文章信息
- 谷君, 许峥嵘, 史丽, 任卫东, 左丽娟, 张秋子
- GU Jun, XU Zhengrong, SHI Li, REN Weidong, ZUO Lijuan, ZHANG Qiuzi
- circFAT1调节miR-211-5p/CCND2轴对糖尿病心肌病大鼠心肌损伤的影响
- circFAT1 affects myocardial injury in rats with diabetic cardiomyopathy by regulating the miR-211-5p/CCND2 axis
- 中国医科大学学报, 2024, 53(6): 516-524
- Journal of China Medical University, 2024, 53(6): 516-524
-
文章历史
- 收稿日期:2023-07-24
- 网络出版时间:2024-05-31 14:53:46
糖尿病心肌病(diabetic cardiomyopathy,DCM)是一种独立于冠状动脉疾病和高血压的糖尿病相关心脏病[1],其特征是心肌纤维化、心室重塑和心功能障碍,可通过多种机制影响心脏健康,包括代谢变化、亚细胞组成异常和微血管损伤[2]。环状RNA(circular RNA,circRNA)是具有共价闭环结构的环状分子,广泛参与癌症、心血管疾病和糖尿病等疾病的发展。目前只有HIPK3和DICAR等少数circRNA被发现与DCM有关[3-4]。近来有研究[5-6]发现circFAT1不仅在多器官系统的肿瘤发生中起重要作用,还可促进高糖诱导的视网膜色素上皮的自噬,抑制其焦亡,可能是糖尿病视网膜病变治疗的潜在靶点。circRNA作用的调节机制主要是作为微RNA(microRNA,miRNA)海绵来抑制mRNA的翻译,在心血管系统中,miRNA可以通过调控多种细胞的功能参与心血管疾病[7]。miR-211-5p是长度为22个核苷酸的miRNA,能够被circPAG1海绵化来抑制糖尿病性白内障中高糖诱导的细胞凋亡和氧化应激[8]。研究[9]发现细胞周期蛋白D2(cyclin D2,CCND2)可调节心肌细胞增殖,并激活细胞周期进程以增强心肌修复,有助于心脏功能障碍恢复。本研究基于生物学信息分析发现circFAT1与miR-211-5p和CCND2之间具有靶向结合位点,拟通过miR-211-5p/CCND2轴探讨circFAT1对DCM大鼠心肌损伤的作用。
1 材料与方法 1.1 材料 1.1.1 动物SPF级SD雄性大鼠,6周龄,体重(230±20)g,购自河北省实验动物中心[SCXK(冀)2021-002],并饲养于河北北方学院[SYXK(冀)2019-004]。饲养条件:温度24~26 ℃,湿度60%,12 h/12 h明暗交替。本研究获得我院动物伦理委员会批准。
1.1.2 主要试剂高糖高脂饲料(货号RD12492,上海锐赛生物科技有限公司);链脲佐菌素(streptozotocin,STZ)(货号60256ES76,翌圣生物科技股份有限公司);一步法实时PCR试剂盒(货号801-001-AR,南京维森特生物技术有限公司);miRNA一链cDNA合成试剂盒、Hieff® miRNA Universal qPCR SYBR Master Mix(货号11148ES10、11171ES03,上海翌圣生物科技股份有限公司);苏木精-伊红(hematoxylin-eosin,HE)、Masson三色染色液(货号TO2055、DC0032,上海江莱生物科技有限公司);TUNEL凋亡检测试剂盒(货号CTCC-M008,无锡菩禾生物医药技术有限公司);白细胞介素-1β(interleukin 1β,IL-1β)、白细胞介素-6(interleukin 6,IL-6)和肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)酶联免疫吸附实验(enzyme linked immunosorbent assay,ELISA)试剂盒(货号EK-R36877、EK-R36902、EK-R38696,上海酶研生物科技有限公司);CCND2兔多抗、山羊抗兔IgG H & L(货号ab230883、ab6721,英国Abcam公司)。
1.2 方法 1.2.1 大鼠模型的建立将大鼠分为对照组(n = 25)和实验组(n = 110),实验组大鼠参照文献[10]方法喂食4周高糖高脂饮食,禁食不禁水12 h后,单次腹腔注射30 mg/kg STZ(溶于0.1 mol/L柠檬酸盐缓冲液,pH=4.5)。对照组大鼠喂食4周普通饲料,并注射等体积的柠檬酸盐缓冲液。72 h后利用血糖仪测量大鼠空腹血糖(fasting blood glucose,FBG),FBG≥16.7 mmol/L为糖尿病大鼠模型复制成功。将模型复制成功的大鼠继续喂养高糖高脂8周,每组随机选取2只大鼠处死,HE染色观察发现心肌组织出现细胞不规则排列、细胞肿胀且间隙增大等结构变化,表示DCM大鼠模型成功建立。然后每组随机选取5只大鼠,采用实时PCR检测大鼠心肌组织中circFAT1、miR-211-5p、CCND2的表达。
1.2.2 动物分组及处理将DCM大鼠随机分为DCM组、circ-NC组、circFAT1组、circFAT1+激动剂-NC组、circFAT1+miR-211-5p激动剂组,每组20只。circ-NC组和circFAT1组大鼠尾静脉注射50 μL携带cir-NC和circFAT1质粒的慢病毒(1×109 TU慢病毒溶于50 μL生理盐水)[11],circFAT1+激动剂-NC组和circFAT1+miR-211-5p激动剂组大鼠在注射circFAT1慢病毒质粒30 min后,分别尾静脉注射5 μL激动剂-NC和miR-211-5p激动剂。对照组和DCM组大鼠均尾静脉注射等体积的生理盐水。在此过程中,对照组大鼠喂食普通饲料,其余5组均继续喂食高脂高糖饲料诱导心肌损伤。
1.2.3 实时PCR检测心肌组织circFAT1、miR-211-5p、CCND2的表达慢病毒质粒干预结束后,禁食禁水12 h,用1%戊巴比妥钠麻醉大鼠后颈椎脱臼处死,腹主动脉采集空腹血2 mL,并解剖获取心脏组织,部分置于4%多聚甲醛中固定,部分冻存于-80 ℃。取冻存的组织样本(n = 5)用TRIzol法提取总RNA。然后以总RNA为模板,用一步法实时PCR试剂盒对circFAT1和CCND2的表达进行定量,随后利用miRNA一链cDNA合成试剂盒进行RNA逆转录,并以cDNA为模板,用Hieff® miRNA Universal qPCR SYBR Master Mix对miR-211-5p进行定量。分别以GAPDH和U6为内参,用2-ΔΔCt法计算circFAT1、CCND2和miR-211-5p的表达量。所有引物序列见表 1。
| Gene | Forward primer(5’-3’) | Reverse primer(5’-3’) |
| circFAT1 | TGAGGGCCAAAGACAAGGGAAAGC | GGTGGGTGCAGGTTCTCATTCACA |
| CCND2 | TTTGCCATGTACCCACCGTC | AGGGCATCACAAGTGAGCG |
| GAPDH | TGGACCTGACCTGCCGTCTAGAAA | GTGGGTGTCGCTGTTGAAGTCAGA |
| miR-211-5p | CTGAATGTGAGGAGGATGT | GTCCTTCGGCATCCCGGCCG |
| U6 | GACAGATTCGGTCTGTGGCAC | GATTACCCGTCGGCCATCGATC |
1.2.4 Western blotting检测心肌组织CCND2蛋白表达
取1.2.3中冻存的心肌组织加入蛋白裂解液进行匀浆,收集上清。通过10% SDS‐PAGE凝胶电泳分离蛋白质,并转至PVDF膜。5%脱脂牛奶室温下封闭膜1 h,后与CCND2一抗在4 ℃下孵育过夜,室温下与山羊抗兔二抗孵育1 h,使用增强化学发光试剂避光显影。以GAPDH为内参,用ImageJ软件对蛋白条带进行定量分析。
1.2.5 大鼠FBG、总胆固醇(total cholesterol,TC)和甘油三酯(triglyceride,TG)水平检测将1.2.3中的空腹血在4 ℃下以1 500 r/min离心15 min,收集上清保存至-20 ℃,采用生化分析仪检测大鼠FBG、TC和TG的水平。
1.2.6 大鼠心脏功能检测大鼠处死之前均进行M型超声心动图检查,以评估心脏功能。1%戊巴比妥钠麻醉大鼠,水平放置在操作台上进行超声检测,超声心动图参数包括左心室舒张末期直径(left ventricular end diastolic diameter,LVEDd)、左心室收缩末期直径(left ventricular end systolic diameter,LVEDs)、左心室射血分数(left ventricular ejection fraction,LVEF)和左心室缩短分数(left ventricular shortening fraction,LVFS),所有测量值均取5个连续心动周期的平均值。
1.2.7 HE和Masson染色观察心肌组织病理形态表现将1.2.3中固定过夜的心脏组织进行石蜡包埋切片(5 μm)。将石蜡切片脱蜡水化后,分别进行HE染色和Masson三色染色。利用光学显微镜观察心脏组织的形态学变化,并使用ImageJ软件测量平均胶原面积,每个切片随机选取5个视野,计算胶原容积分数(collagen volume fraction,CVF),CVF(%)=平均胶原面积/总视野面积×100。
1.2.8 TUNEL染色检测心肌细胞凋亡取1.2.7中的石蜡切片常规脱蜡,根据TUNEL试剂盒操作。用20 g/mL蛋白酶K处理组织切片5 min,再加入TUNEL混合液于37 ℃孵育1 h,DAPI进行核染,PBS清洗后,用抗荧光猝灭封片剂封片,在荧光显微镜下观察细胞凋亡(凋亡细胞呈绿色荧光)。
1.2.9 ELISA法检测IL-1β、IL-6、TNF-α水平取1.2.3中冻存的心肌组织(n = 5)称重,按1∶9(湿重/干重)的比例加入预冷的PBS缓冲液研磨匀浆,收集上清。然后利用ELISA试剂盒检测上清液中IL-1β、IL-6和TNF-α的水平。
1.2.10 双荧光素酶报告基因实验先通过ENCORI信息网站(https://rnasysu.com/encori/)预测miR-211-5p与circFAT1、CCND2在3’UTR区域的结合位点。然后将circFAT1、CCND2野生型(WT)片段和突变型(MUT)片段分别克隆到pmirR-GLO载体中,构建野生型质粒circFAT1-WT、CCND2-WT,和突变型质粒circFAT1-MUT、CCND2-MUT。利用lipofectamine 3000转染试剂将各质粒与miR-211-5p mimic或miR mimic-NC共转染至心肌细胞中,24 h后收集细胞检测荧光素酶活性。
1.3 统计学分析采用Graphpad prism 9.0软件进行统计分析,计量资料均符合正态分布,以x±s表示,多组间比较采用单因素方差分析,两两多重比较采用Tukey’s事后检验。P<0.05为差异有统计学意义。
2 结果 2.1 DCM大鼠心肌组织中circFAT1、miR-211-5p、CCND2的表达与对照组(1.00±0.00)相比,DCM模型大鼠心肌组织中circFAT1和CCND2 mRNA水平(0.34±0.02,0.41±0.03)降低(P<0.05),miR-211-5p表达水平(1.82±0.14)升高(P<0.05)。因此,后续实验通过过表达circFAT1进行。
2.2 各组大鼠心肌组织中circFAT1、miR-211-5p、CCND2 mRNA水平和CCND2蛋白表达与对照组相比,DCM组大鼠circFAT1、CCND2 mRNA及蛋白表达降低,miR-211-5p水平升高(P<0.05);与DCM组相比较,circFAT1组大鼠circFAT1、CCND2 mRNA及蛋白表达升高,miR-211-5p水平降低(P<0.05);与circFAT1组相比,circFAT1+miR-211-5p激动剂组大鼠CCND2 mRNA及蛋白表达降低,miR-211-5p水平升高(P<0.05),见图 1、表 2。
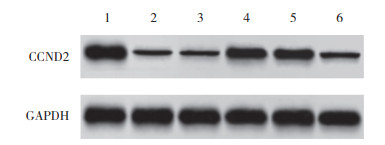
|
| 1, control group; 2, DCM group; 3, Circ-NC group; 4, circFAT1 group; 5, circFAT1+agomir-NC group; 6, circFAT1+miR-211-5p agomir group. 图 1 各组大鼠心肌组织中CCND2蛋白的表达 Fig.1 CCND2 protein expression in myocardial tissue of rats in each group |
| Group | circFAT1 | miR-211-5p | CCND2 mRNA | CCND2 protein |
| Control | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 | 0.96±0.07 |
| DCM | 0.34±0.021) | 1.82±0.141) | 0.41±0.031) | 0.28±0.021) |
| circ-NC | 0.33±0.021) | 1.79±0.131) | 0.43±0.031) | 0.30±0.021) |
| circFAT1 | 0.95±0.072) | 1.24±0.092) | 0.89±0.062) | 0.76±0.052) |
| circFAT1+agomir-NC | 0.94±0.072) | 1.25±0.092) | 0.87±0.062) | 0.75±0.052) |
| circFAT1+miR-211-5p agomir | 0.87±0.063) | 1.75±0.133) | 0.45±0.043) | 0.32±0.023) |
| 1)P<0.05 vs. control group;2)P<0.05 vs. DCM group;3)P<0.05 vs. circFAT1 group. | ||||
2.3 circFAT1对各组大鼠FBG、TC和TG水平的影响
与对照组相比,DCM组大鼠FBG、TC和TG水平升高(P<0.05);与DCM组相比,circFAT1组大鼠FBG、TC和TG水平降低(P<0.05);与circFAT1组相比,circFAT1+miR-211-5p激动剂组大鼠FBG、TC和TG水平升高(P<0.05),见表 3。
| Group | FBG | TC | TG |
| Control | 5.34±0.68 | 2.14±0.18 | 1.28±0.10 |
| DCM | 23.46±2.711) | 9.38±1.141) | 6.83±0.521) |
| circ-NC | 22.87±2.591) | 9.87±1.071) | 6.67±0.561) |
| circFAT1 | 12.65±1.432) | 4.69±0.382) | 2.95±0.182) |
| circFAT1+agomir-NC | 13.28±1.502) | 5.20±0.452) | 2.81±0.212) |
| circFAT1+miR-211-5p agomir | 21.72±2.183) | 8.95±0.823) | 5.72±0.433) |
| 1)P<0.05 vs. control group;2)P<0.05 vs. DCM group;3)P<0.05 vs. circFAT1 group. n = 20. | |||
2.4 circFAT1对各组大鼠心功能指标的影响
与对照组相比,DCM组大鼠LVEDd和LVEDs升高,LVEF和LVFS降低(P<0.05);与DCM组相比,circFAT1组大鼠LVEDd和LVEDs降低,LVEF和LVFS升高(P<0.05);与circFAT1组相比,circFAT1+miR-211-5p激动剂组大鼠LVEDd和LVEDs升高,LVEF和LVFS降低(P<0.05),见表 4。
| Group | LVEDd(mm) | LVEDs(mm) | LVEF(%) | LVFS(%) |
| Control | 2.57±0.18 | 1.46±0.11 | 76.85±5.32 | 43.19±3.14 |
| DCM | 4.26±0.371) | 3.15±0.261) | 42.61±2.871) | 26.05±1.861) |
| circ-NC | 4.18±0.351) | 3.09±0.271) | 43.12±2.751) | 26.07±1.931) |
| circFAT1 | 2.91±0.242) | 1.83±0.142) | 64.80±4.582) | 37.11±2.362) |
| circFAT1+agomir-NC | 2.95±0.232) | 1.85±0.132) | 65.21±4.632) | 37.29±2.452) |
| circFAT1+miR-211-5p agomir | 3.98±0.313) | 2.97±0.253) | 46.74±2.593) | 28.38±1.743) |
| 1)P<0.05 vs. control group;2)P<0.05 vs. DCM group;3)P<0.05 vs. circFAT1 group. n = 20. | ||||
2.5 circFAT1对各组大鼠心肌组织病理形态及纤维化的影响
对照组大鼠心肌组织结构完整,细胞核清晰,心肌纤维排列紧密且规则,无明显的胶原沉积;与对照组(1.63%±0.11%)相比,DCM组大鼠心肌细胞出现水肿,细胞间隙增大,心肌纤维紊乱,胶原纤维积聚明显,CVF(9.85%±0.64%)增大(P<0.05);与DCM组(9.85%±0.64%)和circ-NC组(9.92%±0.67%)相比,circFAT1组大鼠心肌组织损伤明显减轻,心肌纤维规则排列,胶原纤维积聚减少,CVF(2.85%±0.18%)减小(P<0.05);与circFAT1组(2.85%±0.18%)和circFAT1+激动剂-NC组(2.78%±0.19%)相比,circFAT1+miR-211-5p激动剂组大鼠心肌组织损伤加重,胶原纤维积聚,CVF(9.24%±0.58%)增大(P<0.05),见图 2。

|
| A, HE staining; B, Masson staining. 图 2 HE和Masson染色检测各组大鼠心肌组织病理形态和纤维化程度 ×200 Fig.2 HE and Masson staining were used to detect the pathological morphology and fibrosis degree, respectively, of myocardial tissue in each group ×200 |
2.6 circFAT1对各组大鼠心肌细胞凋亡的影响
与对照组(2.14%±0.15%)相比,DCM组心肌细胞凋亡率(14.87%±0.96%)升高(P<0.05);与DCM组(14.87%±0.96%)和circ-NC组(15.12%±0.87%)相比,circFAT1组心肌细胞凋亡率(4.68%±0.31%)降低(P<0.05);与circFAT1组(4.68%±0.31%)和circFAT1+激动剂-NC组(4.72%±0.33%)相比,circFAT1+miR-211-5p激动剂组心肌细胞凋亡率(13.69%±0.79%)升高(P<0.05),见图 3。
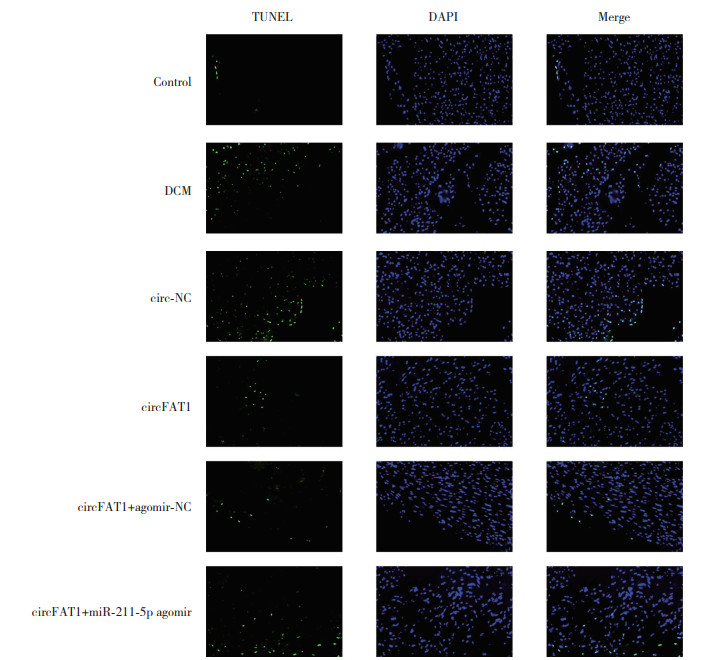
|
| 图 3 TUNEL染色检测各组大鼠心肌细胞凋亡 ×200 Fig.3 TUNEL staining was used to detect myocardial apoptosis in rats in each group ×200 |
2.7 circFAT1对各组大鼠心肌组织中IL-1β、IL-6、TNF-α水平的影响
与对照组相比,DCM组大鼠IL-1β、IL-6、TNF-α水平升高(P<0.05);与DCM组相比,circFAT1组大鼠IL-1β、IL-6、TNF-α水平降低(P<0.05);与circFAT1组相比,circFAT1+miR-211-5p激动剂组大鼠IL-1β、IL-6、TNF-α水平升高(P<0.05),见表 5。
| Group | IL-1β | IL-6 | TNF-α |
| Control | 18.65±1.33 | 21.49±1.63 | 96.83±7.85 |
| DCM | 45.82±3.611) | 86.75±5.211) | 218.96±15.691) |
| circ-NC | 44.76±3.541) | 85.49±5.171) | 214.47±15.281) |
| circFAT1 | 25.96±1.922) | 34.26±2.342) | 124.79±9.252) |
| circFAT1+agomir-NC | 26.13±2.052) | 35.40±2.452) | 127.34±9.672) |
| circFAT1+miR-211-5p agomir | 41.87±3.423) | 79.61±4.863) | 196.53±13.613) |
| 1)P<0.05 vs. control group;2)P<0.05 vs. DCM group;3)P<0.05 vs. circFAT1 group. n = 5. | |||
2.8 circFAT1、miR-211-5p和CCND2之间的靶向关系验证
ENCORI分析发现miR-211-5p与circFAT1、CCND2在3’UTR区域均存在靶向结合位点(图 4A)。双荧光素酶报告基因实验发现,转染circFAT1-WT或CCND2-WT后,共转染miR-211-5p mimic的细胞相对荧光素酶活性显著低于共转染miR-NC的细胞(P<0.05),而转染circFAT1-MUT或CCND2-MUT后,共转染miR-211-5p mimic的细胞与共转染miR-NC的细胞相对荧光素酶活性无统计学差异(P > 0.05),见图 4B、4C。

|
| A, targeted binding sites of miR-211-5p with circFAT1 and CCND2; B, C, the double luciferase reporter gene assay was used to analyze the targeting relationship between miR-211-5p and circFAT1, and miR-211-5p and CCND2. *P < 0.05 vs. miR-NC group. 图 4 circFAT1、miR-211-5p和CCND2之间的靶向关系验证 Fig.4 Verification of the targeting relationship among circFAT1, miR-211-5p, and CCND2 |
3 讨论
DCM是糖尿病的并发症,其心脏的结构改变和功能受损使心脏被迫重塑并减少心输出量,导致心力衰竭和死亡。由于缺乏早期症状和专业的治疗策略,DCM目前在诊断和治疗中有一定难度[12]。研究[13]发现有多种circRNA可有效改善DCM的早期诊断和治疗。circFAT1是一种新的circRNA,来源于FAT1基因的外显子2,在多种癌症的诊断和筛查中具有潜在作用[14]。然而,一项新的研究[5]发现,circFAT1在糖尿病视网膜病变患者中表达下调。因此,为了检测circFAT1在糖尿病其他并发症,本研究利用高糖高脂饮食联合STZ建立DCM大鼠模型,结果显示,DCM大鼠心肌组织中circFAT1的表达及心功能指标LVEF和LVFS水平低于对照组,而FBG、TC、TG、LVEDd、LVEDs水平高于对照组,表明circFAT1可能与DCM的发展有关。随后本研究通过注射携带circFAT1的慢病毒质粒干预DCM大鼠发现,circFAT1组FBG、TC、TG、LVEDd、LVEDs水平降低,LVEF和LVFS水平升高,提示circFAT1可以改善DCM大鼠血糖血脂水平及心脏功能。
心肌细胞肥大、凋亡和纤维化是DCM的3个主要特征,是发展为心力衰竭的主要标志[15]。此外,心肌炎症是DCM发展的关键过程,虽然心肌炎症本身是针对短期应激和异常状况恢复体内平衡的早期反应,但在持续的应激挑战下,会转变为不良的促炎反应[16]。本研究结果显示,circFAT1组大鼠心肌组织细胞肿胀减轻,胶原沉积减少,间质纤维化程度减轻,且细胞凋亡率以及炎性细胞因子IL-1β、IL-6、TNF-α水平较DCM组降低,提示circFAT1过表达可以抑制DCM大鼠心肌细胞肥大、凋亡和纤维化,进而抑制心肌炎症,减轻心肌组织损伤。
研究[11]发现,circRNA主要通过海绵吸附miRNA在基因表达和调控中发挥着重要作用,而miRNA通常直接结合靶mRNAs的3’-UTR区域来抑制转录后水平的基因表达。有研究[17]表明miR-211作为一种新的具有高度敏感性和特异性的生物标志物,可能与糖尿病视网膜病变的发生发展相关。抑制miR-211-5p可以通过靶向相关mRNA抑制高糖介导的细胞障碍。CCND2是细胞从G1期转移到S期所必需的细胞周期调节因子,并参与多种生理病理过程[18],其过表达可以增强人类诱导多能干细胞来源的心肌细胞的再生能力,改善左心室功能[19]。研究[19]已经证明了miR-211可以直接靶向CCND2执行其功能,但miR-211-5p与CCND2及circFAT1的靶向关系尚未见报道。本研究结果显示,DCM大鼠miR-211-5p水平升高,CCND2表达降低;而circFAT1干预后,miR-211-5p水平降低,CCND2表达升高,且双荧光素酶报告基因实验验证了miR-211-5p与circFAT1、CCND2之间均存在靶向关系。提示circFAT1可以靶向下调miR-211-5p,上调CCND2表达。为进一步证明miR-211-5p/CCND2轴的作用,本研究利用miR-211-5p激动剂进行回补实验,发现circFAT1对DCM心肌损伤的缓解作用被逆转,且CCND2的表达低于circFAT1组,提示circFAT1对DCM大鼠心肌组织损伤的抑制作用可能与miR-211-5p/CCND2轴有关。
综上所述,circFAT1可能通过调控miR-211-5p/CCND2轴减轻DCM大鼠心肌组织损伤。本研究为DCM的早期诊断和靶向治疗提供了新的生物标志物。但关于circFAT1在糖尿病及其他并发症中的研究甚少,还需进一步的探讨。
| [1] |
LIU CY, HAN YH, GU XM, et al. Paeonol promotes Opa1-mediated mitochondrial fusion via activating the CK2α-Stat3 pathway in diabetic cardiomyopathy[J]. Redox Biol, 2021, 46: 102098. DOI:10.1016/j.redox.2021.102098 |
| [2] |
MENG LP, LIN H, HUANG XX, et al. METTL14 suppresses pyroptosis and diabetic cardiomyopathy by downregulating TINCR lncRNA[J]. Cell Death Dis, 2022, 13(1): 38. DOI:10.1038/s41419-021-04484-z |
| [3] |
YUAN Q, SUN YW, YANG F, et al. CircRNA DICAR as a novel endogenous regulator for diabetic cardiomyopathy and diabetic pyroptosis of cardiomyocytes[J]. Signal Transduct Target Ther, 2023, 8(1): 99. DOI:10.1038/s41392-022-01306-2 |
| [4] |
JIANG J, GAO GN, PAN Q, et al. Circular RNA circHIPK3 is downregulated in diabetic cardiomyopathy and overexpression of circHIPK3 suppresses PTEN to protect cardiomyocytes from high glucose-induced cell apoptosis[J]. Bioengineered, 2022, 13(3): 6272-6279. DOI:10.1080/21655979.2022.2031395 |
| [5] |
PENG H, ZHANG W, DONG HH, et al. circFAT1 promotes lung adenocarcinoma progression by sequestering miR-7 from repressing IRS2-ERK-mediated CCND1 expression[J]. Int J Biol Sci, 2022, 18(10): 3944-3960. DOI:10.7150/ijbs.70889 |
| [6] |
HUANG CC, QI P, CUI H, et al. circFAT1 regulates retinal pigment epithelial cell pyroptosis and autophagy via mediating m6A reader protein YTHDF2 expression in diabetic retinopathy[J]. Exp Eye Res, 2022, 222: 109152. DOI:10.1016/j.exer.2022.109152 |
| [7] |
ZHANG YX, WANG HY, XIA Y. The expression of miR-211-5p in atherosclerosis and its influence on diagnosis and prognosis[J]. BMC Cardiovasc Disord, 2021, 21(1): 371. DOI:10.1186/s12872-021-02187-z |
| [8] |
TAO D, LIU ZY, WANG L, et al. CircPAG1 interacts with miR-211-5p to promote the E2F3 expression and inhibit the high glucose-induced cell apoptosis and oxidative stress in diabetic cataract[J]. Cell Cycle, 2022, 21(7): 708-719. DOI:10.1080/15384101.2021.2018213 |
| [9] |
NI TJ, HUANG XX, PAN SL, et al. Inhibition of the long non-coding RNA ZFAS1 attenuates ferroptosis by sponging miR-150-5p and activates CCND2 against diabetic cardiomyopathy[J]. J Cell Mol Med, 2021, 25(21): 9995-10007. DOI:10.1111/jcmm.16890 |
| [10] |
叶婷, 陈晶, 马国庆, 等. 黄芪多糖对糖尿病心肌病大鼠自噬的调控机制研究[J]. 时珍国医国药, 2022, 33(2): 312-316. DOI:10.3969/j.issn.1008-0805.2022.02.14 |
| [11] |
FU LF, ZHANG JY, LIN Z, et al. CircularRNA circ_0071269 knockdown protects against from diabetic cardiomyopathy injury by microRNA-145/gasdermin A axis[J]. Bioengineered, 2022, 13(2): 2398-2411. DOI:10.1080/21655979.2021.2024688 |
| [12] |
SUN SC, DAWUTI A, GONG DF, et al. Puerarin-V improve mitochondrial respiration and cardiac function in a rat model of diabetic cardiomyopathy via inhibiting pyroptosis pathway through P2X7 receptors[J]. Int J Mol Sci, 2022, 23(21): 13015. DOI:10.3390/ijms232113015 |
| [13] |
DONG SZ, TU CY, YE X, et al. Expression profiling of circular RNAs and their potential role in early-stage diabetic cardiomyopathy[J]. Mol Med Rep, 2020, 22(3): 1958-1968. DOI:10.3892/mmr.2020.11248 |
| [14] |
DONG WM, ZHANG HQ, DAI YC, et al. circRNA circFAT1 (e2) elevates the development of non-small-cell lung cancer by regulating miR-30e-5p and USP22[J]. Biomed Res Int, 2021, 2021: 6653387. DOI:10.1155/2021/6653387 |
| [15] |
LUO JR, YAN D, LI SS, et al. Allopurinol reduces oxidative stress and activates Nrf2/p62 to attenuate diabetic cardiomyopathy in rats[J]. J Cell Mol Med, 2020, 24(2): 1760-1773. DOI:10.1111/jcmm.14870 |
| [16] |
FILARDI T, GHINASSI B, DI BALDASSARRE A, et al. Cardiomyopathy associated with diabetes: the central role of the cardiomyocyte[J]. Int J Mol Sci, 2019, 20(13): 3299. DOI:10.3390/ijms20133299 |
| [17] |
LIU HN, CAO NJ, LI X, et al. Serum microRNA-211 as a biomarker for diabetic retinopathy via modulating Sirtuin 1[J]. Biochem Biophys Res Commun, 2018, 505(4): 1236-1243. DOI:10.1016/j.bbrc.2018.10.052 |
| [18] |
MA Y, SHAN ZF, LIU Y, et al. CircTHBS1 targeting miR-211/CCND2 pathway to promote cell proliferation and migration potential in primary cystitis glandularis cells[J]. Biosci Rep, 2021, 41(8): BSR20201164. DOI:10.1042/BSR20201164 |
| [19] |
ZHU WQ, ZHAO M, MATTAPALLY S, et al. CCND2 overexpression enhances the regenerative potency of human induced pluripotent stem cell-derived cardiomyocytes: remuscularization of injured ventricle[J]. Circ Res, 2018, 122(1): 88-96. DOI:10.1161/CIRCRESAHA.117.311504 |
 2024, Vol. 53
2024, Vol. 53




