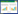文章信息
- 刘晓晨, 金娜, 高源泽, 史宝湘, 单伟
- LIU Xiaochen, JIN Na, GAO Yuanze, SHI Baoxiang, SHAN Wei
- 淫羊藿苷调控BDNF/TrkB信号通路及突触可塑性对创伤后应激障碍大鼠恐惧记忆的改善作用
- Effects of icariin on BDNF/TkB signaling pathway and synaptic plasticity regulation on fear memory improvement in rats with post-traumatic stress disorder
- 中国医科大学学报, 2024, 53(1): 27-33
- Journal of China Medical University, 2024, 53(1): 27-33
-
文章历史
- 收稿日期:2023-04-03
- 网络出版时间:2024-01-04 19:36:57
2. 盘锦市中心医院VIP病房2病区, 辽宁 盘锦 124010;
3. 锦州医科大学第三临床医学院, 辽宁 锦州 121001;
4. 锦州医科大学护理学院, 辽宁 锦州 121001
2. VIP Ward 2 of Panjin Central Hospital, Panjin 124010, China;
3. Third Clinical Medical College of Jinzhou Medical University, Jinzhou 121001, China;
4. School of Nursing, Jinzhou Medical University, Jinzhou 121001, China
创伤后应激障碍(post traumatic stress disorder,PTSD)是重大的公共卫生问题,通常由创伤事件引起,如战争、恐怖袭击、自然灾害等[1]。PTSD的特征是对环境记忆的过度反应和恐惧消除受损[2],而杏仁核在恐惧记忆中发挥重要作用[3]。淫羊藿苷是从传统中草药淫羊藿中提取的类黄酮,可以自由穿过血脑屏障,抑制神经炎症反应,减轻氧化应激损伤,具有很强的神经保护作用[4]。淫羊藿苷在社会失败应激小鼠模型中具有抗抑郁作用,且在多种抑郁症模型中显示出抗抑郁药样活性。淫羊藿苷在大鼠慢性轻度应激抑郁模型中通过调节5-羟色胺能系统功能过度活动,降低神经炎症反应,同样也表现出有效的抗抑郁作用[5]。此外,通过纳米凝胶化合物系统鼻内给药淫羊藿苷,能够改善大鼠抑郁状态[6]。然而,淫羊藿苷在单一连续应激(single-prolonged stress,SPS)诱导的PTSD动物模型中的治疗效果尚不清楚。本研究拟探讨淫羊藿苷对SPS大鼠恐惧记忆的影响,为研究PTSD的治疗方案拓展思路。
1 材料与方法 1.1 动物模型制备及分组选取雄性SD大鼠40只,体质量180~220 g(购自锦州医科大学,许可证号:SYXK [辽] 2019-0007,实验伦理编号2022040201)。应用SPS法构建PTSD大鼠模型[7]。将模型大鼠分为SPS组、淫羊藿苷组及淫羊藿苷+K252a组,每组10只;另取10只正常大鼠作为对照组。淫羊藿苷组及淫羊藿苷+K252a组大鼠均于SPS造模1 d后灌胃给药淫羊藿苷,剂量为20 mg/kg,1次/d,共2周[8]。对照组和SPS组给予等剂量生理盐水。K252a(1 mmol/L)给药方法:脑立体定位仪固定大鼠,用微量注射器缓慢、匀速地注入大鼠侧脑室内(速度约为0.4 μL/min),并留针2 min,K252a为造模7 d后单次给药。2周后停止给药,第15天进行行为学测试,然后处死动物进行相关实验,实验遵循国家《实验动物管理条例》。
1.2 主要试剂及仪器淫羊藿苷(批号:110737-201516),脑源性神经营养因子(brain derived neurotrophic factor,BDNF)一抗(兔抗大鼠)、酪氨酸激酶受体B(tyrosine kinase receptor B,TrkB)一抗(小鼠抗大鼠)、突触后密度蛋白(postsynaptic density protein95,PSD95)一抗(兔抗大鼠)、突触素(synaptophysin,SYN)一抗(兔抗大鼠)、TrkB抑制剂K252a购自英国Abcam公司,冰冻切片机(德国SLEE公司),倒置显微镜(日本Olympus公司),水平电泳仪(美国BIO-RAD公司)。
1.3 旷场实验旷场实验可评估大鼠在陌生环境中的焦虑、恐惧状态。在实验开始前,所有动物在测试室中适应20 min。在安静的环境下,依次将大鼠置于旷场的中心,并通过自动分析系统记录大鼠5 min的行为,包括进入中心区域的次数和中心区域运动距离的百分比等。
1.4 大鼠高架十字迷宫实验应用AMY-maze软件观察并记录5 min内小鼠的行为活动。包括进入开臂的次数(open arm entry,OE)和进入开臂的时间(arm opening time,OT),进入闭臂的次数(closed arm entry,CE)和进入闭臂的时间(arm closing time,CT)(以小鼠躯干中心进入为准),OE%= OE/(OE+CE)×100以及OT% = OT/(OT+CT)×100。
1.5 条件性恐惧测试采用AMY-maze软件记录其5 min僵住时间,并记录排便次数。僵住时间主要反映小鼠重新接触刺激环境的条件恐惧,而排便次数在一定程度上可以反映小鼠的恐惧和焦虑情绪。
1.6 样本制备2周后,每组取5只大鼠,固定后取出大脑进行冰冻切片,用于免疫组化和免疫荧光染色。另每组取5只大鼠活取大脑,裂解制备上清,-20℃保存用于Western blotting检测。
1.7 分子对接选取核心靶点进行分子对接。在PubChem数据库(https://pubchem.ncbi.nlm.nih.gov/)中下载淫羊藿苷的三维结构,结合PDB数据库(https://www.rcsb.org/)中核心靶点的蛋白结构,运用AutoDock Vina对淫羊藿苷与核心靶点进行分子对接验证,并利用Pymol对部分结果进行可视化分析。
1.8 免疫组织化学检测大鼠杏仁核BDNF和TrkB表达切片用PBS洗涤3次,5 min/次;3% H2O2室温避光孵育10 min,10%山羊血清封闭30 min。加入兔抗大鼠BDNF(1∶500)和TrkB(1∶400)4 ℃孵育过夜。复温45 min后用PBS洗涤,加入与HRP偶联的二抗孵育30 min。DAB显色,苏木素染核。光学显微镜下拍照观察。
1.9 Western blotting检测BDNF和TrkB蛋白的相对表达提取杏仁核总蛋白,BCA法测蛋白浓度。10% SDS-PAGE凝胶电泳,后转移至PVDF膜,以含1% BSA的TBST室温封闭3 h;加入一抗4 ℃过夜;TBST洗涤5次;加入HRP标记的二抗室温2 h,TBST洗涤5次;ECL试剂盒显影,Image J软件分析灰度值。
1.10 免疫荧光检测大鼠杏仁核PSD95和SYN表达切片用PBS洗涤3次,5 min/次;0.3% Triton X-100摇床30 min;PBS洗涤3次,5 min/次;10%山羊血清室温孵育30 min;不洗,滴加兔抗大鼠PSD95(1∶400)和小鼠抗大鼠SYN(1∶300),4 ℃过夜;PBS洗涤3次,3 min/次;滴加荧光二抗,室温30 min;PBS洗涤3次,3 min/次;含DAPI的封片剂封片,并应用荧光显微镜拍照及荧光强度测定。
1.11 统计学分析所有符合正态分布的计量资料以x±s表示,采用SPSS 20.0统计软件进行单因素方差分析,组间两两比较采用SNK检验,P < 0.05为差异有统计学意义。
2 结果 2.1 淫羊藿苷对SPS大鼠自主行为和探究行为的影响旷场实验结果显示,与对照组相比,SPS组和淫羊藿苷+K252a组进入中心区域次数和中心区域运动距离百分比明显降低(P < 0.05)。而与SPS组相比,淫羊藿苷组进入中心区域次数和中心区域运动距离百分比明显增加(P < 0.05),见图 1、表 1。
|
| A, control group; B, SPS group; C, icariin group; D, icariin+K252a group. 图 1 4组大鼠运动轨迹 Fig.1 Track map of four groups of rats |
| Group | Number of times entering central area | Percentage of movement distance in central area(%) |
| Control | 6.25±0.47 | 13.28±1.02 |
| SPS | 3.85±0.351) | 8.69±0.851) |
| Icariin | 5.31±0.422) | 11.29±0.962) |
| Icariin+K252a | 3.72±0.391),3) | 8.72±0.781),3) |
| 1)P < 0.05 vs. control group;2)P < 0.05 vs. SPS group;3)P < 0.05 vs. icariin group. | ||
2.2 淫羊藿苷对SPS大鼠焦虑状态的影响
高架十字迷宫实验结果显示,与对照组相比,SPS组和淫羊藿苷+K252a组OE和OT明显降低(P < 0.05)。而与SPS组相比,淫羊藿苷组OE和OT明显增加(P < 0.05),见表 2。
| Group | OE(%) | OT(%) |
| Control | 12.08±1.32 | 10.26±1.14 |
| SPS | 7.23±0.891) | 5.23±0.791) |
| Icariin | 10.84±1.082) | 8.16±0.802) |
| Icariin+K252a | 7.42±0.861),3) | 5.41±0.851),3) |
| 1)P < 0.05 vs. control group;2)P < 0.05 vs. SPS group;3)P < 0.05 vs. icariin group. | ||
2.3 淫羊藿苷对SPS大鼠恐惧状态的影响
条件性恐惧测试结果显示,与对照组相比,SPS组和淫羊藿苷+K252a组僵住时间和排便次数明显增加(P < 0.05)。而与SPS组相比,淫羊藿苷组僵住时间和排便次数明显降低(P < 0.05),见表 3。
| Group | Freeze time(s) | Frequency of stools |
| Control | 3.56±0.35 | 1.50±0.45 |
| SPS | 50.29±2.781) | 4.50±1.501) |
| Icariin | 10.84±1.022) | 2.50±0.852) |
| Icariin+K252a | 51.82±2.951),3) | 4.00±1.501),3) |
| 1)P < 0.05 vs. control group;2)P < 0.05 vs. SPS group;3)P < 0.05 vs. icariin group. | ||
2.4 分子对接验证淫羊藿苷与BDNF的结合活性
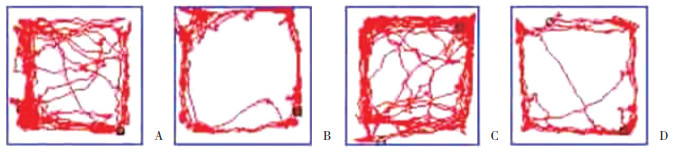
分子对接验证淫羊藿苷与BDNF的关系。一般结合能 < -4.25 kcal/mol表明配体小分子与受体蛋白之间有一定的结合活性;结合能 < -5.0 kcal/mol表明二者之间有较好的结合活性;结合能 < -7.0 kcal/mol表明配体与受体具有强烈的结合活性。分子对接结果显示淫羊藿苷与BDNF的结合能为-5.52 kcal/mol,说明两者之间有较好的结合活性。依据自由结合能的大小,选取部分对接结果进行可视化,利用PyMOL软件绘图(图 2)。结果显示,淫羊藿苷能稳定地对接到BDNF蛋白结构2RTU的口袋中,二者通过氨基酸残基TYR-96、SER-32和ARG-104发生氢键作用,说明在PTSD中BDNF是淫羊藿苷的关键靶点。
|
| 图 2 淫羊藿苷与关键靶标BDNF的分子对接图 Fig.2 Molecular docking diagram of icariin and key target brain-derived neurotrophic factor |
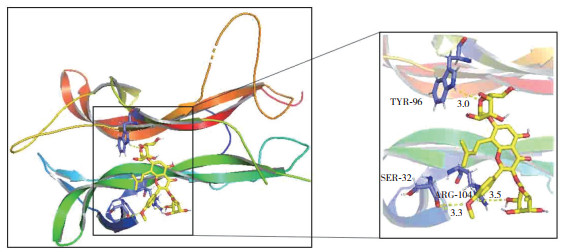
2.5 淫羊藿苷对SPS大鼠杏仁核BDNF和TrkB蛋白表达的影响
与对照组相比,SPS组和淫羊藿苷+K252a组BDNF、TrkB表达明显降低(P < 0.05)。而与SPS组相比,淫羊藿苷组BDNF、TrkB表达明显增加(P < 0.05),见图 3。
|
| A, immunohistochemical staining of BDNF and TrkB (×200);B, Western blotting detection of relative expressions of BDNF and TrkB proteins; C, statistical chart of relative expressions of BDNF and TrkB proteins. BDNF, brain-derived neurotrophic factor; TrkB, tyrosine kinase receptor B. 1, control group; 2 SPS group; 3, icariin group; 4, icariin+K252a group. *P < 0.05 vs. control group; #P < 0.05 vs. SPS group; △P < 0.05 vs. icariin group. 图 3 各组大鼠杏仁核BDNF和TrkB蛋白的表达 Fig.3 Expressions of BDNF and TrkB proteins in the amygdalae of rats by study group |
2.6 淫羊藿苷对SPS大鼠杏仁核PSD95和SYN表达的影响
将对照组BDNF免疫荧光强度值设定为(100.00±0.00)%,荧光强度值分析结果显示,与对照组相比,SPS组和淫羊藿苷+K252a组PSD95和SYN荧光强度明显降低(P < 0.05)。而与SPS组相比,淫羊藿苷组PSD95和SYN荧光强度明显增加(P < 0.05)。SPS组和淫羊藿苷+K252a组比较,差异无统计学意义(P > 0.05),见图 4。
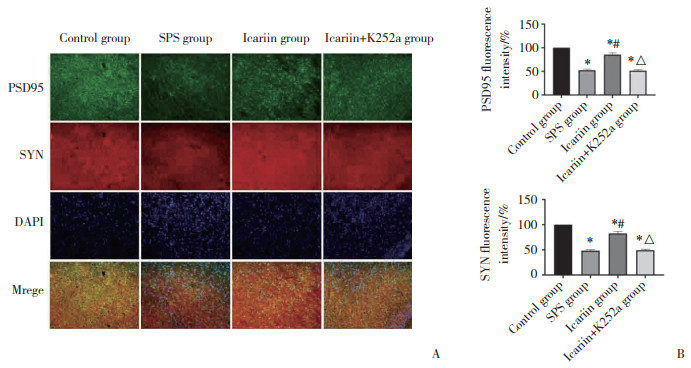
|
| A, PSD95 and SYN immunofluorescence staining (×200);B, PSD95 and SYN fluorescence intensity statistical charts. PSD95, postsynaptic density protein 95;SYN, synaptophysin. *P < 0.05 vs. control group; #P < 0.05 vs. SPS group; △P < 0.05 vs. icariin group. 图 4 大鼠杏仁核PSD95和SYN的免疫荧光染色 Fig.4 Immunofluorescence staining of PSD95 and SYN in rat amygdalae |
3 讨论
无法消除恐惧是PTSD等许多精神疾病的特征。杏仁核是恐惧记忆的关键结构,参与恐惧记忆消除[9]。本研究结果显示,SPS诱导的大鼠出现了恐惧记忆异常,提示模型诱导成功,这为下一步治疗奠定了基础。
淫羊藿苷,俗称角山羊草或阴阳火,是一种从传统中草药淫羊藿中提取的类黄酮,具有多种药理学作用[10]。在抑郁啮齿动物的强迫游泳试验和悬尾试验中,淫羊藿苷缩短了僵住时间,证明了可能的抗抑郁作用[5]。PTSD造成的恐惧记忆异常是一种精神障碍,从理论上讲,淫羊藿苷可以对其起到防治作用。本研究对SPS诱导的大鼠给予淫羊藿苷灌胃治疗后,与SPS组相比,淫羊藿苷治疗组大鼠进入中心区域次数和中心区域运动距离百分比明显增加,OE和OT明显增加,僵住时间和排便次数明显降低,这提示淫羊藿苷对SPS诱导的大鼠出现的恐惧记忆异常具有防治作用,但其具体机制尚不清楚。
BDNF已被证明能够促进神经元存活、分化、功能和可塑性[11-12]。在PTSD患者的大脑和血液中也观察到促炎细胞因子水平升高,这些细胞因子通过氧化应激增强等机制介导PTSD的发生[13]。淫羊藿苷还逆转了皮质酮诱导的海马BDNF水平降低,与FST中的不动性降低有关[14]。这些结果证实了淫羊藿苷类抗抑郁活性中抗神经炎症机制的重要性。抗抑郁药已被证明可以逆转和保护海马体中抑郁诱导的BDNF下调[15]。此外,研究[16]表明,外源性淫羊藿苷治疗可显著上调大鼠海马中BDNF的表达。本研究发现淫羊藿苷减弱了SPS诱导的大鼠杏仁核BDNF水平下降,表明BDNF在介导淫羊藿苷的抗PTSD中起重要作用。TrkB是BDNF的特异性受体,为了验证淫羊藿苷调控BDNF的可靠性,本研究在大鼠给予淫羊藿苷后又加入了TrkB抑制剂,结果发现,淫羊藿苷的保护作用被成功逆转,这说明BDNF/TrkB通路确实在淫羊藿苷抑制SPS诱导的大鼠恐惧记忆障碍中起关键作用。
突触相关蛋白,尤其是突触前SYN和突触后PSD95,可以促进突触可塑性形成。SYN和PSD95缺陷与恐惧记忆的形成有关[17]。研究[18]发现,电针对PTSD大鼠恐惧记忆消退的影响可能是通过其对杏仁核突触可塑性的修复。本研究结果显示,淫羊藿苷给药后明显抑制SPS诱导的杏仁核中SYN和PSD95的下调。此外,在给予淫羊藿苷后又加入了TrkB抑制剂,结果发现,淫羊藿苷的保护作用被成功逆转,这说明BDNF/TrkB通路与突触可塑性存在调控关系。
综上所述,淫羊藿苷可以有效缓解SPS诱导的大鼠恐惧记忆障碍,这种保护作用可能与激活BDNF/TrkB通路及上调突触相关蛋白SYN和PSD95有关。当然,PTSD发病机制复杂,淫羊藿苷对其的保护作用有待进一步深入探讨。
| [1] |
MAERCKER A, CLOITRE M, BACHEM R, et al. Complex post-traumatic stress disorder[J]. Lancet, 2022, 400(10345): 60-72. DOI:10.1016/S0140-6736(22)00821-2 |
| [2] |
LIN CC, LIU YP. Pharmacological implications of adjusting abnormal fear memory: towards the treatment of post-traumatic stress disorder[J]. Pharmaceuticals (Basel), 2022, 15(7): 788. DOI:10.3390/ph15070788 |
| [3] |
赖树盛, 王晗, 褚建祎, 等. 磁共振影像组学诊断脑卒中后创伤后应激障碍[J]. 中国医科大学学报, 2022, 51(7): 604-610. DOI:10.12007/j.issn.0258-4646.2022.07.006 |
| [4] |
赵天池. 淫羊藿苷药理活性研究进展[J]. 中国民康医学, 2021, 33(12): 84-85. DOI:10.3969/j.issn.1672-0369.2021.12.034 |
| [5] |
WANG YS, SHEN CY, JIANG JG. Antidepressant active ingredients from herbs and nutraceuticals used in TCM: pharmacological mechanisms and prospects for drug discovery[J]. Pharmacol Res, 2019, 150(4): 104520. DOI:10.1016/j.phrs.2019.104520 |
| [6] |
XU D, LU YR, KOU N, et al. Intranasal delivery of icariin via a nanogel-thermoresponsive hydrogel compound system to improve its antidepressant-like activity[J]. Int J Pharm, 2020, 586(8): 119550. DOI:10.1016/j.ijpharm.2020.119550 |
| [7] |
SHAN W, HAN F, XU Y, et al. Stathmin regulates spatiotemporal variation in the memory loop in single-prolonged stress rats[J]. J Mol Neurosci, 2020, 70(4): 576-589. DOI:10.1007/s12031-019-01459-w |
| [8] |
WU X, WU J, XIA S, et al. Icaritin opposes the development of social aversion after defeat stress via increases of GR mRNA and BDNF mRNA in mice[J]. Behav Brain Res, 2013, 256(11): 602-608. DOI:10.1016/j.bbr.2013.09.034 |
| [9] |
RESSLER RL, MAREN S. Synaptic encoding of fear memories in the amygdala[J]. Curr Opin Neurobiol, 2019, 54(4): 54-59. DOI:10.1016/j.conb.2018.08.012 |
| [10] |
HE CY, WANG Z, SHI JS. Pharmacological effects of icariin[J]. Adv Pharmacol, 2020, 87: 179-203. DOI:10.1016/bs.apha.2019.10.004 |
| [11] |
彭娜, 徐琳琳, 魏博, 等. 舒芬太尼预处理对脑缺血-再灌注损伤大鼠脑内BDNF-CREB信号通路的影响[J]. 解剖科学进展, 2021, 27(6): 685-688, 692. DOI:10.16695/j.cnki.1006-2947.2021.06.012 |
| [12] |
ZHUANG X, ZHAN B, JIA Y, et al. IL-33 in the basolateral amygdala integrates neuroinflammation into anxiogenic circuits via modulating BDNF expression[J]. Brain Behav Immun, 2022, 102(5): 98-109. DOI:10.1016/j.bbi.2022.02.019 |
| [13] |
NOTARAS M, VAN DEN BUUSE M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders[J]. Mol Psychiatry, 2020, 25(10): 2251-2274. DOI:10.1038/s41380-019-0639-2 |
| [14] |
GONG MJ, HAN B, WANG SM, et al. Icariin reverses corticosterone-induced depression-like behavior, decrease in hippocampal brain-derived neurotrophic factor (BDNF) and metabolic network disturbances revealed by NMR-based metabonomics in rats[J]. J Pharm Biomed Anal, 2016, 123(4): 63-73. DOI:10.1016/j.jpba.2016.02.001 |
| [15] |
ZHANG XX, SUN HL, SU Q, et al. Antidepressant-like activity of icariin mediated by group I mGluRs in prenatally stressed offspring[J]. Brain Dev, 2017, 39(7): 593-600. DOI:10.1016/j.braindev.2017.03.021 |
| [16] |
LU Q, ZHU HL, LIU XJ, et al. Icariin sustains the proliferation and differentiation of Aβ25-35-treated hippocampal neural stem cells via the BDNF-TrkB-ERK/Akt signaling pathway[J]. Neurol Res, 2020, 42(11): 936-945. DOI:10.1080/01616412.2020.1792701 |
| [17] |
WU PF, GUAN XL, WANG F, et al. N-acetylcysteine facilitates extinction of cued fear memory in rats via reestablishing basolateral amygdala glutathione homeostasis[J]. Acta Pharmacol Sin, 2022, 43(2): 260-272. DOI:10.1038/s41401-021-00661-0 |
| [18] |
LI M, LI K, ZHANG H, et al. Study on the mechanism of TMRK electroacupuncture in repairing synaptic plasticity in amygdala and hippocampus to relieve fear memory in PTSD rats[J]. Technol Health Care, 2019, 27: 425-443. DOI:10.3233/thc-199038 |
 2024, Vol. 53
2024, Vol. 53