文章信息
- 谭冰, 方凌燕, 陈明华, 曾巧莉, 郭润民
- TAN Bing, FANG Lingyan, CHEN Minghua, ZENG Qiaoli, GUO Runmin
- 长链非编码RNA FGD5-AS1调节miR-195-5p/PIM1轴对高糖诱导的心肌细胞损伤的影响
- Effect of long non-coding RNA FGD5-AS1 on high glucose-induced myocardial cell injury through regulation of the miR-195-5p/PIM1 axis
- 中国医科大学学报, 2024, 53(6): 487-494
- Journal of China Medical University, 2024, 53(6): 487-494
-
文章历史
- 收稿日期:2023-09-08
- 网络出版时间:2024-05-31 11:20:56
2. 广东医科大学附属第二医院心血管科,广东 湛江 524000;
3. 广东医科大学附属第二医院神经内科,广东 湛江 524000;
4. 顺德妇女儿童医院,佛山市顺德区妇幼保健院科教科,广东 佛山 528300
2. Department of Cardiology, The Second Affiliated Hospital of Guangdong Medical University, Zhanjiang 524000, China;
3. Department of Neurology, The Second Affiliated Hospital of Guangdong Medical University, Zhanjiang 524000, China;
4. Department of Science and Education, Women and Children's Hospital of Guangdong Medical University, Maternity and Child Healthcare Hospital of Shunde Foshan, Foshan 528300, China
糖尿病心肌病(diabetes cardiomyopathy,DCM)是糖尿病的一种严重的心血管并发症[1-2]。高血糖引发的心肌细胞损伤是DCM重要的病理基础之一,凋亡信号的激活和炎症是造成心肌细胞损伤的主要致病因素,抑制高糖诱导的炎症及心肌细胞凋亡可有效治疗DCM[3-4]。长链非编码RNA FGD5反义RNA1(long non-coding RNA FGD5 antisense RNA1,lncRNA FGD5-AS1)是心肌梗死及糖尿病相关心血管疾病的重要调控因子,在2型糖尿病和心血管疾病患者体内其水平呈逐步下降趋势,上调其表达可促使高糖诱导的人心肌细胞生长并减轻其凋亡[5]。miR-195-5p可被lncRNA FGD5-AS1靶向下调,参与抑制lncRNA FGD5-AS1过表达对缺氧诱导的人心肌细胞的损伤[6]。miR-195-5p在DCM大鼠心肌中高度表达,抑制miR-195-5p可改善其心功能障碍[7];Pim-1原癌基因(Pim-1 proto-oncogene,PIM1)作为重要的炎症调节因子,在炎症反应中表达降低,过表达PIM1可减轻脂多糖诱导的肺上皮细胞凋亡与炎症损伤[8],通过减少DCM大鼠心肌细胞凋亡改善其心功能[9]。检索starBase数据库发现lncRNA FGD5-AS1与miR-195-5p间、miR-195-5p与PIM1间存在结合位点,故推测lncRNA FGD5-AS1可能通过调节miR-195-5p/PIM1轴减轻高糖诱导的心肌细胞损伤。本研究拟通过体外培养大鼠心肌细胞H9c2及人心肌细胞并建立其DCM细胞模型,验证此推测。
1 材料与方法 1.1 材料 1.1.1 细胞及试剂大鼠心肌细胞H9c2、人心肌细胞(武汉普诺赛生命科技有限公司);葡萄糖[纯度99%,批号20190314,赛恩科实验器材(济南)有限公司];lncRNA FGD5-AS1过表达质粒、miR-195-5p inhibitor、mimics及其阴性对照(上海吉凯基因医学科技股份有限公司);高灵敏通用型实时定量PCR(real-time quantitative PCR,RT-qPCR)预混液、快速逆转录试剂盒[翌圣生物科技(上海)股份有限公司];Andy Fluor™ 594标记EdU细胞增殖分析试剂盒(广州复能基因有限公司);乳酸脱氢酶(lactate dehydrogenase,LDH)活性检测试剂盒、双荧光素酶报告基因检测试剂盒、大鼠及人肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)酶联免疫吸附测定(enzyme linked immunosorbent assay,ELISA)试剂盒、大鼠及人白细胞介素-6(interleukin 6,IL-6)ELISA试剂盒、MTT试剂盒(北京索莱宝科技有限公司);TUNEL检测试剂盒、HRP标记大鼠抗兔二抗、兔抗大鼠anti-PIM1、Bax、Bcl-2、β-actin一抗、兔抗人anti-PIM1、Bax、Bcl-2、β-actin一抗(美国Abcam公司)。
1.1.2 主要实验仪器RT-qPCR仪(型号Z-genesig-q32,广州点成生物有限公司);全自动酶标分析仪(型号UMR-9600,杭州优米仪器有限公司);倒置荧光显微镜(型号MF53-N,广州市明美光电技术有限公司);小型垂直电泳套装(型号165-8033,美国Bio-Rad公司);微孔板发光检测仪(型号GloMax 96,北京原平皓生物技术有限公司)。
1.2 方法 1.2.1 构建DCM体外细胞模型并分组处理快速解冻复苏H9c2细胞、人心肌细胞,传代后接种于24孔板培养,随机分为对照组、模型组、lncRNA FGD5-AS1过表达组、miR-195-5p inhibitor组、阴性对照组、lncRNA FGD5-AS1过表达+miR-195-5p mimics组,对照组细胞以含5.5 mmol/L葡萄糖的培养基正常培养,除对照组外的各组细胞均以含30 mmol/L葡萄糖的培养基培养,以诱导建立DCM体外细胞模型[10-11]。同时采用脂质体3000试剂进行分组转染:lncRNA FGD5-AS1过表达组转染lncRNA FGD5-AS1过表达质粒,miR-195-5p inhibitor组转染miR-195-5p inhibitor,阴性对照组转染空载质粒和miR-195-5p阴性对照,lncRNA FGD5-AS1过表达+miR-195-5p mimics组转染lncRNA FGD5-AS1过表达质粒和miR-195-5p mimics,对照组和模型组细胞以等量脂质体3000试剂处理,各组细胞均处理24 h后进行后续实验。
1.2.2 检测各组细胞lncRNA FGD5-AS1、miR-195-5p和PIM1表达提取各组细胞总RNA,反转录获得模板DNA,与高灵敏通用型RT-qPCR预混液、基因引物混匀,行qPCR反应,β-actin用于lncRNA FGD5-AS1、PIM1内参照,U6用于miR-195-5p内参照。用2-ΔΔCt算法分析得到其相对表达,引物序列见表 1。
| Gene | Sequence(5’-3’) |
| lncRNA FGD5-AS1 | |
| F | CGTGGAGAAGAATTGGGC |
| R | CGTGGAGAAGAATTGGGC |
| PIM1 | |
| F | TCTCAGGGACAGGCACCATT |
| R | GCGGCGAAATCAAACTCATC |
| β-actin | |
| F | GCGCGGCTACAGCTTCA |
| R | CTTAATGTCACGCACGATTTCC |
| miR-195-5p | |
| F | GGGGTAGCAGCACAGAAAT |
| R | TCCAGTGCGTGTCGTGGA |
| U6 | |
| F | CTCGCTTCGGCAGCACA |
| R | AACGCTTCACGAATTTGCGT |
1.2.3 检测各组细胞增殖情况
(1)MTT法,将H9c2及人心肌细胞分别传代后接种于96孔板,按照1.2.2方法分组处理后,以MTT工作液孵育2 h后测定各组细胞吸光值,计算细胞存活率,细胞存活率(%)=实验处理组细胞吸光度值/对照组细胞吸光度值×100。(2)Edu染色,以Edu试剂孵育1.2.2中分组处理后的各组细胞3.5 h,行Edu染色,洗涤后行DAPI染色,以荧光显微镜采集各组细胞图像,用ImageJ软件定量各组细胞Edu阳性数与总数,计算细胞增殖率,细胞增殖率(%)=Edu阳性细胞数/细胞总数×100。
1.2.4 检测各组细胞凋亡情况以TUNEL染色试剂孵育1.2.2中分组处理后的各组H9c2及人心肌细胞,10%甲醛固定,DAPI染色,荧光显微镜下采集各组细胞图像,用Image J软件定量各组细胞凋亡(即TUNEL阳性)数与总数,计算细胞凋亡率,细胞凋亡率(%)=细胞凋亡数/细胞总数×100。
1.2.5 检测各组细胞LDH及炎性细胞因子TNF-α、IL-6表达水平收集1.2.2中分组处理后的各组H9c2及人心肌细胞培养液,4 ℃、1 000 r/min离心10 min,采用LDH活性检测试剂盒检测上清中LDH活性水平,采用ELISA试剂盒检测TNF-α及IL-6水平。
1.2.6 免疫印迹法检测各组H9c2细胞PIM1和凋亡相关蛋白表达提取1.2.2中分组处理后的各组H9c2及人心肌细胞总蛋白,测定其浓度后变性,每组取20 µg上样电泳,转膜,蛋白封闭后剪下待测蛋白PIM1、Bax、Bcl-2和内参蛋白β-actin,加入对应一抗、二抗孵育,显色,拍照,用Image Pro Plus 6.0软件量化各组蛋白灰度值,计算相对表达水平,相对表达水平=待测蛋白灰度值/内参蛋白灰度值。
1.2.7 双荧光素酶报告基因实验验证H9c2和人心肌细胞lncRNA FGD5-AS1对miR-195-5p、miR-195-5p对PIM1的靶向调控H9c2及人心肌细胞分别传代后接种至96孔板,将野生型、突变型miR-195-5p分别和空载质粒、lncRNA FGD5-AS1过表达质粒共转染到H9c2和人心肌细胞中,野生型、突变型PIM1 3’-UTR报告质粒分别和miR-195-5p mimics及其阴性对照共转染至H9c2和人心肌细胞中,24 h后收集各组细胞,以双荧光素酶报告基因检测试剂盒于微孔板发光检测仪中测定各组双荧光素酶相对活性。
1.3 统计学分析采用SPSS 24.0软件进行统计分析。所有数据均用x±s表示,采用单因素方差分析进行多组间差异比较,采用SNK-q检验进一步比较2组间差异。P < 0.05为差异有统计学意义。
2 结果 2.1 各组H9c2和人心肌细胞lncRNA FGD5-AS1、miR-195-5p、PIM1表达水平比较与对照组相比,模型组细胞lncRNA FGD5-AS1、PIM1 mRNA表达降低,miR-195-5p表达升高(均P < 0.05)。与模型组相比,lncRNA FGD5-AS1过表达组细胞lnc-RNA FGD5-AS1、PIM1 mRNA表达升高,miR-195-5p表达降低(均P < 0.05);miR-195-5p inhibitor组细胞lncRNA FGD5-AS1表达无统计学差异(P > 0.05),PIM1 mRNA表达升高(P < 0.05),miR-195-5p表达降低(P < 0.05);阴性对照组细胞lncRNA FGD5-AS1、miR-195-5p、PIM1 mRNA表达无统计学差异(P > 0.05)。与lnc-RNA FGD5-AS1过表达组相比,lncRNA FGD5-AS1过表达+miR-195-5p mimics组细胞lncRNA FGD5-AS1表达无统计学差异(P > 0.05),miR-195-5p表达升高(P < 0.05),PIM1 mRNA表达降低(P < 0.05)。见表 2。
| Group | H9c2 cells | Human cardiomyocytes | |||||
| lncRNA FGD5-AS1/GAPDH | miR-195-5p/U6 | PIM1/GAPDH | lncRNA FGD5-AS1/GAPDH | miR-195-5p/U6 | PIM1/GAPDH | ||
| Control | 1.00±0.14 | 0.98±0.16 | 0.99±0.15 | 1.01±0.17 | 1.02±0.16 | 0.97±0.15 | |
| Model | 0.34±0.111) | 2.32±0.201) | 0.30±0.101) | 0.37±0.121) | 2.30±0.211) | 0.35±0.111) | |
| lncRNA FGD5-AS1 overexpression | 0.95±0.132) | 1.06±0.182) | 0.93±0.132) | 0.96±0.162) | 1.10±0.182) | 0.92±0.132) | |
| miR-195-5p inhibitor | 0.36±0.12 | 1.02±0.172) | 0.96±0.142) | 0.39±0.13 | 1.07±0.172) | 0.94±0.142) | |
| Negative control | 0.32±0.10 | 2.35±0.21 | 0.29±0.09 | 0.35±0.11 | 2.32±0.20 | 0.34±0.10 | |
| lncRNA FGD5-AS1-over-expression+miR-195-5p mimics | 0.97±0.152) | 2.27±0.193) | 0.33±0.113) | 0.94±0.142) | 2.25±0.193) | 0.38±0.123) | |
| 1)P<0.05 vs. control group;2)P<0.05 vs. model group;3)P<0.05 vs. the lncRNA FGD5-AS1 overexpression group. n = 6. | |||||||
2.2 各组H9c2细胞和人心肌细胞增殖、损伤情况
与对照组比较,模型组H9c2和人心肌细胞存活率、增殖率降低(P < 0.05)。与模型组比较,lncRNA FGD5-AS1过表达组、miR-195-5p inhibitor组H9c2和人心肌细胞存活率、增殖率升高(P < 0.05),阴性对照组H9c2和人心肌细胞存活率、增殖率无统计学差异(P > 0.05)。与lncRNA FGD5-AS1过表达组比较,lnc-RNA FGD5-AS1过表达+miR-195-5p mimics组H9c2和人心肌细胞存活率、增殖率降低(P < 0.05)。见图 1、图 2、表 3。
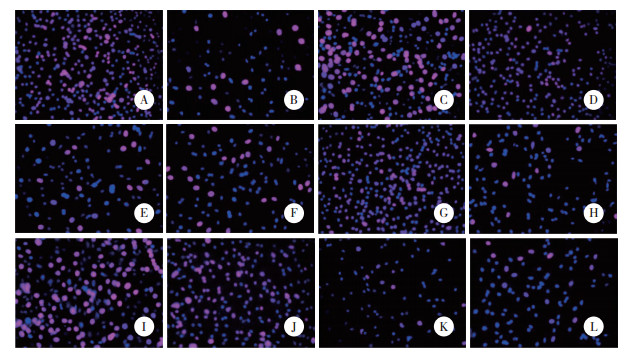
|
| A to F, H9c2 cells; G to L, human cardiomyocytes; A, G, control group; B, H, model group; C, I, lncRNA FGD5-AS1 overexpression group; D, J, miR-195-5p inhibitor group; E, K, negative control group; F, L, lncRNA FGD5-AS1 overexpression+miR-195-5p mimics group. 图 1 Edu染色检测H9c2和人心肌细胞增殖 ×200 Fig.1 Edu staining detection of H9c2 cells and human cardiomyocytes proliferation ×200 |
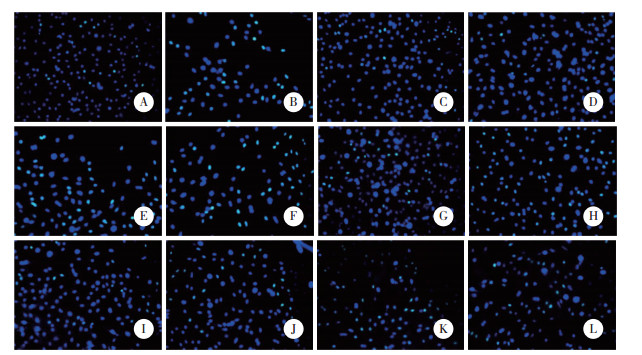
|
| A to F, H9c2 cells; G to L, human cardiomyocytes; A, G, control group; B, H, model group; C, I, lncRNA FGD5-AS1 overexpression group; D, J, miR-195-5p inhibitor group; E, K, negative control group; F, L, lncRNA FGD5-AS1 overexpression+miR-195-5p mimics group. 图 2 TUNEL染色检测H9c2和人心肌细胞凋亡 ×200 Fig.2 TUNEL staining detection of H9c2 and human myocardial cell apoptosis ×200 |
| Item | Control | Model | lncRNA FGD5-AS1 overexpression | miR-195-5p inhibitor | Negative control | lncRNA FGD5-AS1 overexpression+ miR-195-5p mimics |
| H9c2 cells | ||||||
| Survival rate(%) | 100.00±19.11 | 51.20±13.521) | 87.93±15.232) | 93.04±14.412) | 48.15±13.70 | 59.42±14.843) |
| Proliferation rate(%) | 69.85±9.61 | 15.46±5.121) | 57.19±9.302) | 63.78±8.942) | 15.32±5.08 | 20.53±6.143) |
| LDH(U/L) | 186.34±39.15 | 619.25±45.281) | 199.36±37.432) | 192.01±38.162) | 625.62±43.51 | 608.17±42.943) |
| Apoptosis rate(%) | 8.91±2.66 | 44.67±4.931) | 13.02±3.302) | 10.95±3.422) | 47.28±5.03 | 38.23±4.623) |
| Human cardiomyocytes | ||||||
| Survival rate(%) | 100.00±17.05 | 49.10±12.971) | 86.95±16.832) | 91.50±15.362) | 47.82±13.20 | 54.06±14.183) |
| Proliferation rate(%) | 63.21±8.74 | 12.15±4.031) | 51.98±7.752) | 46.02±6.922) | 11.96±3.93 | 15.03±4.873) |
| LDH(U/L) | 195.63±37.52 | 637.41±42.391) | 210.02±38.142) | 202.15±35.972) | 651.04±43.45 | 628.10±40.743) |
| Apoptosis rate(%) | 9.10±2.93 | 49.36±5.101) | 14.12±4.022) | 12.07±3.842) | 54.85±5.21 | 42.78±4.223) |
| 1)P<0.05 vs. control group;2)P<0.05 vs. model group;3)P<0.05 vs. lncRNA FGD5-AS1 overexpression group. n = 6. | ||||||
2.3 各组H9c2细胞和人心肌细胞炎性细胞因子表达水平比较
与对照组比较,模型组H9c2和人心肌细胞TNF-α、IL-6水平升高(P < 0.05)。与模型组比较,lncRNA FGD5-AS1过表达组、miR-195-5p inhibitor组H9c2和人心肌细胞TNF-α、IL-6表达水平降低(P < 0.05),阴性对照组H9c2和人心肌细胞TNF-α、IL-6表达水平无统计学差异(P > 0.05)。与lncRNA FGD5-AS1过表达组比较,lncRNA FGD5-AS1过表达+miR-195-5p mimics组H9c2和人心肌细胞TNF-α、IL-6表达水平降低(P < 0.05)。见表 4。
| Group | H9c2 cells | Human cardiomyocytes | |||
| TNF-α | IL-6 | TNF-α | IL-6 | ||
| Control | 58.25±11.71 | 44.17±10.64 | 62.24±12.50 | 51.32±11.22 | |
| Model | 146.82±14.191) | 131.48±13.271) | 158.46±16.111) | 143.64±14.861) | |
| lncRNA FGD5-AS1 overexpression | 68.01±12.202) | 52.03±10.332) | 70.86±13.042) | 60.89±10.282) | |
| miR-195-5p inhibitor | 63.59±13.162) | 48.92±11.152) | 66.02±11.872) | 55.32±10.152) | |
| Negative control | 150.23±15.04 | 134.75±13.41 | 163.11±15.96 | 149.24±15.03 | |
| lncRNA FGD5-AS1 overexpression+miR-195-5p mimics | 137.98±13.433) | 123.84±12.823) | 150.32±14.613) | 135.95±13.763) | |
| 1)P<0.05 vs. control group;2)P<0.05 vs. model group;3)P<0.05 vs. lncRNA FGD5-AS1 overexpression group. n = 6. | |||||
2.4 各组H9c2细胞和人心肌细胞PIM1及凋亡相关蛋白表达水平比较
与对照组比较,模型组H9c2和人心肌细胞PIM1、Bcl-2蛋白表达降低,Bax蛋白表达升高(均P < 0.05)。与模型组比较,lncRNA FGD5-AS1过表达组、miR-195-5p inhibitor组H9c2和人心肌细胞PIM1、Bcl-2蛋白表达升高,Bax蛋白表达降低(均P < 0.05),阴性对照组H9c2和人心肌细胞PIM1、Bcl-2、Bax蛋白表达无统计学差异(均P > 0.05)。与lncRNA FGD5-AS1过表达组比较,lncRNA FGD5-AS1过表达+miR-195-5p mimics组H9c2和人心肌细胞PIM1、Bcl-2蛋白表达降低,Bax蛋白表达升高(均P < 0.05)。见图 3、表 5。
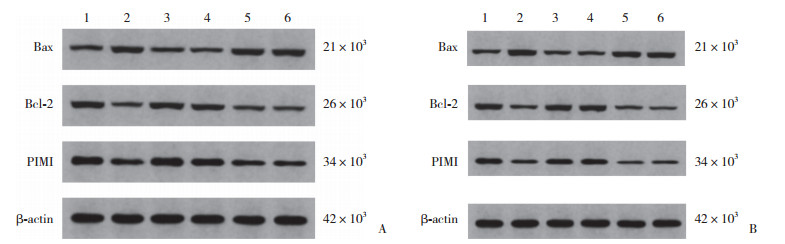
|
| A, H9c2 cells; B, human cardiomyocytes. 1, control group; 2, model group; 3, lncRNA FGD5-AS1 overexpression group; 4, miR-195-5p inhibitor group; 5, negative control group; 6, lncRNA FGD5-AS1 overexpression+miR-195-5p mimics group. 图 3 免疫印迹法检测H9c2和人心肌细胞PIM1、Bax、Bcl-2蛋白表达 Fig.3 Immunoblotting assay for detecting the expression of PIM1, Bax, and Bcl-2 proteins in H9c2 cells and human cardiomyocytes |
| Group | H9c2 cells | Human cardiomyocytes | |||||
| PIM1/β-actin | Bax/β-actin | Bcl-2/β-actin | PIM1/β-actin | Bax/β-actin | Bcl-2/β-actin | ||
| Control | 0.93±0.16 | 0.29±0.07 | 0.76±0.13 | 0.71±0.09 | 0.31±0.08 | 0.83±0.15 | |
| Model | 0.41±0.121) | 0.63±0.101) | 0.26±0.081) | 0.19±0.061) | 0.67±0.111) | 0.39±0.111) | |
| lncRNA FGD5-AS1 overexpression | 0.87±0.142) | 0.33±0.082) | 0.71±0.102) | 0.66±0.082) | 0.35±0.092) | 0.78±0.132) | |
| miR-195-5p inhibitor | 0.89±0.152) | 0.31±0.092) | 0.73±0.112) | 0.67±0.102) | 0.33±0.072) | 0.79±0.142) | |
| Negative control | 0.40±0.11 | 0.65±0.12 | 0.27±0.09 | 0.17±0.05 | 0.69±0.12 | 0.38±0.12 | |
| lncRNA FGD5-AS1 overexpression+miR-195-5p mimics | 0.45±0.133) | 0.59±0.113) | 0.28±0.093) | 0.21±0.073) | 0.64±0.133) | 0.41±0.133) | |
| 1)P<0.05 vs. control group;2)P<0.05 vs. model group;3)P<0.05 vs. lncRNA FGD5-AS1 overexpression group. n = 6. | |||||||
2.5 lncRNA FGD5-AS1对H9c2和人心肌细胞miR-195-5p、miR-195-5p靶向调节PIM1的影响
通过检索starBase数据库获得lncRNA FGD5-AS1与miR-195-5p间、miR-195-5p与PIM1间结合位点,见图 4。与野生型miR-195-5p+空载组比较,野生miR-195-5p+lncRNA FGD5-AS1过表达组H9c2和人心肌细胞相对荧光素酶活性显著降低(P < 0.05);突变型miR-195-5p+空载组与突变型miR-195-5p+lncRNA FGD5-AS1过表达组H9c2和人心肌细胞相对荧光素酶活性无统计学差异(P > 0.05)。与野生型PIM1+miR-195-5p mimics阴性对照组比较,野生型PIM1+miR-195-5p mimics组H9c2和人心肌细胞相对荧光素酶活性显著降低(P < 0.05);突变型PIM1+miR-195-5p mimics阴性对照组与突变型PIM1+miR-195-5p mimics组H9c2和人心肌细胞间相对荧光素酶活性无统计学差异(P > 0.05)。见表 6。

|
| 图 4 lncRNA FGD5-AS1与miR-195-5p间、miR-195-5p与PIM1间结合位点 Fig.4 Binding sites between lncRNA FGD5-AS1 and miR-195-5p, as well as between miR-195-5p and PIM1 |
| Group | H9c2 cells | Human cardiomyocytes |
| Wild miR-195-5p+empty load group | 1.01±0.13 | 1.00±0.11 |
| Wild miR-195-5p+lncRNA FGD5-AS1 overexpression group | 0.30±0.091) | 0.27±0.081) |
| Mutation miR-195-5p+no-load group | 1.02±0.14 | 0.97±0.10 |
| Mutated miR-195-5p+lncRNA FGD5-AS1 overexpression group | 1.03±0.15 | 0.98±0.12 |
| Wild PIM1+miR-195-5p mimics negative control group | 0.98±0.13 | 0.99±0.15 |
| Wild PIM1+miR-195-5p mimics group | 0.24±0.072) | 0.32±0.102) |
| Mutation PIM1+miR-195-5p mimics negative control group | 0.99±0.14 | 1.00±0.14 |
| Mutation PIM1+miR-195-5p mimics group | 0.97±0.12 | 1.01±0.11 |
| 1)P<0.05 vs. wild miR-195-5p+empty load group;2)P<0.05 vs. wild PIM1+miR-195-5p mimics negative control group. | ||
3 讨论
我国糖尿病发病率呈不断上升趋势,所引发的心血管并发症DCM的发病率和致死率也持续上升,已成为严重的公共卫生问题之一,且迄今为止还没有针对DCM的特异性治疗方法,因此深入探讨DCM致病机制并寻找更有效的新型治疗手段具有重大意义[12-14]。本研究以30 mmol/L葡萄糖诱导建立DCM体外细胞模型,结果显示,以含30 mmol/L葡萄糖的培养基培养大鼠心肌细胞H9c2及人心肌细胞,均可诱导炎性细胞因子TNF-α、IL-6过量表达,引发严重细胞炎症,进而降低2种细胞的存活率、增殖率,并提高其凋亡率、LDH及Bax表达水平,导致细胞严重损伤并大量凋亡,表明DCM体外细胞模型构建成功。
lncRNA FGD5-AS1是调控炎症与细胞凋亡、生长的一种非编码RNA,在DCM、心肌梗死等疾病中发挥重要调控作用,其过表达可减少缺氧/复氧诱导的心肌细胞凋亡和炎症,并改善其心功能[15-16],还能抑制高糖诱导的人心肌细胞凋亡并促进其存活[5],因此,lncRNA FGD5-AS1可能成为重要的DCM治疗靶点。本研究结果显示,转染lncRNA FGD5-AS1过表达质粒增强H9c2及人心肌细胞lncRNA FGD5-AS1表达,可提高细胞存活率、增殖率、Bcl-2蛋白表达水平,降低细胞凋亡率、LDH及TNF-α、IL-6、Bax水平,表明过表达lncRNA FGD5-AS1可减少促炎性细胞因子产生释放,阻碍炎症反应发生发展,进而抑制高糖诱导的心肌细胞损伤凋亡,并促进其存活生长,发挥细胞保护作用,提示lncRNA FGD5-AS1在DCM的临床治疗中可能具有良好的研发前景。
miR-195-5p作为一种重要的炎性细胞因子,在糖尿病及其并发症中具有重要的调控作用。高血糖可诱导miR-195-5p高表达,引发妊娠期糖尿病小鼠后代心脏发育障碍[17]。研究[18]显示,糖尿病足溃疡伤口液中含有miR-195-5p,可对患者血管生成和伤口愈合发挥抑制作用。PIM1是一种心脏保护激酶,可通过维持端粒长度拮抗心肌细胞衰老[19],增强PIM1的表达和活性,抑制G蛋白偶联受体激动剂诱导的新生大鼠心室心肌细胞肥大和肌节功能受损,并改善压力超负荷诱导的小鼠心功能障碍[20],而CAI等[6]的研究表明lncRNA FGD5-AS1可通过靶向下调miR-195-5p表达,减轻缺氧诱导的人心肌细胞凋亡。生物信息学分析预知lncRNA FGD5-AS1可能通过miR-195-5p调控PIM1表达,因此推测lncRNA FGD5-AS1减轻高糖诱导的心肌细胞损伤的分子机制可能是调节miR-195-5p/PIM1轴。本研究结果显示,过表达lncRNA FGD5-AS1可上调H9c2和人心肌细胞PIM1 mRNA及蛋白表达水平,降低miR-195-5p表达水平;miR-195-5p inhibitor的作用与lncRNA FGD5-AS1过表达质粒相似,亦可减轻高糖诱导的心肌细胞炎症损伤及凋亡,双荧光素酶报告基因实验证实了H9c2及人心肌细胞内lncRNA FGD5-AS1可靶向下调miR-195-5p表达,而miR-195-5p可靶向下调PIM1表达,表明miR-195-5p/PIM1轴参与介导lncRNA FGD5-AS1对高糖诱导的心肌细胞炎症损伤及凋亡的抑制过程;联合转染过表达质粒和miR-195-5p mimics可减弱单独转染lncRNA FGD5-AS1过表达质粒对高糖诱导心肌细胞的抗炎作用,拮抗其对心肌细胞损伤凋亡的抑制作用,逆转其对心肌细胞的保护作用,揭示lncRNA FGD5-AS1抑制高糖诱导的心肌细胞损伤及凋亡可能通过上调miR-195-5p实现。
综上所述,lncRNA FGD5-AS1可通过调节miR-195-5p/PIM1轴介导DCM的发生和发展,过表达lnc-RNA FGD5-AS1可通过下调miR-195-5p而提升PIM1表达水平,进而减少炎性细胞因子产生释放,抑制炎症发生与进展,减轻高糖诱导的心肌细胞损伤及凋亡,对心肌细胞发挥保护作用。本研究为深入阐释DCM发病机制提供了新的思路和依据,并发现了新型诊断和治疗靶点。
| [1] |
KE J, PAN J, LIN H, et al. Diabetic cardiomyopathy: a brief summary on lipid toxicity[J]. ESC Heart Fail, 2023, 10(2): 776-790. DOI:10.1002/ehf2.14224 |
| [2] |
KETENCI M, ZABLOCKI D, SADOSHIMA J. Mitochondrial quality control mechanisms during diabetic cardiomyopathy[J]. JMA J, 2022, 5(4): 407-415. DOI:10.31662/jmaj.2022-0155 |
| [3] |
WANG Y, CAI F, LI G, et al. Novel dual glucagon-like peptide-1/glucose-dependent insulinotropic polypeptide receptor agonist attenuates diabetes and myocardial injury through inhibiting hyperglycemia, inflammation and oxidative stress in rodent animals[J]. Bioengineered, 2022, 13(4): 9184-9196. DOI:10.1080/21655979.2022.2051859 |
| [4] |
ZUO Y, XIAO T, QIU XD, et al. Adiponectin reduces apoptosis of diabetic cardiomyocytes by regulating miR-711/TLR4 axis[J]. Diabetol Metab Syndr, 2022, 14(1): 131. DOI:10.1186/s13098-022-00904-y |
| [5] |
WANG YD, WANG J. Diagnostic significance of serum FGD5-AS1 and its predictive value for the development of cardiovascular disea-ses in patients with type 2 diabetes[J]. Diabetol Metab Syndr, 2022, 14(1): 20-29. DOI:10.1186/s13098-022-00789-x |
| [6] |
CAI XY, ZHANG P, WANG S, et al. lncRNA FGD5 antisense RNA 1 upregulates RORA to suppress hypoxic injury of human cardiomyocyte cells by inhibiting oxidative stress and apoptosis via miR-195[J]. Mol Med Rep, 2020, 22(6): 4579-4588. DOI:10.3892/mmr.2020.11558 |
| [7] |
DING HS, YAO JH, XIE HX, et al. MicroRNA-195-5p downregulation inhibits endothelial mesenchymal transition and myocardial fibrosis in diabetic cardiomyopathy by targeting Smad7 and inhibiting transforming growth factor beta 1-smads-snail pathway[J]. Front Physiol, 2021, 12: 709123-709137. DOI:10.3389/fphys.2021.709123 |
| [8] |
YANG J, CHEN X. SIRT6 attenuates LPS-induced inflammation and apoptosis of lung epithelial cells in acute lung injury through ACE2/STAT3/PIM1 signaling[J]. Immun Inflamm Dis, 2023, 11(3): e809-e820. DOI:10.1002/iid3.809 |
| [9] |
XIA XF, LIANG Y, ZHENG WH, et al. MiR-410-5p promotes the development of diabetic cardiomyopathy by suppressing PIM1-induced anti-apoptosis[J]. Mol Cell Probes, 2020, 52: 101558. DOI:10.1016/j.mcp.2020.101558 |
| [10] |
杜美玲, 王晓元, 李会贤, 等. circ_0031739通过下调miR-98-5p促进高糖诱导的心肌细胞损伤[J]. 中国老年学杂志, 2022, 42(23): 5774-5778. DOI:10.3969/j.issn.1005-9202.2022.23.029 |
| [11] |
高莉晶, 李婷, 原丽, 等. 刺芒柄花素对高糖诱导的H902心肌细胞损伤的作用机制[J]. 中国临床药理学杂志, 2022, 38(23): 2820-2824. DOI:10.13699/j.cnki.1001-6821.2022.23.007 |
| [12] |
LORENZO-ALMORÓS A, CEPEDA-RODRIGO JM, LORENZO Ó. Diabetic cardiomyopathy[J]. Rev Clin Esp (Barc), 2022, 222(2): 100-111. DOI:10.1016/j.rceng.2019.10.012 |
| [13] |
PENG C, ZHANG YX, LANG XY, et al. Role of mitochondrial metabolic disorder and immune infiltration in diabetic cardiomyopathy: new insights from bioinformatics analysis[J]. J Transl Med, 2023, 21(1): 66-83. DOI:10.1186/s12967-023-03928-8 |
| [14] |
CAI C, WU F, HE J, et al. Mitochondrial quality control in diabetic cardiomyopathy: from molecular mechanisms to therapeutic strategies[J]. Int J Biol Sci, 2022, 18(14): 5276-5290. DOI:10.7150/ijbs.75402 |
| [15] |
ZHAO Y, WANG CC, CUI TJ, et al. LncRNA FGD5-AS1 reduces cardiomyocyte apoptosis and inflammation by modulating Akt and miR-223-3p expression[J]. Am J Transl Res, 2022, 14(9): 6175-6186. |
| [16] |
HAO L, WANG J, BI SJ, et al. Upregulation of long noncoding RNA FGD5-AS1 ameliorates myocardial ischemia/reperfusion injury via microRNA-106a-5p and microRNA-106b-5p[J]. J Cardiovasc Pharmacol, 2021, 78(1): e45-e54. DOI:10.1097/fjc.0000000000001036 |
| [17] |
HE LK, WANG XT, JIN Y, et al. Identification and validation of the miRNA-mRNA regulatory network in fetoplacental arterial endothelial cells of gestational diabetes mellitus[J]. Bioengineered, 2021, 12(1): 3503-3515. DOI:10.1080/21655979.2021.1950279 |
| [18] |
LIU J, WANG JH, FU W, et al. MiR-195-5p and miR-205-5p in extracellular vesicles isolated from diabetic foot ulcer wound fluid decrease angiogenesis by inhibiting VEGFA expression[J]. Aging, 2021, 13(15): 19805-19821. DOI:10.18632/aging.203393 |
| [19] |
EBEID DE, KHALAFALLA FG, BROUGHTON KM, et al. Pim1 maintains telomere length in mouse cardiomyocytes by inhibiting TGFβ signalling[J]. Cardiovasc Res, 2021, 117(1): 201-211. DOI:10.1093/cvr/cvaa066 |
| [20] |
KORF-KLINGEBIEL M, REBOLL MR, POLTEN F, et al. Myeloid-derived growth factor protects against pressure overload-induced heart failure by preserving sarco/endoplasmic reticulum Ca2+-ATPase expression in cardiomyocytes[J]. Circulation, 2021, 144(15): 1227-1240. DOI:10.1161/circulationaha.120.053365 |
 2024, Vol. 53
2024, Vol. 53




