文章信息
- 肖雯, 田源, 蒋宇, 陈芳
- XIAO Wen, TIAN Yuan, JIANG Yu, CHEN Fang
- GDF11调控NF-κB通路影响巨噬细胞M1极化介导的炎症反应
- GDF11 affects M1 polarization mediated inflammatory response in macrophages by regulating the NF-κB pathway
- 中国医科大学学报, 2024, 53(4): 342-348, 354
- Journal of China Medical University, 2024, 53(4): 342-348, 354
-
文章历史
- 收稿日期:2023-06-15
- 网络出版时间:2024-04-10 18:34:01
2. 湖南师范大学生命科学学院生理学系,长沙 410081;
3. 湖南中医药大学临床医学院内科教研室,长沙 410007
2. Department of Physiology, College of Life Science, Hunan Normal University, Changsha 410081, China;
3. Department of Internal Medicine, Clinical Medicine School of Hunan University of Chinese Medicine, Changsha 410007, China
脓毒症是由感染引起的机体反应失衡,可导致器官功能障碍,危及患者生命[1],是重症监护病房最常见的并发症及致死原因[2]。目前认为失控的全身炎症反应是脓毒症致死的主要原因之一[3]。巨噬细胞在炎症反应中扮演重要角色,具有可塑性和功能异质性,可因刺激物或微环境的变化而活化成生物学行为完全不同的2种类型,即经典极化的M1型巨噬细胞和替代极化的M2型巨噬细胞[4]。M1型巨噬细胞能分泌大量的肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)和白细胞介素(interleukin,IL)-6等,具有较强的促炎和组织损伤作用[5]。在脓毒症中,M1型巨噬细胞过度极化释放大量炎性细胞因子,造成细胞因子风暴和感染性休克是引起脓毒症患者死亡的重要原因[6]。生长转化因子11(growth differentiation factor 11,GDF11)属于转化生长因子-β(transforming growth factor-β,TGF-β)超家族,可能与巨噬细胞极化有关[7-10]。研究[11]发现,激活核转录因子(nuclear factor-κB,NF-κB)可促进M1型巨噬细胞极化。GDF11能够抑制TLR/NF-κB信号,从而减轻炎症反应[12]。因此,本研究利用小鼠RAW264.7巨噬细胞探讨脓毒症中GDF11是否能通过调控NF-κB信号通路影响巨噬细胞极化介导的炎症反应,旨在进一步认识脓毒症巨噬细胞极化和炎症反应发生发展过程中的分子机制,为寻找更有效的干预治疗措施提供理论基础和实验依据。
1 材料与方法 1.1 材料RAW264.7巨噬细胞购自ATCC细胞库。DMEM培养液、胎牛血清、磷酸盐缓冲液(phosphate buffered solution,PBS)、山羊血清购自美国Gibco公司;脂多糖(lipopolysaccharide,LPS)(NO.MFCD00164401)、DAPI(NO.D9542)购自美国Sigma公司;γ干扰素(interferon-γ,IFN-γ)(NO.RP01070)、GDF11同源重组蛋白(NO.RP00378LQ)购自中国ABcional公司;SDS-PAGE快速凝胶制备试剂盒(BL565A)购自中国Biosharp公司;TRIzol(NO.15596-026)、细胞流式抗体PE-CY7 anti-mouseF4/80(NO.25-4801-82)和PE anti-mouse iNOS(NO. 61-5920-82)购自美国Invitrogen公司;逆转录试剂盒(NO.6110A)、Taq酶(NO.R500A)购自日本Takara公司;抗GDF11、NF-κB、P-NF-κB和GAPDH抗体以及二抗购自英国Abcam公司。实时定量PCR仪购自美国ABI公司,流式细胞仪购自美国BD公司,蛋白电泳分离、转膜和化学发光成像系统购自美国Bio-rad公司,荧光显微成像系统购自德国徕卡公司。
1.2 方法 1.2.1 细胞培养采用含10%胎牛血清的DMEM培养基在5% CO2、37 ℃细胞培养箱中常规传代培养RAW264.7巨噬细胞。
1.2.2 实验分组将细胞分为对照组(不做任何处理),LPS+IFN组(100 ng/mL LPS+30 ng/mL IFN-γ处理细胞24 h),GDF11组(50 ng/mL同源重组GDF11蛋白处理细胞24 h)、LPS+IFN+GDF11组(100 ng/mL LPS+30 ng/mL IFN-γ+50 ng/mL同源重组GDF11蛋白处理24 h)。
1.2.3 流式细胞术检测M1极化标记物iNOS阳性表达率按1×106/孔接种细胞于6孔板,分组处理细胞,24 h后收集细胞,离心后去上清,PBS清洗3次,将细胞数调整至1×106/mL,取100 μL细胞悬液加入PE-CY7 anti-mouse F4/80及PE anti-mouse iNOS抗体混匀,室温孵育20 min,通过流式细胞术检测细胞F4/80及iNOS的阳性表达率。
1.2.4 实时PCR(real-time quantitative PCR,RT-qPCR)检测TNF-α、IL-6、GDF11和NF-κB mRNA表达按1×106/孔接种细胞于6孔板,分组处理细胞,24 h后收集细胞,TRIzol法提取总RNA,根据逆转录试剂盒和RT-qPCR试剂盒说明书进行mRNA定量分析。在NCBI查询各基因的CDS,用Primer Premier 5.0软件设计引物,序列见表 1。反应条件:预变性95 ℃2 min;变性95 ℃10 s,退火60 ℃34 s,72 ℃延伸30 s,循环40次,最后延伸10 min。β-actin作为内参照,2-ΔΔCt法计算mRNA相对表达水平。
| Gene | Primer sequence(5’-3’) |
| β-actin | |
| Forward | GGCTGTATTCCCCTCCATCG |
| Reverse | CCAGTTGGTAACAATGCCATGT |
| TNF-α | |
| Forward | CAGGCGGTGCCTATGTCTC |
| Reverse | CGATCACCCCGAAGTTCAGTAG |
| IL-6 | |
| Forward | TCTATACCACTTCACAAGTCGGA |
| Reverse | GAATTGCCATTGCACAACTCTTT |
| GDF11 | |
| Forward | CTGCGCCTAGAGAGCATCAAG |
| Reverse | TTGGAAGTCGTGCAGATCCAG |
| NF-κB | |
| Forward | TGCGATTCCGCTATAAATGCG |
| Reverse | ACAAGTTCATGTGGATGAGGC |
1.2.5 Western blotting检测细胞GDF11、NF-κB、P-NF-κB蛋白表达
按1×106/孔接种细胞于6孔板,分组处理细胞,24 h后收集细胞,RIPA裂解液提取总蛋白,BCA定量蛋白后变性。10%SDS-PAGE电泳分离后,转移至PVDF膜,5%脱脂奶粉室温封闭1 h,一抗4 ℃孵育过夜,二抗室温孵育1 h,ECL化学发光并采集图像。采用Image J软件分析灰度值,以GAPDH作内参照,计算各目的蛋白的相对表达水平。
1.2.6 免疫荧光标记检测GDF11、NF-κB表达按1×106/孔接种细胞于6孔板,分组处理细胞,24 h后收集细胞,4%多聚甲醛固定15 min,0.5% Triton X-100通透10 min,3%山羊血清室温封闭30 min,按抗体说明书稀释至推荐浓度的一抗室温孵育1 h,对应的荧光二抗避光孵育30 min,加DAPI避光孵育5 min染色,荧光显微镜下拍照采集图像。
1.3 统计学分析采用GraphPad Prism 9软件进行统计学分析和图形绘制。计量资料采用x±s表示,单因素方差分析用于多组间比较,Bonferroni校正的t检验用于组间两两比较,P < 0.05为差异有统计学意义。
2 结果 2.1 LPS+IFN对RAW264.7巨噬细胞极化的影响(图 1)
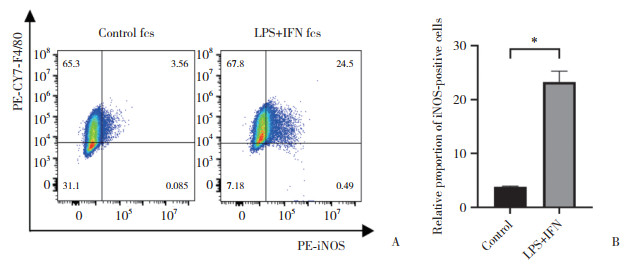
|
| A, flow cytometry analysis of positive expression rate of M1-polarization marker iNOS in macrophages; B, statistical chart of flow cytometry results. *P < 0.000 1. 图 1 LPS+IFN对RAW264.7巨噬细胞极化的影响 Fig.1 Effects of LPS+IFN on the polarization of RAW264.7 macrophages |
流式细胞术结果显示,与对照组相比,LPS+IFN组RAW264.7巨噬细胞M1极化标志物iNOS阳性比例显著增加(P < 0.05),表明LPS+IFN-γ处理诱导巨噬细胞M1极化。见图 1。
2.2 LPS+IFN对RAW264.7巨噬细胞介导的炎症反应的影响(图 2)
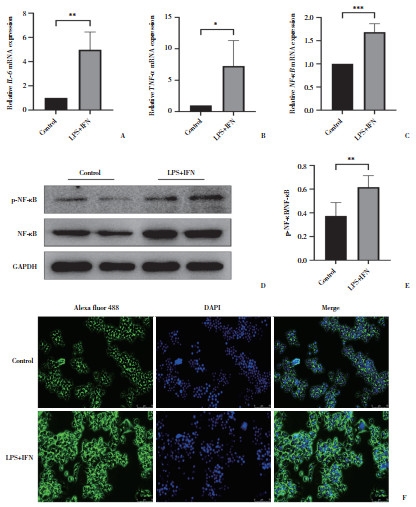
|
| A to C, expression analysis of inflammatory factors IL-6, TNF-α, and NF-κB mRNA by RT-qPCR; D to E, protein expression of NF-κB and P-NF-κB assessed by Western blotting; F, immunofluorescence detection of NF-κB protein expression and intracellular distribution (×20). *P < 0.05, **P < 0.01, ***P < 0.000 1. 图 2 LPS+IFN对RAW264.7巨噬细胞介导的炎症反应的影响 Fig.2 Effects of LPS+IFN on RAW264.7 macrophage-mediated inflammatory response |
RT-qPCR结果显示,与对照组相比,LPS+IFN组细胞IL-6、TNF-α、NF-κB mRNA表达显著升高(P < 0.05)。Western blotting结果显示,LPS+IFN组P-NF-κB/NF-κB显著升高(P < 0.05)。免疫荧光检测结果提示,与对照组相比,LPS+IFN组RAW264.7巨噬细胞NF-κB蛋白表达显著上升,NF-κB入核增加。
2.3 LPS+IFN对RAW264.7巨噬细胞GDF11表达的影响(图 3)
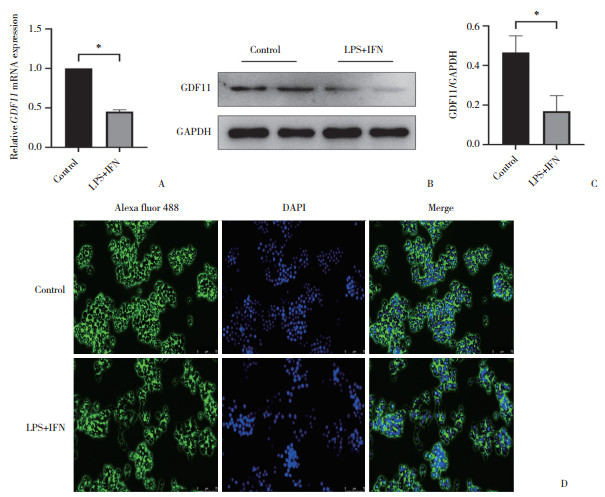
|
| A, expression analysis of GDF11 mRNA expression by RT-qPCR; B, C, protein expression of GDF11 assessed by Western blotting; D, immunofluore-scence detection of GDF11 protein expression and distribution (×20). *P < 0.000 1. 图 3 LPS+IFN对RAW264.7巨噬细胞GDF11表达的影响 Fig.3 Effects of LPS+IFN on GDF11 expression in RAW264.7 macrophages |
RT-qPCR、Western blotting及免疫荧光结果显示,与对照组相比,LPS+IFN组巨噬细胞GDF11 mRNA和蛋白表达均减少(P < 0.05),提示GDF11可能与LPS+IFN诱导的巨噬细胞M1极化相关。
2.4 GDF11对LPS+IFN诱导的RAW264.7巨噬细胞极化的影响(图 4)
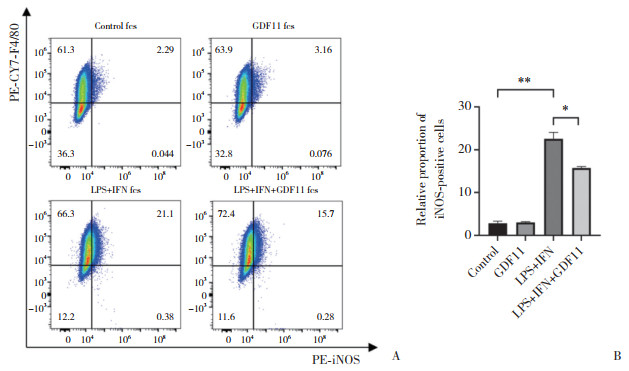
|
| A, flow cytometry analysis of positive expression rate of M1-polarization marker iNOS in macrophages; B, statistical chart of flow cytometry results. *P < 0.001, **P < 0.000 1. 图 4 GDF11对LPS+IFN诱导的RAW264.7巨噬细胞极化的影响 Fig.4 Effect of GDF11 on polarization of RAW264.7 macrophages induced by LPS+IFN |
流式细胞术结果显示,与对照组相比,GDF11组巨噬细胞iNOS阳性比例无显著差异(P > 0.05);而与LPS+IFN组相比,LPS+IFN+GDF11组iNOS阳性比例显著降低,细胞M1极化显著较少(P < 0.05)。
2.5 GDF11对LPS+IFN诱导的巨噬细胞炎性细胞因子表达的影响RT-qPCR结果显示,与对照组相比,GDF11组RAW264.7巨噬细胞IL-6(1.82±0.41 vs. 1.00±0.06,P = 0.123)、TNF-α(1.24±0.12 vs. 1.00±0.06,P = 0.189)表达差异无统计学意义;而LPS+IFN+GDF11组细胞IL-6(52.25±21.91 vs. 147.9±7.26,P = 0.014)、TNF-α(2.39±0.31 vs. 5.19±1.01,P = 0.038)表达与LPS+IFN组相比显著降低。
2.6 GDF11对LPS+IFN诱导的RAW264.7巨噬细胞NF-κB表达的影响(图 5)
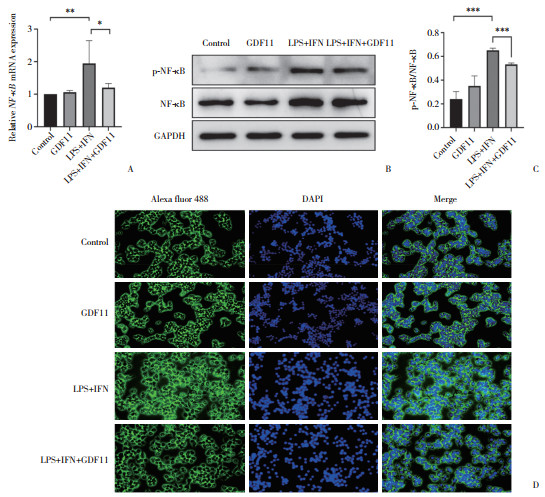
|
| A, expression analysis of NF-κB mRNA expression by RT-qPCR; B, C, protein expression of NF-κB and P-NF-κB assessed by Western blotting; D, immunofluorescence detection of NF-κB protein expression and distribution (×20). *P < 0.05, **P < 0.001, ***P < 0.000 1. 图 5 GDF11对LPS+IFN诱导的RAW264.7巨噬细胞NF-κB表达的影响 Fig.5 Effect of GDF11 on NF-κB expression in RAW264.7 macrophages induced by LPS+IFN |
与对照组相比,GDF11组巨噬细胞NF-κB mRNA及P-NF-κB/NF-κB无明显变化(P > 0.05);而与LPS+IFN组相比,LPS+IFN+GDF11组细胞NF-κB mRNA、P-NF-κB/NF-κB表达显著降低(P < 0.05);免疫荧光检测结果显示NF-κB蛋白表达显著减少,NF-κB入核减少(P < 0.05)。
3 讨论M1型巨噬细胞是一种典型的抗原呈递细胞,能产生多种细胞因子和介质[13-14],参与机体免疫防御和炎症反应的调节,在脓毒症中起至关重要的作用[15]。然而,M1型巨噬细胞的过度激活也会导致炎症反应的过度放大,导致炎症风暴,危及患者生命[16]。M1型巨噬细胞的极化由细菌成分、细胞因子等多种因素调节[17]。本研究使用LPS+IFN-γ成功诱导了RAW 264.7巨噬细胞M1型极化,并发现炎性细胞因子IL-6、TNF-α mRNA表达升高,NF-κB被激活进入细胞核。NF-κB可调节炎症相关基因的表达,导致细胞因子和趋化因子增加,吸引更多的免疫细胞到达感染部位,是脓毒症炎症风暴的核心[18]。既往研究[19-21]表明,抑制或激活NF-κB通路可调节巨噬细胞的极化、吞噬能力及细胞因子产生等功能,但具体机制目前仍不明确,因此,本研究探讨了巨噬细胞NF-κB通路的调控机制以及相关的信号通路,以深入了解其在巨噬细胞功能调控中的作用。
GDF11具有抗衰老和再生能力[22]及抗炎作用[23-24]。研究[12]发现,GDF11能够减少LPS的肺部炎症反应,降低肺部白细胞浸润,减少炎性细胞因子的产生。GDF11也可以减少内皮细胞中LPS诱导的炎症反应,保护内皮细胞免受氧化应激的损伤等[25-26]。本研究探讨了GDF11是否与巨噬细胞的M1型极化及其介导的炎症反应相关,结果发现,在LPS+IFN-γ诱导的巨噬细胞中,GDF11 mRNA及蛋白表达都显著降低,提示巨噬细胞M1型极化与GDF11之间存在相关性。为了证实GDF11与巨噬细胞极化的关联,本研究用GDF11的同源重组蛋白体外处理RAW264.7巨噬细胞,结果发现,GDF11降低LPS+IFN-γ诱导的巨噬细胞M1极化,并降低巨噬细胞炎性细胞因子IL-6和TNF-α mRNA的表达,证实GDF11确实是调控巨噬细胞M1极化及其介导的炎症反应的的靶点,但其调控机制尚不明确。
既往研究[27]报道,GDF11可能通过JNK和NF-κB信号通路在体外降低棕榈酸诱导的RAW264.7巨噬细胞炎性细胞因子表达,GDF11过表达可抑制NF-κB活性,降低炎症反应和氧化应激反应,从而减轻组织损伤和延缓疾病进程[10, 12]。而在某些疾病状态下,GDF11的缺乏可能导致NF-κB异常激活,从而加剧炎症反应和组织损伤[28-29]。本研究结果也表明,外源性予以GDF11处理显著降低NF-κB磷酸化比值,并减少NF-κB的入核。证实GDF11是NF-κB的调控靶点,而GDF11的外源性补充可以减少巨噬细胞M1型极化及相关炎性细胞因子释放。
总之,本研究结果发现,GDF11通过调控NF-κB通路影响巨噬细胞M1极化,阐明了脓毒症炎症反应的新机制。为进一步认识脓毒症巨噬细胞极化和炎症反应发生发展过程的分子机制以及寻找更有效的干预治疗措施,提供了理论基础和实验依据。
| [1] |
SINGER M, DEUTSCHMAN CS, SEYMOUR CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)[J]. JAMA, 2016, 315(8): 801-810. DOI:10.1001/jama.2016.0287 |
| [2] |
STOLLER J, HALPIN L, WEIS M, et al. Epidemiology of severe sepsis: 2008-2012[J]. J Crit Care, 2016, 31(1): 58-62. DOI:10.1016/j.jcrc.2015.09.034 |
| [3] |
SEYMOUR CW, GESTEN F, PRESCOTT HC, et al. Time to treatment and mortality during mandated emergency care for sepsis[J]. N Engl J Med, 2017, 376(23): 2235-2244. DOI:10.1056/nejmoa1703058 |
| [4] |
沈灵芝, 李莉, 严静. 巨噬细胞极化在脓毒症免疫机制中的作用[J]. 中华重症医学电子杂志(网络版), 2019, 5(2): 185-189. DOI:10.3877/cma.j.issn.2096-1537.2019.02.018 |
| [5] |
JIANG T, SUN L, ZHU J, et al. MicroRNA-23a-3p promotes macrophage M1 polarization and aggravates lipopolysaccharide-induced acute lung injury by regulating PLK1/STAT1/STAT3 signalling[J]. Int J Exp Pathol, 2022, 103(5): 198-207. DOI:10.1111/iep.12445 |
| [6] |
SU Y, SONG XX, TENG JL, et al. Mesenchymal stem cells-derived extracellular vesicles carrying microRNA-17 inhibits macrophage apoptosis in lipopolysaccharide-induced sepsis[J]. Int Immunopharmacol, 2021, 95: 107408. DOI:10.1016/j.intimp.2021.107408 |
| [7] |
EGERMAN MA, GLASS DJ. The role of GDF11 in aging and skeletal muscle, cardiac and bone homeostasis[J]. Crit Rev Biochem Mol Biol, 2019, 54(2): 174-183. DOI:10.1080/10409238.2019.1610722 |
| [8] |
LIU WH, FENG L, WANG XA, et al. GDF11 improves ischemia-reperfusion-induced acute kidney injury via regulating macrophage M1/M2 polarization[J]. Kidney Blood Press Res, 2023, 48(1): 209-219. DOI:10.1159/000529444 |
| [9] |
GOLDSTEIN JM, SENGUL H, MESSEMER KA, et al. Steady-state and regenerative hematopoiesis occurs normally in mice in the absence of GDF11[J]. Blood, 2019, 134(20): 1712-1716. DOI:10.1182/blood.2019002066 |
| [10] |
LI WW, WANG WH, LIU L, et al. GDF11 antagonizes TNF-α-induced inflammation and protects against the development of inflammatory arthritis in mice[J]. FASEB J, 2019, 33(3): 3317-3329. DOI:10.1096/fj.201801375rr |
| [11] |
HUANG YL, TIAN C, LI QM, et al. TET1 knockdown inhibits Porphyromonas gingivalis LPS/IFN-γ-induced M1 macrophage polarization through the NF-κB pathway in THP-1 cells[J]. Int J Mol Sci, 2019, 20(8): 2023. DOI:10.3390/ijms20082023 |
| [12] |
XU HB, QIN B, ZHANG JZ, et al. Growth differentiation factor 11 relieves acute lung injury in mice by inhibiting inflammation and apoptosis[J]. Eur Rev Med Pharmacol Sci, 2020, 24(12): 6908-6918. DOI:10.26355/eurrev_202006_21682 |
| [13] |
CHEN YN, HU MR, WANG L, et al. Macrophage M1/M2 polarization[J]. Eur J Pharmacol, 2020, 877: 173090. DOI:10.1016/j.ejphar.2020.173090 |
| [14] |
DEY A, NI ZR, JOHNSON MS, et al. A multi-colour confocal microscopy method for identifying and enumerating macrophage subtypes and adherent cells in the stromal vascular fraction of human adipose[J]. J Immunol Meth, 2021, 491: 112988. DOI:10.1016/j.jim.2021.112988 |
| [15] |
CHEN XS, LIU YC, GAO YL, et al. The roles of macrophage polarization in the host immune response to sepsis[J]. Int Immunopharmacol, 2021, 96: 107791. DOI:10.1016/j.intimp.2021.107791 |
| [16] |
JAGGI U, MATUNDAN HH, YU J, et al. Essential role of M1 macrophages in blocking cytokine storm and pathology associated with murine HSV-1 infection[J]. PLoS Pathog, 2021, 17(10): e1009999. DOI:10.1371/journal.ppat.1009999 |
| [17] |
WANG C, MA C, GONG LH, et al. Macrophage polarization and its role in liver disease[J]. Front Immunol, 2021, 12: 803037. DOI:10.3389/fimmu.2021.803037 |
| [18] |
CAO YY, WANG Z, WANG ZH, et al. Inhibition of miR-155 alleviates sepsis-induced inflammation and intestinal barrier dysfunction by inactivating NF-κB signaling[J]. Int Immunopharmacol, 2021, 90: 107218. DOI:10.1016/j.intimp.2020.107218 |
| [19] |
JIAO Y, ZHANG T, ZHANG CM, et al. Exosomal miR-30d-5p of neutrophils induces M1 macrophage polarization and primes macrophage pyroptosis in sepsis-related acute lung injury[J]. Crit Care Lond Engl, 2021, 25(1): 356. DOI:10.1186/s13054-021-03775-3 |
| [20] |
LIU LL, GUO HM, SONG AM, et al. Progranulin inhibits LPS-induced macrophage M1 polarization via NF-κB and MAPK pathways[J]. BMC Immunol, 2020, 21(1): 32. DOI:10.1186/s12865-020-00355-y |
| [21] |
YANG K, XU JJ, FAN M, et al. Lactate suppresses macrophage pro-inflammatory response to LPS stimulation by inhibition of YAP and NF-κB activation via GPR81-mediated signaling[J]. Front Immunol, 2020, 11: 587913. DOI:10.3389/fimmu.2020.587913 |
| [22] |
ROCHETTE L, MAZINI L, MELOUX A, et al. Anti-aging effects of GDF11 on skin[J]. Int J Mol Sci, 2020, 21(7): 2598. DOI:10.3390/ijms21072598 |
| [23] |
MACHELAK W, SZCZEPANIAK A, JACENIK D, et al. The role of GDF11 during inflammation - an overview[J]. Life Sci, 2023, 322: 121650. DOI:10.1016/j.lfs.2023.121650 |
| [24] |
DUAN FX, WANG XW, WANG HW, et al. GDF11 ameliorates severe acute pancreatitis through modulating macrophage M1 and M2 polarization by targeting the TGFβR1/SMAD-2 pathway[J]. Int Immunopharmacol, 2022, 108: 108777. DOI:10.1016/j.intimp.2022.108777 |
| [25] |
LI L, GAO Y, LIU ZC, et al. GDF11 alleviates neointimal hyperplasia in a rat model of artery injury by regulating endothelial NLRP3 inflammasome activation and rapid re-endothelialization[J]. J Transl Med, 2022, 20(1): 28. DOI:10.1186/s12967-022-03229-6 |
| [26] |
SUN BY, XING K, QI C, et al. Down-regulation of miR-215 attenuates lipopolysaccharide-induced inflammatory injury in CCD-18co cells by targeting GDF11 through the TLR4/NF-κB and JNK/p38 signaling pathways[J]. Histol Histopathol, 2020, 35(12): 1473-1481. DOI:10.14670/HH-18-278 |
| [27] |
MEI W, XIANG GD, LI YX, et al. GDF11 protects against endothelial injury and reduces atherosclerotic lesion formation in apolipoprotein E-null mice[J]. Mol Ther, 2016, 24(11): 1926-1938. DOI:10.1038/mt.2016.160 |
| [28] |
MEI W, ZHU B, SHU Y, et al. GDF11 protects against glucotoxicity-induced mice retinal microvascular endothelial cell dysfunction and diabetic retinopathy disease[J]. Mol Cell Endocrinol, 2021, 537: 111422. DOI:10.1016/j.mce.2021.111422 |
| [29] |
WANG WH, QU RZ, WANG X, et al. GDF11 antagonizes psoriasis-like skin inflammation via suppression of NF-κB signaling pathway[J]. Inflammation, 2019, 42(1): 319-330. DOI:10.1007/s10753-018-0895-3 |
 2024, Vol. 53
2024, Vol. 53




