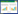文章信息
- 邹佳芮, 陈克研, 张振
- ZOU Jiarui, CHEN Keyan, ZHANG Zhen
- 5-HT1A受体拮抗剂对七氟烷致老年认知功能障碍模型大鼠突触可塑性的作用及其机制
- Effects of the 5-HT1A receptor antagonist on synaptic plasticity in sevoflurane-induced cognitive dysfunction in aged rats and its mechanism
- 中国医科大学学报, 2024, 53(1): 60-66, 74
- Journal of China Medical University, 2024, 53(1): 60-66, 74
-
文章历史
- 收稿日期:2023-07-17
- 网络出版时间:2024-01-04 20:22:02
2. 中国医科大学实验动物部, 沈阳 110122
2. Department of Experimental Animal, China Medical University, Shenyang 110122, China
术后认知障碍(postoperative cognitive dysfunction,POCD)是老年患者手术后常见的神经系统并发症,主要表现为学习、记忆力、注意力和执行能力下降[1]。临床研究[2]表明,POCD发病率和死亡率增加,术后恢复时间延长导致患者生活质量严重下降。麻醉是POCD的主要风险因素,七氟烷是吸入麻醉药物,广泛应用于各种手术,更容易引发认知功能障碍。目前,POCD具体的发病机制仍不清楚,近些年公认的POCD发病机制认为与Aβ蛋白代谢、神经炎症、氧化应激、神经递质释放障碍以及突触可塑性的变化密切相关[3-6]。突触可塑性具有维持神经元和神经回路稳定的作用,在学习、记忆、认知功能以及神经退行性疾病中意义重大[7]。近年来通过改善海马突触可塑性提高认知功能的策略得到广泛认同,其中环磷酸腺苷反应原件结合蛋白(cAMP response element binding protein,CREB)/脑源性神经营养因子(brain-derived neurotrophic factor,BDNF)信号通路尤为重要。5-羟色胺(5-hydroxytryptamine,5-HT)主要分布于海马体,是一种常见的神经递质,参与认知功能与情绪调节等生物活动。5-HT1A受体在海马组织表达最多。本研究应用七氟烷构建老年认知功能障碍大鼠模型,探讨5-HT1A受体拮抗剂对老年认知功能障碍模型大鼠海马突触可塑性的作用及其机制。
1 材料与方法 1.1 实验动物18月龄Sprague-Dawley雄性大鼠30只,体质量600~700 g,购自北京华阜康生物科技股份有限公司。大鼠在18~25 ℃,湿度40%~60%,自然昼夜节律变化光照条件下饲养,自由进食、饮水。本研究获得中国医科大学实验动物伦理委员会批准(CMUXN2022803)。
1.2 试剂七氟烷购自恒瑞医药(上海)有限公司,HE染色试剂盒、尼氏染色液、高尔基染色液购自武汉塞维尔生物科技有限公司,MecP2、p250GAP、PSD-95、GAP-43、Syn、PKA、CREB1、p-CREB1以及BDNF抗体购自美国Abcam公司,5-HT1A受体拮抗剂(WAY100635)、免疫荧光染色试剂盒(FITC)、免疫荧光染色试剂盒(Alexa Fluor 555)、BCA蛋白测定浓度试剂盒购自碧云天生物技术有限公司,总RNA提取试剂盒、FastKing cDNA第一链合成试剂盒、SuperReal荧光定量预混试剂购自天跟生化科技(北京)有限公司,合成引物购自上海生工股份有限公司。
1.3 动物分组及模型建立大鼠随机分为对照组、模型组、治疗组,每组10只。模型组按照文献[8]方法建立认知功能障碍模型,水迷宫实验用于评估造模成功。吸入50%空气、氧气混合物(2 L/min)和2%七氟烷,4 h后将大鼠固定于脑立体定位仪,分开颅骨,于左侧脑室(距颅骨表面深度4 mm)注射5 μL生理盐水,注射时间5 min,速度1 μL/min。治疗组在吸入50%空气、氧气混合物(2 L/min)和2%七氟烷4 h后左侧脑室注射5-HT1A受体拮抗剂(3 μg)。模型组和治疗组注射完成后取少量牙托粉水混合剂凝固于颅骨孔道,并进行缝合。对照组仅吸入50%空气、氧气混合物(2 L/min)4 h。
1.4 Morris水迷宫(Morris water maze,MWM)实验参照文献[9],各组大鼠在构建模型前利用MWM实验进行4 d的定位航行训练,模型建立后1 d进行定位航行试验及空间探索实验来评价大鼠学习记忆能力。
1.5 HE和尼氏染色各组取3只大鼠,麻醉并进行心脏灌流,取脑组织,经4%多聚甲醛固定24 h后石蜡包埋,切片后经脱蜡、水合,根据HE、尼氏染色试剂盒说明书进行染色,染色后的切片经脱水、透明和封片后,显微镜下观察。
1.6 高尔基染色各组取3只大鼠,不经麻醉直接断头取脑组织,脑组织经4%多聚甲醛固定24 h后置于高尔基染色液避光染色14 d,期间每日更换高尔基染色液。14 d后取出脑组织置于4 ℃ 30%蔗糖溶液避光3 d,然后切片(4 μm),甘油明胶封片后显微镜下观察。
1.7 免疫荧光染色各组大鼠脱蜡、脱水后的脑组织切片,5% BSA封闭修复2 h,PBS清洗3次,每次5 min,加入稀释后的一抗于4 ℃孵育过夜,次日PBS清洗后加入混有0.1 μg/mL DAPI的荧光二抗,室温避光孵育1 h,PBS洗片后滴加抗荧光淬灭剂进行封片,然后荧光显微镜下观察切片。
1.8 实时荧光定量PCR各组取3只大鼠,麻醉并进行心脏灌流,取脑海马组织提取总RNA,采用FastKing cDNA第一链合成试剂盒将提取的RNA逆转录成cDNA,以cDNA为模板进行PCR扩增。引物序列如下:PKA,正向5’-GAGCAGGAGAGCGTGAAAGA-3’,反向5’-TCCTTGTGCTTCACGAGCAT-3’;CREB1,正向5’-GAGGTGTAGTTTGACGCGGT-3’,反向5’-GGTTGTCTGCTCCAGAGTCC-3’;BDNF,正向5’-GCTGAGCGTGTGTGACAGTA-3’,反向5’-ACTGGGTAGTTCGGCACTGG-3’;GAPDH,正向5’-GCTGAGCGTGTGTGACAGTA-3’,反向5’-ACTGGGTAGTTCGGCACTGG-3’。根据SuperReal荧光定量预混试剂说明书进行定量PCR反应,结果根据2-ΔΔCt方法计算。
1.9 Western blotting各组大鼠脑海马组织经RIPA裂解液裂解提取总蛋白,采用BCA蛋白试剂盒测定蛋白浓度,取30 μg蛋白作为样品,沸水煮样5 min使蛋白充分变性,变性后的样品进行SDS-PAGE凝胶电泳、转膜,5% 脱脂牛奶封闭后一抗4 ℃孵育过夜,次日洗膜后室温二抗孵育30 min。ECL显色后采用全自动化学发光仪发光。采用Image J软件计算获得的蛋白条带的灰度值,目的蛋白条带灰度值与内参蛋白条带灰度值比值即为目的蛋白相对表达量。
1.10 统计学分析应用GraphPad Prism 9.0软件进行统计学处理。计量资料采用x±s表示,组间两两比较采用LSD-t检验,多组间比较采用方差分析。P < 0.05为差异有统计学意义。
2 结果 2.1 各组大鼠空间学习记忆能力比较结果显示,认知功能障碍模型建立前,各组大鼠随着训练时间的延长逃避潜伏期均逐渐缩短。认知功能障碍模型建立后,与对照组比较,模型组大鼠逃避潜伏期明显延长(P < 0.01);与模型组比较,治疗组大鼠逃避潜伏期显著缩短(P < 0.05)。见图 1A。与对照组比较,模型组大鼠平台穿越次数显著减少(P < 0.05);与模型组比较,治疗组大鼠平台穿越次数显著增加(P < 0.05)。见图 1B、1C。表明5-HT1A受体拮抗剂可以改善七氟烷致老年认知功能障碍模型大鼠学习记忆能力。
|
| A, escape latency of the rats in each group; B, frequency of platform crossing of the rats in each group; C, water maze pathway of the rats in each group. **P < 0.01 vs. control group; #P < 0.05 vs. model group. 图 1 各组大鼠逃避潜伏期和平台穿越次数比较 Fig.1 Comparisons of escape latencies and platform crossing frequency among the rats in each group |
2.2 各组大鼠脑组织形态比较
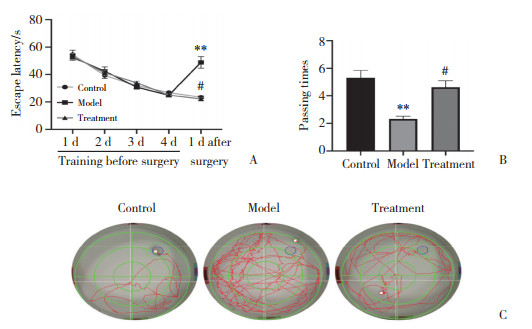
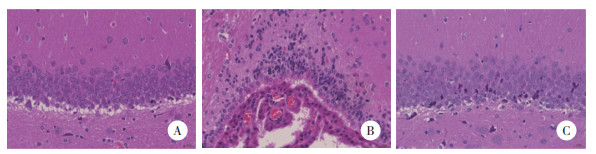
结果显示,对照组大鼠海马组织神经元形态结构清晰完整,排列整齐致密,神经细胞分布均匀,核大规则。神经元尼氏体染色清晰,数量较多。与对照组比较,模型组大鼠海马组织神经元形态结构不规则,排列紊乱疏松,周围组织间隙扩大,细胞核出现深染、固缩。神经元尼氏体减少或消失。与模型组比较,治疗组大鼠海马神经元形态规则,结构相对完整,排列均匀,神经元内尼氏体数量增多。见图 2、3。表明5-HT1A受体拮抗剂具有一定的神经保护作用。
|
| A, control group; B, model group; C, treatment group. 图 2 各组海马神经元的形态改变HE ×400 Fig.2 Morphological changes to neurons in the hippocampal region of each group HE ×400 |
|
| A, control group; B, model group; C, treatment group. 图 3 各组海马神经元的形态改变尼氏染色×400 Fig.3 Morphological changes to neurons in the hippocampal region of each group Nissl staining ×400 |
2.3 各组大鼠海马神经元树突分支与树突棘密度比较
结果显示,与对照组比较,模型组大鼠海马神经元树突分支数量和树突棘密度较显著减少。与模型组比较,治疗组大鼠海马神经元树突分支数量和树突棘密度显著增加。见图 4。表明5-HT1A受体拮抗剂具有增强七氟烷致老年认知功能障碍模型大鼠海马神经元突触可塑性的作用。
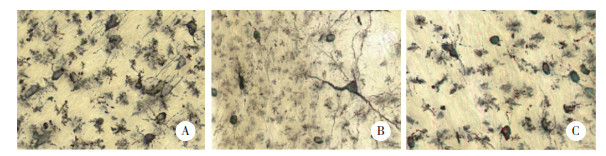
|
| A, control group; B, model group; C, treatment group. 图 4 各组大鼠海马神经元树突分支与树突棘密度比较高尔基染色× 400 Fig.4 Number of dendritic branches and the dendritic spine densities in the hippocampal neurons from each group Golgi ×400 |
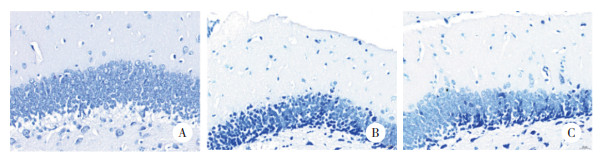
2.4 各组大鼠脑组织中MecP2、p250GAP、PSD-95、GAP-43与Syn蛋白的表达
结果显示,与对照组比较,模型组大鼠脑组织MeCP2、PSD-95、GAP-43、Syn蛋白表达明显减少(均P < 0.01),p250GAP蛋白表达明显增加(P < 0.01)。与模型组比较,治疗组大鼠脑组织MeCP2、PSD-95、GAP-43、Syn蛋白表达显著增加(均P < 0.01),p250GAP蛋白表达明显减少(P < 0.05)。见图 5。
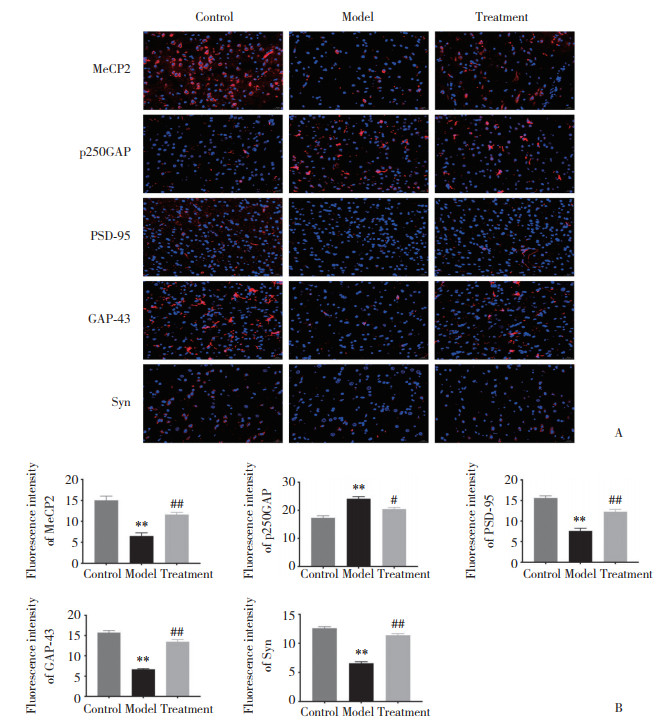
|
| A, immunofluorescence staining results (×400);B, statistical results. **P < 0.01 vs. control group; ##P < 0.01, #P < 0.05 vs. model group. 图 5 各组大鼠脑组织中MecP2、p250GAP、PSD-95、GAP-43与Syn蛋白的表达 Fig.5 Comparisons among the MecP2, p250GAP, PSD-95, GAP-43, and Syn protein expressions in rat brain tissues from each group |
2.5 各组大鼠大脑海马组织中PKA、CREB1和BDNF mRNA的表达
结果显示,与对照组比较,模型组大鼠海马组织中PKA、CREB1、BDNF mRNA表达均显著降低(均P < 0.01)。与模型组比较,治疗组大鼠PKA、CREB1、BDNF mRNA表达均明显升高(均P < 0.01),见图 6。表明5-HT1A受体拮抗剂可增加七氟烷致老年认知功能障碍模型大鼠大脑海马组织中PKA、CREB1、BDNF mRNA表达。见图 6。
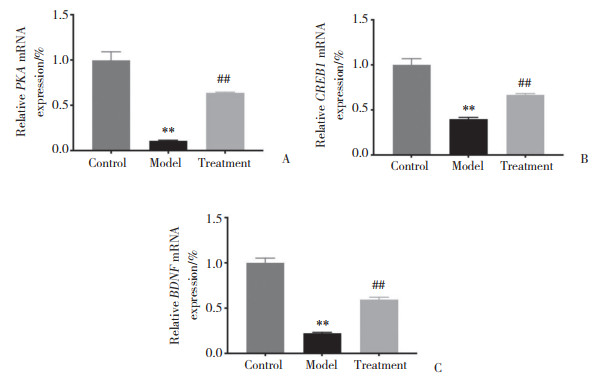
|
| A, PKA; B, CREB1;C, BDNF. **P < 0.01 vs. control group; ##P < 0.01 vs. model group. 图 6 各组大鼠大脑海马组织中PKA、CREB1和BDNF mRNA表达 Fig.6 Comparisons among the PKA, CREB1, and BDNF mRNA expressions in hippocampal tissue from each group |
2.6 各组大鼠大脑海马组织中PKA、CREB1、p-CREB1和BDNF蛋白的表达
结果显示,与对照组比较,模型组大鼠海马组织中PKA、CREB1、p-CREB1、BDNF蛋白表达均明显减少(均P < 0.01);与模型组比较,治疗组大鼠海马组织中PKA、CREB1、p-CREB1、BDNF蛋白表达均显著增加(均P < 0.05),见图 7。可见,5-HT1A受体拮抗剂具有上调七氟烷致老年认知功能障碍模型大鼠大脑海马组织中PKA、CREB1、p-CREB1和BDNF蛋白表达的作用。
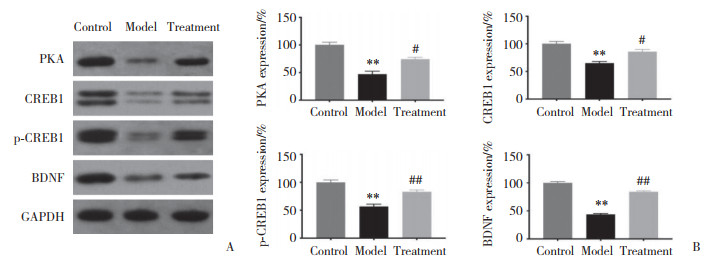
|
| A, protein expression band diagram; B, statistical results of protein expression. **P < 0.01 vs. control group; #P < 0.05, ##P < 0.01 vs. model group. 图 7 各组大鼠大脑海马组织中PKA、CREB1、p-CREB1和BDNF的蛋白表达 Fig.7 Comparisons among the PKA, CREB1, p-CREB1, and BDNF protein expressions in hippocampal tissue from each group |
3 讨论
POCD是老年手术患者麻醉后常见的并发症。已有研究[10]发现七氟烷对小鼠认知功能有影响;更有研究[8]显示七氟烷会导致老年大鼠出现认知功能障碍,且大脑海马区5-HT1A表达上调。5-HT1A受体主要参与情感与认知功能,通过刺激5-HT1A受体可以改善认知障碍大鼠的记忆功能[11]。本研究结果显示,5-HT1A受体拮抗剂可改善七氟烷致老年认知功能障碍模型大鼠的学习记忆能力,与以往研究结果一致。
POCD发病机制错综复杂,越来越多研究[12]表明突触可塑性缺陷与POCD之间存在相关性。已有研究[13]发现手术创伤会下调PSD-95的表达,导致认知能力下降。突触可塑性是神经元之间连接增强或削弱的能力,可反映神经元树突棘形态和密度的变化[14]。本研究结果显示,5-HT1A受体拮抗剂不仅具有七氟烷致老年认知功能障碍模型大鼠的神经保护能力,还可以增加树突分支数量和树突棘密度。MeCP2是大脑中高浓度的转录抑制因子,调节突触蛋白的表达和神经可塑性[15]。p250GAP是一种Rho家族的GTP酶激活蛋白,与多种突触蛋白相作用并水解下游底物上的GTP,从而启动底物相关的信号通路引起细胞骨架解聚,减小树突棘密度及体积[16]。Syn与PSD-95在突触可塑性和认知功能中发挥重要作用,可促进突触可塑性;认知障碍患者脑内Syn与PSD-95水平均较低[17]。GAP-43与轴突发芽和神经末梢可塑性有关,其表达上调促进突触发生[18]。本研究结果显示,5-HT1A受体拮抗剂可促进七氟烷致老年认知功能障碍模型大鼠脑组织中MeCP2、PSD-95、GAP-43和Syn表达并抑制p250GAP表达。
研究[19]发现CREB依赖性转录和BDNF对突触可塑性调节至关重要。BDNF信号促进了PSD-95在突触和树突棘中的表达,并调节了PSD-95的突触后定位。Syn也受BDNF通路调节,在神经元的突触发育和可塑性中起着关键作用。PKA可催化CREB磷酸化,磷酸化的CREB对海马依赖性长期记忆的形成至关重要,可调控下游BDNF蛋白合成,进而促进中枢神经系统的突触传递和发生,调节神经元的发育,进而促进突触可塑性[20]。CREB通路既可正向抑制p250GAP蛋白合成保证突触形态的稳定及正常的生长发育,又可反向上调上游MeCP2蛋白表达显著提高BDNF水平,进而影响突触可塑性及学习记忆[15, 21]。本研究结果显示,5-HT1A受体拮抗剂可以通过上调海马区神经元细胞内PKA、CREB1、BDNF mRNA表达激活CREB/BDNF通路。
综上所述,5-HT1A受体拮抗剂可以改善七氟烷致老年认知功能障碍模型大鼠脑组织海马神经元细胞突触可塑性,其机制可能是通过激活CREB/BDNF信号通路,提高PKA、CREB1、BDNF mRNA及蛋白表达和CREB1磷酸化,促进PSD-95、GAP-43和Syn蛋白表达,进而减缓突触损伤,保护神经元来实现的。本研究仅检测了5-HT1A受体拮抗剂对七氟烷致老年认知功能障碍模型大鼠脑组织中PKA/CREB/BDNF通路的变化以及多种突触相关蛋白表达情况,而未证明5-HT1A受体拮抗剂通过PKA/CREB/BDNF通路影响突触相关蛋白表达,仍须进一步研究论证。
| [1] |
ECKENHOFF RG, MAZE M, XIE Z, et al. Perioperative neurocognitive disorder: state of the preclinical science[J]. Anesthesiology, 2020, 132(1): 55-68. DOI:10.1097/ALN.0000000000002956 |
| [2] |
LIN X, CHEN Y, ZHANG P, et al. The potential mechanism of postoperative cognitive dysfunction in older people[J]. Exp Gerontol, 2020, 130: 110791. DOI:10.1016/j.exger.2019.110791 |
| [3] |
YANG Y, LIU Y, ZHU J, et al. Neuroinflammation-mediated mitochondrial dysregulation involved in postoperative cognitive dysfunction[J]. Free Radic Biol Med, 2022, 178: 134-146. DOI:10.1016/j.freeradbiomed.2021.12.004 |
| [4] |
ZHANG J, ZHU S, JIN P, et al. Graphene oxide improves postoperative cognitive dysfunction by maximally alleviating amyloid beta burden in mice[J]. Theranostics, 2020, 10(26). |
| [5] |
HE J, GAO J, ZHU H, et al. Effects of NBP on postoperative cognitive dysfunction in rats via Nrf 2/ARE pathway[J]. Aging (Albany NY), 2023, 15(1): 276-286. DOI:10.18632/aging.204481 |
| [6] |
QIU LL, PAN W, LUO D, et al. Dysregulation of BDNF/TrkB signaling mediated by NMDAR/Ca (2+)/calpain might contribute to post-operative cognitive dysfunction in aging mice[J]. J Neuroinflammation, 2020, 17(1): 23. DOI:10.1186/s12974-019-1695-x |
| [7] |
MANSVELDER HD, VERHOOG MB, GORIOUNOVA NA. Synaptic plasticity in human cortical circuits: cellular mechanisms of learning and memory in the human brain?[J]. Curr Opin Neurobiol, 2019, 54: 186-193. DOI:10.1016/j.conb.2018.06.013 |
| [8] |
QIU Y, WANG Y, WANG X, et al. Role of the hippocampal 5-HT1A receptor-mediated cAMP/PKA signalling pathway in sevoflurane-induced cognitive dysfunction in aged rats[J]. J Int Med Res, 2018, 46(3): 1073-1085. DOI:10.1177/0300060517744037 |
| [9] |
JIAO YN, ZHANG JS, QIAO WJ, et al. Kai-Xin-San inhibits tau pathology and neuronal apoptosis in aged SAMP8 mice[J]. Mol Neurobiol, 2022, 59(5): 3294-3309. DOI:10.1007/s12035-021-02626-0 |
| [10] |
YU Y, YANG Y, TAN H, et al. Tau Contributes to sevoflurane-induced neurocognitive impairment in neonatal mice[J]. Anesthesiology, 2020, 133(3): 595-610. DOI:10.1097/ALN.0000000000003452 |
| [11] |
LIU XS, XUE QS, ZENG QW, et al. Sevoflurane impairs memory consolidation in rats, possibly through inhibiting phosphorylation of glycogen synthase kinase-3beta in the hippocampus[J]. Neurobiol Learn Mem, 2010, 94(4): 461-467. DOI:10.1016/j.nlm.2010.08.011 |
| [12] |
XUE Z, SHUI M, LIN X, et al. Role of BDNF/ProBDNF imbalance in postoperative cognitive dysfunction by modulating synaptic plasticity in aged mice[J]. Front Aging Neurosci, 2022, 14: 780972. DOI:10.3389/fnagi.2022.780972 |
| [13] |
XIONG C, LIU J, LIN D, et al. Complement activation contributes to perioperative neurocognitive disorders in mice[J]. J Neuroinflammation, 2018, 15(1): 254. DOI:10.1186/s12974-018-1292-4 |
| [14] |
LIU X, WANG J. N-methyl-D-aspartate receptors mediate synaptic plasticity impairment of hippocampal neurons due to arsenic exposure[J]. Neuroscience, 2022, 498: 300-310. DOI:10.1016/j.neuroscience.2022.07.017 |
| [15] |
YAO ZH, YAO XL, ZHANG Y, et al. miR-132 down-regulates methyl CpG binding protein 2(MeCP2) during cognitive dysfunction following chronic cerebral hypoperfusion[J]. Curr Neurovasc Res, 2017, 14(4): 385-396. DOI:10.2174/1567202614666171101115308 |
| [16] |
SUN W, YANG J, HONG Y, et al. Lanthanum chloride impairs learning and memory and induces dendritic spine abnormality by down-regulating Rac1/PAK signaling pathway in hippocampus of offspring rats[J]. Cell Mol Neurobiol, 2020, 40(3): 459-475. DOI:10.1007/s10571-019-00748-7 |
| [17] |
LIU Y, ZHANG Y, ZHENG X, et al. Galantamine improves cognition, hippocampal inflammation, and synaptic plasticity impairments induced by lipopolysaccharide in mice[J]. J Neuroinflammation, 2018, 15(1): 112. DOI:10.1186/s12974-018-1141-5 |
| [18] |
MIRSHAFA A, MOHAMMADI H, SHOKRZADEH M, et al. Tropisetron protects against brain aging via attenuating oxidative stress, apoptosis and inflammation: the role of SIRT1 signaling[J]. Life Sci, 2020, 248: 117452. DOI:10.1016/j.lfs.2020.117452 |
| [19] |
JIN G, ZHU L, LIU P, et al. Xanthoceraside prevented synaptic loss and reversed learning-memory deficits in APP/PS1 transgenic mice[J]. J Physiol Sci, 2019, 69(3): 477-488. DOI:10.1007/s12576-019-00664-x |
| [20] |
SHARMA VK, SINGH TG. CREB: a multifaceted target for Alzheimer's disease[J]. Curr Alzheimer Res, 2020, 17(14): 1280-1293. DOI:10.2174/1567205018666210218152253 |
| [21] |
MARLER KJ, SUETTERLIN P, DOPPLAPUDI A, et al. BDNF promotes axon branching of retinal ganglion cells via miRNA-132 and p250GAP[J]. J Neurosci, 2014, 34(3): 969-979. DOI:10.1523/JNEUROSCI.1910-13.2014 |
 2024, Vol. 53
2024, Vol. 53