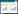文章信息
- 刘明, 刘熙鹏, 李淳, 张秀峰, 曹兵, 乔建新, 王雪
- LIU Ming, LIU Xipeng, LI Chun, ZHANG Xiufeng, CAO Bing, QIAO Jianxin, WANG Xue
- 水飞蓟素通过调控miR-124-3p/WEE1轴影响胶质瘤细胞恶性生长的机制研究
- Mechanism of silymarin on malignant growth of glioma cells by regulating miR-124-3p/WEE1 axis
- 中国医科大学学报, 2024, 53(2): 142-148
- Journal of China Medical University, 2024, 53(2): 142-148
-
文章历史
- 收稿日期:2023-03-14
- 网络出版时间:2024-01-10 16:08:41
胶质瘤是中枢神经系统最常见的恶性原发性肿瘤,具有较高的侵袭性。目前主要的治疗方法是手术切除和放化疗联合治疗,但由于胶质瘤细胞不受控制的增殖、细胞异质性和扩散能力导致预后较差,生存率很低[1-2]。因此,胶质瘤的发病机制和治疗方法一直是神经科学的研究热点。水飞蓟素(silymarin,SM)是从菊科草本植物水飞蓟果实及种子中提取的一类二氢黄酮醇与苯丙素衍生物缩合而成的黄酮木脂素类成分,具有抗氧化、抗炎和抗癌等多种生物学活性[3]。研究[4]发现,SM可抑制乳腺癌、结肠癌、卵巢癌和肺癌等多种癌细胞的增殖,但在胶质瘤中的研究较少。
微RNA(microRNA,miRNA)在肿瘤细胞的增殖、分化、转移和凋亡过程中发挥重要的调节作用。如miR-124-3p在胶质瘤细胞中低表达,上调其表达能抑制胶质瘤细胞的增殖、侵袭和转移[5-6]。WEE1是酪氨酸激酶家族的一员,参与调节细胞周期进程,在多种肿瘤中表达上调[7]。文献[8]报道,WEE1在胶质瘤中充当致癌基因,且可被多种miRNA靶向下调,抑制胶质瘤的发展。但其与miR-124-3p的靶向关系尚不清楚。本研究拟探讨SM对胶质瘤细胞恶性生长的影响,以及对miR-124-3p/WEE1轴的调控机制。
1 材料与方法 1.1 材料 1.1.1 实验动物NOD/SCID免疫缺陷雄性小鼠15只,6周龄,20~25 g,购自北京维通利华实验动物技术有限公司[许可证号:SCXK(京)2021-0006]。所有小鼠均在26 ℃、湿度60%的环境中饲养。本研究经河北北方学院附属第一医院医学伦理委员会批准(编号W2023048)。
1.1.2 细胞及主要试剂胶质瘤U87细胞系(货号CL-0238,武汉普诺赛生命科技有限公司)。SM(纯度NMR≥98%,货号WKQ-0010632,四川省维克奇生物科技有限公司);Lipofectamine 3000转染试剂(货号L3000015,上海恒斐生物科技有限公司);CCK-8细胞增殖检测试剂盒(货号KTC011001,艾美捷科技有限公司);Transwell-24孔板(货号3422,北京伊塔生物科技有限公司);细胞周期检测试剂盒(货号SH-0213,北京凯诗源生物科技有限公司);细胞周期蛋白D1(cyclin D1)、cleaved caspase-3兔单克隆抗体(货号ab16663、ab32042,英国abcam公司);cleaved caspase-8、cleaved caspase-9兔多克隆抗体(货号ABP50008、ABP50009,艾美捷科技生物有限公司);羊抗兔IgG二抗(货号E-AB-1075,武汉伊莱瑞特生物科技股份有限公司);双荧光素酶活性检测试剂盒(货号LM130595-100,上海联迈生物工程有限公司);miR-124-3p、WEE1、U6、GAPDH引物(上海生工生物工程股份有限公司合成)。
1.2 方法 1.2.1 细胞分组与处理将胶质瘤U87细胞接种至含10%胎牛血清的DEME培养基,于37 ℃、5% CO2培养箱中培养。待细胞生长至对数期时,将其分为:(1)对照组;(2)SM低、中、高浓度组,分别用浓度为40、60、80 µg/mL的SM处理[9];(3)SM高浓度+miR-124-3p抑制剂组(SM高+miR-124-3p inhibitor组),先用Lipofectamine 3000转染试剂转染50 pmol的miR-124-3p抑制剂 [5]后,再用80 µg/mL SM处理细胞48 h。
1.2.2 CCK-8检测细胞的增殖活性接种细胞至96孔板(2×104/孔),按照分组培养48 h后,加入CCK-8试剂(100 µL/孔),37 ℃孵育2 h,使用酶标仪测量450 nm处每孔吸光度,并计算细胞存活率(%)=(实验组OD-空白组OD)/(对照组OD-空白组OD)×100。每组实验重复3次。
1.2.3 Transwell检测细胞迁移、侵袭能力分组培养48 h后,收集各组细胞并悬浮于不含胎牛血清的培养基。接种至Transwell上室(1×105/孔)行迁移实验,或接种至涂有基质胶Matrigel的上室行侵袭实验,然后,下室均加入200 µL完全培养基。孵育24 h后,弃上室中残余细胞,将膜下表面迁移和侵入的细胞用4%多聚甲醛固定,结晶紫染色。倒置显微镜下观察,并随机选择每个孔的5个视野进行细胞计数。
1.2.4 流式细胞术检测细胞周期变化将细胞接种至6孔板中,分组培养6 h(1个细胞周期)。胰蛋白酶消化收集细胞,PBS清洗后,70%乙醇4 ℃固定过夜。300 µL PI/RNase染色15 min,将细胞充分悬浮后,用流式细胞仪检测细胞各周期变化,并计算细胞周期百分比。
1.2.5 Western blotting检测细胞中cyclin D1及凋亡相关蛋白的表达收集各组细胞,加入RIPA裂解液,14 000 g离心30 min。用BCA试剂盒检测蛋白含量后,10% SDS-PAGE分离蛋白并转移至PVDF膜,5%脱脂牛乳封闭,4 ℃孵育过夜加入一抗(cyclin D1稀释1︰250,cleaved caspase-8、cleaved caspase-9、cleaved caspase-3稀释1︰2 000),室温孵育2 h。羊抗兔IgG二抗(1︰500稀释)室温下孵育2 h。ECL显色。以GAPDH为内参。凝胶成像仪成像,用Image J软件分析计算蛋白相对表达量。
1.2.6 实时定量PCR(quantitative real-time polyme-rase chain reaction,qRT-PCR)检测细胞中miR-124-3p和WEE1 mRNA表达收集各组细胞,用TRIzol提取总RNA。利用一步法qRT-PCR试剂盒配制反应体系:2×SYBR Green Mix 12.5 µL,enzyme 1 µL,上、下游引物各1 µL,RNA模板1 µL,补水至25 µL。95 ℃预变性30 s,95 ℃变性10 s、60 ℃退火30 s,40个循环。miR-124-3p以U6为内参,WEE1以GAPDH为内参,采用2-ΔΔCt法计算mRNA相对表达量。PCR引物序列如下:miR-124-3p,正向,5’-GGGTATTGAGGAAGGTGTT-3’,反向,5’-CAGTGCGTGTCGTGGAGT-3’;U6,正向,5’-GAGCCACAGCGTAACG-3’,反向,5’-CTAGCACATAGTACACG-3’;WEE1,正向,5’-GCTTGCCCTCACAGTGGTATG-3’,反向,5’-CCGAGGTAATCTACCCGTCTGA-3’;GAPDH,正向,5’-GGTCTCCTCTGACTTCAACA-3’,反向,5’-GCCAAATTCGTTGTCATAC-3’。
1.2.7 双荧光素酶活性实验验证miR-124-3p和WEE1间的靶向关系用ENCORI数据库预测miR-124-3p和WEE1之间的结合位点。将扩增后的WEE1基因片段插入至荧光素酶报告载体p-mirGLO中,分别构建野生型(WEE1-WT)和突变型(WEE1-MUT)质粒。将miR-124-3p mimic和miR-124-3p mimic-NC分别与构建的野生型或突变型质粒共转染到细胞中,培养48 h,检测荧光素酶活性。
1.2.8 建立NOD/SCID小鼠颅内移植瘤模型并进行给药和分析参照文献[10]方法建立小鼠胶质瘤颅内移植模型。腹腔注射1%戊巴比妥钠(40 mg/kg)麻醉NOD/SCID小鼠,固定在立体定向仪上,做颅骨中线切口。选取距前囟后2 mm、距冠状缝外侧2 mm、距暴露的硬脑膜背腹侧4 mm的位置,用微量注射器通过颅骨1.2 mm钻孔注射5 µL胶质瘤细胞悬液(5×107/mL)至目标部位,缝合头皮并术后清洁。3 d后,将小鼠随机分为模型组和SM治疗组,每组5只,并随机挑选5只健康小鼠作为对照组,SM治疗组小鼠口服SM(25 mg/kg,5次/周)[3],模型组和对照组均灌胃等量生理盐水,共持续21 d。每天监测小鼠的健康状况和行为。实验结束时,麻醉并脱颈处死小鼠。将小鼠称重后,立即取出肿瘤组织称重,并用游标卡尺测量肿瘤的长径(a)和短径(b),计算肿瘤体积(v,mm3)= ab2/2。
1.3 统计学分析采用GraphPad Prism 8.0软件行统计学分析。计量资料采用x±s表示。2组间比较,若方差齐时,采用t’检验。多组间比较采用单因素方差分析,两两多重比较采用SNK-q检验。P < 0.05为差异有统计学意义。
2 结果 2.1 SM对细胞增殖活性的影响与对照组相比,SM不同浓度组细胞的增殖活性显著降低,且呈剂量依赖性(P < 0.05);与SM高浓度组相比,SM高+miR-124-3p inhibitor组细胞的增殖活性显著升高(P < 0.05)。见表 1。
| Group | Proliferation activity(%) |
| Control | 98.37±2.23 |
| Low concentration SM | 71.65±2.181) |
| Medium concentration SM | 54.91±1.641),2) |
| High concentration SM | 28.76±1.371),2),3) |
| High SM+miR-124-3p inhibitor | 82.19±2.061),4) |
| 1)compared with control group,P < 0.05;2)compared with low concentration SM group,P < 0.05;3)compared with medium concentration SM group,P < 0.05;4)compared with high concentration SM group,P < 0.05. | |
2.2 SM对细胞迁移和侵袭能力的影响
与对照组相比,SM不同浓度组细胞迁移和侵袭数显著降低,且呈剂量依赖性(P < 0.05);与SM高浓度组相比,SM高+miR-124-3p inhibitor组细胞迁移和侵袭数显著升高(P < 0.05),见图 1、表 2。
|
| 图 1 各组细胞迁移和侵袭的能力 结晶紫染色 ×200 Fig.1 The ability of migration and invasion of cells in each group Crystal violet stain×200 |
| Group | Migration number | Invasion number |
| Control | 214.26±8.21 | 159.67±6.32 |
| Low concentration SM | 182.73±8.11) | 128.43±5.771) |
| Medium concentration SM | 167.42±7.831),2) | 102.84±5.611),2) |
| High concentration SM | 139.17±7.541),2),3) | 81.59±5.431),2),3) |
| High SM+miR-124-3p inhibitor | 196.39±8.061),4) | 142.73±6.241),4) |
| 1)compared with control group,P < 0.05;2)compared with low concentration SM group,P < 0.05;3)compared with medium concentration SM group,P < 0.05;4)compared with high concentration SM group,P < 0.05. | ||
2.3 SM对细胞周期的影响
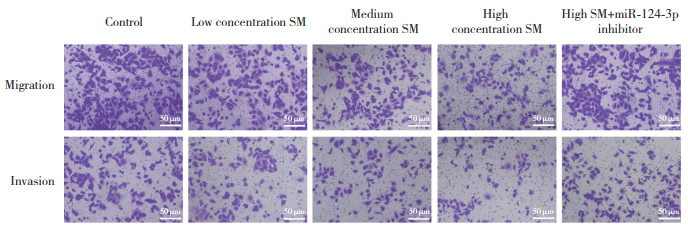
与对照组相比,SM不同浓度组G0/G1期细胞数升高,S和G2/M期细胞数降低,且呈剂量依赖性(P < 0.05);与SM高浓度组相比,SM高+miR-124-3p inhibitor组G0/G1期细胞百分数降低,S和G2/M期细胞数升高(P < 0.05),见图 2、表 3。
|
| A, control group; B, low concentration SM group; C, medium concentration SM group; D, high concentration SM group; E, high SM+miR-124-3p inhibitor group. 图 2 流式细胞术检测各组细胞周期的变化 Fig.2 Detection of cell cycle changes in each group by flow cytometry |
| Group | G0/G1 | S | G2/M |
| Control | 38.94±2.16 | 35.70±1.89 | 25.36±1.37 |
| Low concentration SM | 57.61±2.241) | 24.84±1.571) | 17.52±1.231) |
| Medium concentration SM | 69.72±2.371),2) | 17.35±1.261),2) | 12.93±0.981),2) |
| High concentration SM | 83.46±2.681),2),3) | 10.23±0.871),2),3) | 6.31±0.571),2),3) |
| High SM+miR-124-3p inhibitor | 46.73±2.271),4) | 30.57±1.641),4) | 22.70±1.211),4) |
| 1)compared with control group,P < 0.05;2)compared with low concentration SM group,P < 0.05;3)compared with medium concentration SM group,P < 0.05;4)compared with high concentration SM group,P < 0.05. | |||
2.4 SM对细胞中cyclin D1及凋亡相关蛋白的影响
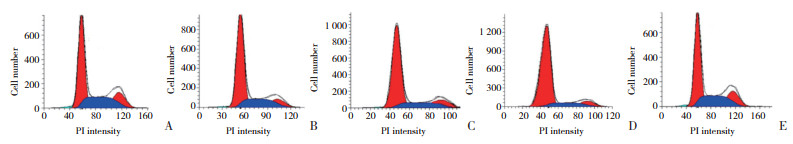
与对照组相比,SM不同浓度组细胞中cyclin D1表达降低,cleaved caspase-8、cleaved caspase-9、cleaved caspase-3表达升高,且均呈剂量依赖性(P < 0.05);与SM高浓度组相比,SM高+miR-124-3p inhibitor组细胞中cyclin D1表达升高,cleaved caspase-8、cleaved caspase-9、cleaved caspase-3表达降低(P < 0.05)。见图 3和表 4。
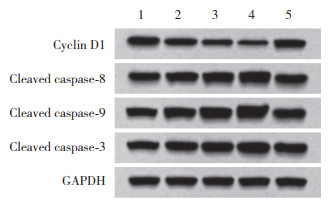
|
| 1, control group; 2, low concentration SM group; 3, medium concentration SM group; 4, high concentration SM group; 5, high SM+miR-124-3p inhibitor group. 图 3 各组细胞中cyclin D1及凋亡相关蛋白表达 Fig.3 Expression of cyclin D1 and apoptosis-related proteins in cells of each group |
| Group | Cyclin D1 | Cleaved caspase-8 | Cleaved caspase-9 | Cleaved caspasec-3 |
| Control | 1.03±0.07 | 0.96±0.06 | 1.01±0.05 | 0.97±0.05 |
| Low concentration SM | 0.82±0.061) | 1.24±0.081) | 1.28±0.091) | 1.19±0.061) |
| Medium concentration SM | 0.65±0.041),2) | 1.43±0.101),2) | 1.52±0.111),2) | 1.37±0.081),2) |
| High concentration SM | 0.46±0.021),2),3) | 1.61±0.111),2),3) | 1.69±0.121),2),3) | 1.58±0.101),2),3) |
| High SM+miR-124-3p inhibitor | 0.94±0.061),4) | 1.08±0.071),4) | 1.10±0.081),4) | 1.06±0.061),4) |
| 1)compared with control group,P < 0.05;2)compared with low concentration SM group,P < 0.05;3)compared with medium concentration SM group,P < 0.05;4)compared with high concentration SM group,P < 0.05. | ||||
2.5 SM对miR-124-3p和WEE1的mRNA表达的影响
与对照组相比,SM不同浓度组细胞中miR-124-3p表达升高,WEE1 mRNA表达降低,且均呈剂量依赖性(P < 0.05);与SM高浓度组相比,SM高+miR-124-3p inhibitor组细胞的miR-124-3p表达降低,WEE1 mRNA表达升高(P < 0.05)。见表 5。
| Group | miR-124-3p | WEE1 |
| Control | 0.98±0.06 | 1.01±0.06 |
| Low concentration SM | 1.26±0.081) | 0.75±0.041) |
| Medium concentration SM | 1.43±0.091),2) | 0.57±0.031),2) |
| High concentration SM | 1.62±0.111),2),3) | 0.32±0.021),2),3) |
| High SM+miR-124-3p inhibitor | 1.17±0.081),4) | 0.86±0.051),4) |
| 1)compared with control group,P < 0.05;2)compared with low concentration SM group,P < 0.05;3)compared with medium concentration SM group,P < 0.05;4)compared with high concentration SM group,P < 0.05. | ||
2.6 miR-124-3p和WEE1之间的靶向关系
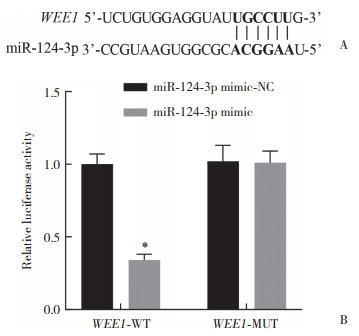
ENCORI分析发现,miR-124-3p与WEE1存在靶向结合位点。双荧光素酶报告基因实验结果显示,转染WEE1-WT后,共转染miR-124-3p mimic的细胞相对荧光素酶活性显著低于共转染miR-124-3p mimic-NC的细胞(P < 0.05),而转染WEE1-MUT后,共转染miR-124-3p mimic的细胞与共转染miR-124-3p mimic-NC的细胞相对荧光素酶活性无显著差异(P > 0.05)。见图 4。
|
| A, prediction of targeted binding sequence of miR-124-3p and WEE1 by ENCORI analysis; B, double luciferase reporter gene experiment verified the targeting relationship between miR-124-3p and WEE1. *compared with WEE1-WT+miR-124-3p mimic-NC, P < 0.05. 图 4 miR-124-3p与WEE1的靶向关系 Fig.4 Targeting relationship between miR-124-3p and WEE1 |
2.7 SM对胶质瘤移植小鼠体内肿瘤大小的影响
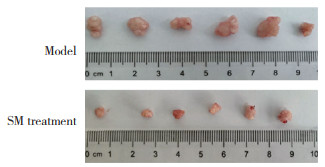
与对照组相比,模型组和SM组小鼠的体质量无显著差异(P > 0.05);与模型组相比,SM组肿瘤的质量和体积均显著减小(P < 0.05)。见图 5、表 6。
|
| 图 5 各组小鼠肿瘤的生长情况对比 Fig.5 Comparison of tumor growth of mice in each group |
| Group | Body weight(g) | Tumor weight(g) | Tumor volume(mm3) |
| Control | 22.93±1.92 | 0±0 | 0±0 |
| Model | 25.47±2.13 | 1.84±2.13 | 891.47±2.13 |
| SM treatment | 23.62±1.86 | 0.35±0.021) | 287.92±7.681) |
| 1)compared with model group,P < 0.05. | |||
3 讨论
胶质瘤是中枢神经系统最常见的原发性肿瘤,也是颅内肿瘤患者死亡的主要原因[11]。因此,不断探索新的胶质瘤治疗方法对提高生存率非常重要。SM由药用植物水飞蓟中提取,具有降血脂、抗氧化、抗肿瘤、抗炎等作用[12]。研究[13-14]显示,SM可抑制胶质母细胞瘤细胞的增殖和迁移,促进胶质瘤细胞的死亡。本研究发现,与对照组相比,SM组的细胞增殖活性、迁移和侵袭能力均显著降低,提示SM能够抑制胶质瘤细胞的增殖、迁移和侵袭。
细胞周期是一个高度调控的过程,可促进细胞生长、遗传物质复制和细胞分裂。cyclin D1是调控细胞周期G0/G1期的关键蛋白,在癌症的发病机制中起核心作用[15]。细胞凋亡是一种为维持内环境稳态而发生的程序性细胞死亡,在所有类型癌症的化疗过程中均可观察到[16]。参与细胞凋亡的主要途径包括外源性途径和内源性途径,在外源性途径中,caspase-8先被激活,随后引起下游级联反应;而内源性途径由线粒体介导,caspase-9和caspase-3被激活[17]。本研究结果显示,SM组G0/G1期细胞数增加,S期和G2/M期细胞数减少,且cyclin D1表达低于对照组,cleaved caspase-8、cleaved caspase-9、cleaved caspase-3表达高于对照组。表明SM能将胶质瘤细胞周期阻滞于G0/G1期,并通过激活caspase-8、caspase- 9、caspase-3诱导细胞凋亡,从而抑制胶质瘤的发展。
在人类恶性肿瘤的发生和发展中,miRNA异常表达至关重要。miR-124-3p可通过靶向下调CREB3募集因子,促进胶质瘤细胞的凋亡[6, 18]。WEE1激酶是细胞周期的关键调节蛋白,其表达也与多种肿瘤的增殖有关,如着丝粒蛋白E可通过与WEE1结合促进胶质母细胞瘤细胞的增殖[19]。本研究发现,与对照组相比,SM组细胞中miR-124-3p表达上调,WEE1 mRNA表达下调,提示SM可促进miR-124-3p表达,抑制WEE1表达。进一步转染miR-124-3p inhibitor后,SM对胶质瘤细胞恶性行为的抑制作用被逆转,同时WEE1 mRNA水平升高。ENCORI分析和荧光素酶报告基因实验进一步验证了miR-124-3p和WEE1之间存在靶向关系。因此,推测SM对胶质瘤发展的抑制作用是通过上调miR-124-3p并靶向下调WEE1实现的。
本研究中,将胶质瘤移植至小鼠颅内并分析肿瘤的生长情况,结果发现,SM能够抑制肿瘤的生长,但不影响小鼠的体质量。表明SM可抑制胶质瘤在体内的发展,且具有良好的耐受性。
综上所述,SM可通过调控miR-124-3p/WEE1轴抑制胶质瘤细胞的恶性生长。本研究通过体内外实验为SM成为治疗胶质瘤的潜在药物提供了充分的证据。但SM具有广泛的药理活性,在其他肿瘤中的治疗机制还需要更进一步的研究。
| [1] |
ZHAO NN, ZHANG JJ, ZHAO Q, et al. Mechanisms of long non-coding RNAs in biological characteristics and aerobic glycolysis of glioma[J]. Int J Mol Sci, 2021, 22(20): 11197. DOI:10.3390/ijms222011197 |
| [2] |
ZHA CJ, MENG XQ, LI LL, et al. Neutrophil extracellular traps mediate the crosstalk between glioma progression and the tumor microenvironment via the HMGB1/RAGE/IL-8 axis[J]. Cancer Biol Med, 2020, 17(1): 154-168. DOI:10.20892/j.issn.2095-3941.2019.0353 |
| [3] |
KIM SH, CHOO GS, YOO ES, et al. Silymarin inhibits proliferation of human breast cancer cells via regulation of the MAPK signaling pathway and induction of apoptosis[J]. Oncol Lett, 2021, 21(6): 492. DOI:10.3892/ol.2021.12753 |
| [4] |
AMIR ES, MAHBOOBEH GR, SOGHRA M, et al. A review of therapeutic potentials of milk thistle (Silybum marianum L.) and its main constituent, silymarin, on cancer, and their related patents[J]. Iran J Basic Med Sci, 2022, 25(10): 1166-1176. DOI:10.22038/IJBMS.2022.63200.13961 |
| [5] |
GAO C, SHEN J, MENG ZX, et al. Sevoflurane inhibits glioma cells proliferation and metastasis through miRNA-124-3p/ROCK1 axis[J]. Pathol Oncol Res, 2020, 26(2): 947-954. DOI:10.1007/s12253-019-00597-1 |
| [6] |
ZENG H, HUANG MY, GONG X. MicroRNA-124-3p promotes apoptosis and autophagy of glioma cells by down-regulating CREBRF[J]. Neurol Res, 2022, 44(12): 1094-1103. DOI:10.1080/01616412.2022.2112374 |
| [7] |
WU M, LI XJ, LIU Q, et al. MiR-526b-3p serves as a prognostic factor and regulates the proliferation, invasion, and migration of glioma through targeting WEE1[J]. Cancer Manag Res, 2019, 11: 3099-3110. DOI:10.2147/cmar.s192361 |
| [8] |
GONG JW, TANG Z, YU ZT, et al. MiR-138-5p inhibits the growth and invasion of glioma cells by regulating WEE1[J]. Anal Cell Pathol, 2022, 2022: 1-12. DOI:10.1155/2022/7809882 |
| [9] |
KIM S, CHOO G, YOO E, et al. Silymarin induces inhibition of growth and apoptosis through modulation of the MAPK signaling pathway in AGS human gastric cancer cells[J]. Oncol Rep, 2019, 42(5): 1904-1914. DOI:10.3892/or.2019.7295 |
| [10] |
SANCHEZ VE, LYNES JP, WALBRIDGE S, et al. Author correction: gl261 luciferase-expressing cells elicit an anti-tumor immune response: an evaluation of murine glioma models[J]. Sci Rep, 2022, 12: 5615. DOI:10.1038/s41598-020-67411-w |
| [11] |
XU XH, BAN YC, ZHAO ZL, et al. MicroRNA-1298-3p inhibits proliferation and invasion of glioma cells by downregulating Nidogen-1[J]. Aging, 2020, 12(9): 7761-7773. DOI:10.18632/aging.103087 |
| [12] |
GUO H, CAO H, CUI XW, et al. Silymarin's inhibition and treatment effects for alzheimer's disease[J]. Molecules, 2019, 24(9): 1748. DOI:10.3390/molecules24091748 |
| [13] |
CZARNIK-KWAŚNIAK J, KWAŚNIAK K, KWASEK P, et al. The influence of lycopene,[J]. Nutrients, 2019, 12(1): 96. DOI:10.3390/nu12010096 |
| [14] |
WANG CC, HE C, LU S, et al. Autophagy activated by silibinin contributes to glioma cell death via induction of oxidative stress-mediated BNIP3-dependent nuclear translocation of AIF[J]. Cell Death Dis, 2020, 11(8): 630. DOI:10.1038/s41419-020-02866-3 |
| [15] |
MONTALTO FI, DE AMICIS F. Cyclin D1 in cancer: a molecular connection for cell cycle control, adhesion and invasion in tumor and stroma[J]. Cells, 2020, 9(12): 2648. DOI:10.3390/cells9122648 |
| [16] |
GU J, RAUNIYAR S, WANG Y, et al. Chrysophanol induced glioma cells apoptosis via activation of mitochondrial apoptosis pathway[J]. Bioengineered, 2021, 12(1): 6855-6868. DOI:10.1080/21655979.2021.1972079 |
| [17] |
LIU XH, ZHAO PY, WANG XJ, et al. Celastrol mediates autophagy and apoptosis via the ROS/JNK and Akt/mTOR signaling pathways in glioma cells[J]. J Exp Clin Cancer Res, 2019, 38(1): 1-18. DOI:10.1186/s13046-019-1173-4 |
| [18] |
KHAN AQ, AHMED EI, ELAREER NR, et al. Role of miRNA-regulated cancer stem cells in the pathogenesis of human malignancies[J]. Cells, 2019, 8(8): 840. DOI:10.3390/cells8080840 |
| [19] |
MA CL, WANG JC, ZHOU J, et al. CENPE promotes glioblastomas proliferation by directly binding to WEE1[J]. Transl Cancer Res, 2020, 9(2): 717-725. DOI:10.21037/tcr.2019.11.40 |
 2024, Vol. 53
2024, Vol. 53