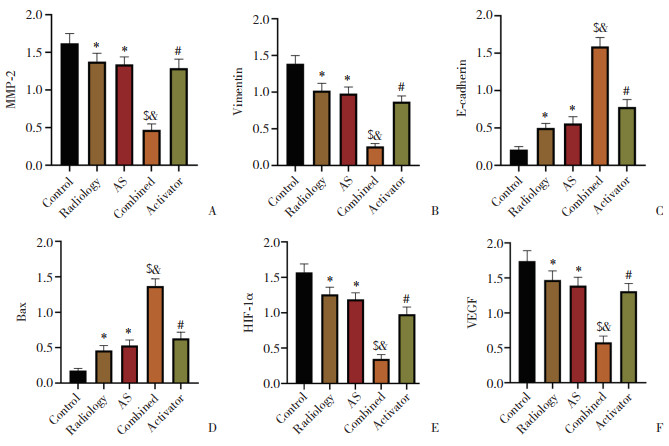
|
| A, MMP-2; B, vimentin; C, E-cadherin; D, Bax; E, HIF-1α; F, VEGF. *P < 0.05 vs. control group; $ P < 0.05 vs. radiology group; &P < 0.05 vs. AS group; #P < 0.05 vs. combined group. 图 5 各组EC9706细胞中MMP-2、vimentin、E-cadherin、Bax、HIF-1α、VEGF表达比较 Fig.5 Comparison of the expressions of MMP-2, vimentin, E-cadherin, Bax, HIF-1α, and VEGF in EC9706 cells of each group |
3 讨论
AS是积雪草的主要活性成分,可通过miR-635/高迁移率族蛋白1轴抑制胃癌进展,是对抗胃癌的潜在治疗药物[10]。EMT与肿瘤、侵袭、转移和耐药性有关,可促进肿瘤细胞侵袭和迁移。在EMT发生时E-cadherin表达降低,vimentin表达升高[12]。AS通过阻断p65和p38丝裂原活化蛋白激酶激活,抑制胰腺癌PANC-1细胞EMT特性[7]。本研究发现,AS可抑制EC9706细胞增殖、迁移和侵袭,降低转移相关蛋白(MMP-2、Vimentin) 表达,促进细胞凋亡及凋亡蛋白Bax及E-cadherin蛋白表达,且联合X射线照射对EC9706细胞恶性行为的抑制作用更明显。提示AS通过降低EMT抑制细胞迁移和侵袭,促进细胞凋亡,增加放疗敏感性,从而抑制EC进展。目前EC患者的传统治疗方法包括手术、化疗、放化疗等,近期手术、免疫治疗与放化疗联合能够提高EC患者生存率,但放化疗对人体损害较大[2, 13]。AS作为天然提取物,不良反应小,具有成为治疗EC靶向药物的潜力,未来可能单独或者通过与放化疗联合发挥抗EC作用。
VEGF能促进MMP分泌,导致细胞外基质降解并为细胞侵入附近组织提供通道。MMP-2和MMP-9与EC等癌细胞的迁移和侵袭能力密切相关[14]。VEGF是HIF-1α的关键下游效应物,HIF-1α/VEGF信号通路激活可诱导非小细胞肺癌的EMT [15]。靶向抑制HIF-1α/VEGF信号通路可以提高子宫内膜癌细胞的放疗敏感性[9]。基于这些发现,HIF-1α/VEGF信号通路可能是治疗EC转移的重要靶点。本研究结果显示,AS可抑制EC9706细胞HIF1-α、VEGF表达。而使用HIF1-α/VEGF信号通路激活剂后减弱了AS对EMT的抑制作用。提示AS可能通过抑制HIF1-α/VEGF通路抑制EMT,从而增强EC细胞的放疗敏感性。
综上所述,AS可能通过抑制HIF1-α/VEGF信号通路抑制EMT,从而增强EC细胞的放疗敏感性。本研究为EC的治疗提供新的药物参考,然而AS增强EC细胞的放疗敏感性可能涉及到其他通路,有待进一步验证。
| [1] |
LIU CQ, MA YL, QIN Q, et al. Epidemiology of esophageal cancer in 2020 and projections to 2030 and 2040[J]. Thorac Cancer, 2023, 14(1): 3-11. DOI:10.1111/1759-7714.14745 |
| [2] |
WANG HL, XU YF, ZUO FL, et al. Immune-based combination thera- py for esophageal cancer[J]. Front Immunol, 2022, 13: 1020290. DOI:10.3389/fimmu.2022.1020290 |
| [3] |
AN LB, LI MY, JIA QG. Mechanisms of radiotherapy resistance and radiosensitization strategies for esophageal squamous cell carcinoma[J]. Mol Cancer, 2023, 22(1): 140. DOI:10.1186/s12943-023-01839-2 |
| [4] |
HE ZL, HU YY, NIU ZQ, et al. A review of pharmacokinetic and pharmacological properties of asiaticoside, a major active constituent of Centella asiatica (L.) Urb[J]. J Ethnopharmacol, 2023, 302(Pt A): 115865. DOI:10.1016/j.jep.2022.115865 |
| [5] |
HAN AR, LEE SH, HAN SJ, et al. Triterpenoids from the leaves of Centella asiatica inhibit ionizing radiation-induced migration and invasion of human lung cancer cells[J]. Evid Based Complement Alternat Med, 2020, 2020: 3683460. DOI:10.1155/2020/3683460 |
| [6] |
YE CM, YAO ZC, WANG YY, et al. Asiaticoside promoted ferroptosis and suppressed immune escape in gastric cancer cells by downregulating the Wnt/β-catenin pathway[J]. Int Immunopharmacol, 2024, 134: 112175. DOI:10.1016/j.intimp.2024.112175 |
| [7] |
HE YG, PENG XH, ZHENG L, et al. Asiaticoside inhibits epithe- lial-mesenchymal transition and stem cell-like properties of pancreatic cancer PANC-1 cells by blocking the activation of p65 and p38MAPK[J]. J Gastrointest Oncol, 2021, 12(1): 196-206. DOI:10.21037/jgo-20-533 |
| [8] |
CHEN MT, ZHONG KY, TAN JC, et al. Baicalein is a novel TLR4- targeting therapeutics agent that inhibits TLR4/HIF-1α/VEGF signaling pathway in colorectal cancer[J]. Clin Transl Med, 2021, 11(11): e564. DOI:10.1002/ctm2.564 |
| [9] |
MIYASAKA A, ODA K, IKEDA Y, et al. PI3K/mTOR pathway inhibition overcomes radioresistance via suppression of the HIF1-α/VEGF pathway in endometrial cancer[J]. Gynecol Oncol, 2015, 138(1): 174-180. DOI:10.1016/j.ygyno.2015.04.015 |
| [10] |
ZHANG C, JI XL, CHEN ZG, et al. Asiaticoside suppresses gastric cancer progression and induces endoplasmic reticulum stress through the miR-635/HMGA1 axis[J]. J Immunol Res, 2022, 2022: 1917585. DOI:10.1155/2022/1917585 |
| [11] |
LI J, LU X, WEI LQ, et al. PHD2 attenuates high-glucose-induced blood retinal barrier breakdown in human retinal microvascular endothelial cells by regulating the HIF-1α/VEGF pathway[J]. Inflamm Res, 2022, 71(1): 69-79. DOI:10.1007/s00011-021-01518-2 |
| [12] |
XU JC, CHEN TY, LIAO LT, et al. NETO2 promotes esophageal cancer progression by inducing proliferation and metastasis via PI3K/AKT and ERK pathway[J]. Int J Biol Sci, 2021, 17(1): 259-270. DOI:10.7150/ijbs.53795 |
| [13] |
YANG H, LIU H, CHEN YP, et al. Long-term efficacy of neoadjuvant chemoradiotherapy plus surgery for the treatment of locally advanced esophageal squamous cell carcinoma: the NEOCRTEC5010 randomized clinical trial[J]. JAMA Surg, 2021, 156(8): 721-729. DOI:10.1001/jamasurg.2021.2373 |
| [14] |
MOHAMMADI F, JAVID H, AFSHARI AR, et al. Substance P accelerates the progression of human esophageal squamous cell carcinoma via MMP-2, MMP-9, VEGF-A, and VEGFR1 overexpression[J]. Mol Biol Rep, 2020, 47(6): 4263-4272. DOI:10.1007/s11033-020-05532-1 |
| [15] |
WANG ML, WANG WD, DING JM, et al. Downregulation of Rab17 promotes cell proliferation and invasion in non-small cell lung cancer through STAT3/HIF-1α/VEGF signaling[J]. Thorac Cancer, 2020, 11(2): 379-388. DOI:10.1111/1759-7714.13278 |
 2025, Vol. 54
2025, Vol. 54