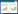文章信息
- 邱忠凯, 刘俊龙, 丁全明, 梁伟
- QIU Zhongkai, LIU Junlong, DING Quanming, LIANG Wei
- 膀胱癌患者血清中肿瘤异常蛋白和细胞角蛋白19的表达及其临床意义
- Expression and clinical significance of tumor abnormal protein and cytokeratin 19 in the serum of patients with bladder cancer
- 中国医科大学学报, 2023, 52(7): 628-632
- Journal of China Medical University, 2023, 52(7): 628-632
-
文章历史
- 收稿日期:2022-04-14
- 网络出版时间:2023-07-06 14:14:17
膀胱癌是全球范围内常见的癌症类型之一[1]。根据美国癌症协会预测,2022年美国将有约81 180例膀胱癌新发病例和17 100例膀胱癌相关死亡病例[2]。男性膀胱癌发病率较高,发病年龄多为60岁以上,吸烟、老龄化和接触化学工业产品等是膀胱癌的风险因素[3]。近年来,膀胱癌的临床诊断和治疗方式取得了一定进展,但膀胱癌的高转移率一直是困扰临床治疗的难题[4]。因此,全面了解膀胱癌的发病机制、寻找有意义的临床辅助诊断指标有助于改善患者的预后。近年研究[5]发现癌细胞在代谢过程中,释放出复杂的异常糖蛋白和钙阻蛋白,组成肿瘤异常蛋白(tumor abnormal protein,TAP)。当肿瘤细胞产生的TAP达到一定量时可在患者外周血或末梢血中检出。研究[6]表明TAP在乳腺癌、结肠癌、胃癌等恶性肿瘤患者血清中表达上调。细胞角蛋白(cytokeratin,CK)是一类由CK基因编码的水溶性聚合多肽。CK19是一种CK蛋白小分子,主要分布于上皮组织,是构成细胞骨架的中间微丝,广泛表达于肺癌、结肠癌和乳腺癌等恶性肿瘤中[7]。TAP和CK19在膀胱癌患者血清中的表达水平研究鲜有报道。血清肿瘤标志物检测具有便捷、快速的优点,近年来已获得临床认可[8]。因此,对早期恶性肿瘤进行血清学肿瘤标志物检测具有重要参考价值。本研究探讨膀胱癌患者血清中TAP及CK19的表达情况,分析两者之间及与膀胱癌的临床病理特征间的相关性,旨在为膀胱癌的诊断提供新的参考及依据。
1 材料与方法 1.1 临床资料及分组选取2020年1月至2021年1月我院收治的70例膀胱癌患者作为膀胱癌组。纳入标准:(1)病理学确诊为膀胱癌且为初发;(2)无其他恶性肿瘤;(3)未接受放、化疗等其他治疗。排除标准:(1)合并泌尿系统其他基础疾病;(2)数据不完整。其中男42例,女28例;年龄46~79岁,平均年龄(64.60±9.05)岁;肌层浸润25例,非肌层浸润45例;淋巴结转移阳性16例,肿瘤直径 < 3 cm 44例。选取同期我院体检的63例健康人群作为对照组,其中男34例,女29例;年龄48~78岁,平均年龄(62.65±7.49)岁。选取同期我院诊治的泌尿系统良性疾病患者54例为良性组,其中男29例,女25例;年龄45~77岁,平均年龄(61.57±8.14)岁。本研究已获得我院医学伦理委员会批准,患者及家属均知情同意并签署知情同意书。3组年龄、性别比较差异均无统计学意义(均P > 0.05)。
1.2 主要试剂及仪器TAP检测试剂盒(20173401893)及TAP分析系统仪(产品型号RS2011-A)购自浙江瑞生公司,CK19酶联免疫吸附试验(enzyme limked immunosorbent assay,ELISA)试剂盒购自武汉博士康生物工程有限公司,酶标仪购自美国Bio-Rad公司,荧光显微镜购自日本Olympus公司。
1.3 检测方法抽取患者清晨空腹静脉血(约3 mL),EDTA抗凝,按照试剂盒说明书进行血清TAP及CK19指标检测。每个标本重复3次并计算平均值。
1.3.1 凝集法检测血清TAP水平取约1 mL新鲜全血制备载玻片,均匀涂片后室温下干燥15 min,载玻片中加入凝固剂,2 h后观察测量形成的凝集粒子面积。凝聚物颗粒面积≥121 μm2时判定TAP高表达,否则为TAP低表达。
1.3.2 ELISA检测血清CK19水平全血离心后分离上层血清,设置标准品孔和样本孔,标准品孔及样本孔加入CK19抗体后按照说明书步骤加入终止液,随后用酶标仪检测各孔的吸光度(optical density,OD)值。根据样本OD值计算对应的浓度值。CK19正常范围为0~7 ng/mL,> 7 ng/mL为高表达,否则为低表达。
1.4 统计学分析利用SPSS 23.0和GraphPad Prism 7.0软件进行统计分析,正态分布的计量资料采用x±s表示,多组间比较采用单因素方差分析;不符合正态分布的计量资料采用M(P25~P75)表示,组间比较采用Mann-Whitney U检验。计数资料组间比较采用χ2检验。采用Spearman线性相关分析TAP及CK19相关性。受试者操作特征(receiver operating characteristic,ROC)曲线分析及图形绘制采用MedCalc 20.106统计软件。P < 0.05为差异有统计学意义。
2 结果 2.1 3组血清中TAP及CK19表达水平比较结果显示,与对照组和良性组比较,膀胱癌组TAP及CK19表达水平显著增高(均P < 0.001),而对照组和良性组TAP及CK19表达水平比较无统计学差异(均P > 0.05)。见图 1。
|
| A,TAP expression;B,CK19 expression. *** P < 0.001. 图 1 3组TAP和CK19表达水平比较 Fig.1 Expression levels of TAP and CK19 in different groups |
2.2 膀胱癌患者血清中TAP及CK19表达水平与各项临床指标的相关性
结果显示,TAP和CK19表达水平与肿瘤直径明显相关(均P < 0.05);TAP表达与肿瘤浸润深度明显相关(P < 0.01)。肿瘤越大,TAP及CK19表达水平越高。而TAP表达水平与年龄、性别、淋巴结转移均不相关(均P > 0.05);CK19表达与年龄、性别、淋巴结转移和肿瘤肿瘤浸润深度均不相关(均P > 0.05)。见表 1。
| Item | n | TAP expression | P | CK19 expression | P | ||
| Low | High | Low | High | ||||
| Sex | |||||||
| Male | 42 | 13 | 29 | 0.589 2 | 15 | 27 | 0.533 1 |
| Female | 28 | 7 | 21 | 8 | 20 | ||
| Age | |||||||
| ≥60 year | 48 | 15 | 33 | 0.463 7 | 18 | 30 | 0.221 9 |
| < 60 year | 22 | 5 | 17 | 5 | 17 | ||
| Invasion depth | |||||||
| Non muscle | 45 | 18 | 27 | 0.004 5 | 13 | 32 | 0.342 9 |
| Muscle | 25 | 2 | 23 | 10 | 15 | ||
| Lymphatic invasion | |||||||
| Positive | 16 | 3 | 13 | 0.322 1 | 6 | 10 | 0.652 6 |
| Negative | 54 | 17 | 37 | 17 | 37 | ||
| Tumor size | |||||||
| ≥3 cm | 26 | 3 | 23 | 0.015 3 | 2 | 24 | 0.000 6 |
| < 3 cm | 44 | 17 | 27 | 21 | 23 | ||
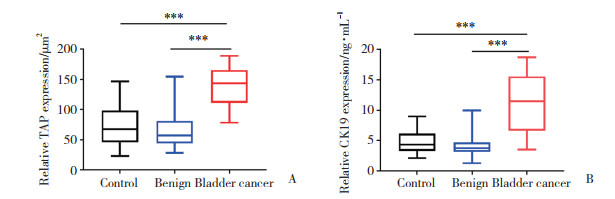
2.3 膀胱癌患者血清中TAP及CK19水平的相关性
Spearman线性相关分析膀胱癌患者血清TAP及CK19水平的相关性。结果显示,血清TAP和CK19水平呈正相关(r = 0.362 3,P = 0.002 1),见图 2。
|
| 图 2 膀胱癌患者血清中TAP和CK19表达的相关分析 Fig.2 Correlation between TAP and CK19 expression in the serum of bladder cancer patients |
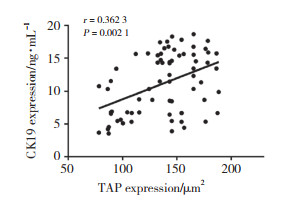
2.4 血清TAP和CK19单独及联合检验对膀胱癌的诊断价值
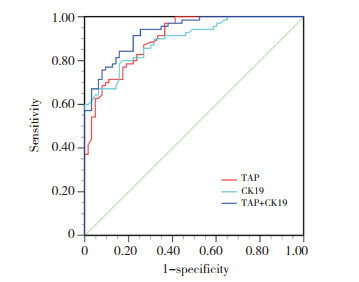
以TAP ≥ 121 μm2,CK19 > 7 ng/mL为阳性诊断标准绘制ROC曲线,TAP、CK19和二者联合检测的曲线下面积(area under curve,AUC)分别为0.904、0.895、0.932,约登指数分别为0.606、0.627、0.692,灵敏度分别为0.971、0.786、0.914,特异度分别为0.635、0.841、0.778。联合检测的诊断价值明显优于TAP及CK19单独检测见图 3、表 2。
|
| 图 3 TAP、CK19单独及联合检测的ROC曲线 Fig.3 ROC curve of TAP, CK19 and the combination of TAP and CK19 |
| Methods | Sensitivity | Specificity | Cut-off | AUC | 95%CI | P | Youden | |
| Lower | Upper | |||||||
| TAP(μm2) | 0.971 | 0.635 | 126.5 | 0.904 | 0.840 | 0.948 | < 0.001 | 0.606 |
| CK19(ng/mL) | 0.786 | 0.841 | 6.6 | 0.895 | 0.829 | 0.941 | < 0.001 | 0.627 |
| TAP+CK19 | 0.914 | 0.778 | - | 0.932 | 0.874 | 0.968 | < 0.001 | 0.692 |
3 讨论
目前,膀胱镜下膀胱黏膜病理活检以及辅助的尿脱落细胞学检测被认为是膀胱癌诊断的金标准[9]。膀胱癌治疗方式主要包括手术治疗(膀胱镜下电切、膀胱全切、淋巴结清扫等)和术后生物免疫治疗(膀胱内灌注化疗药物、卡介苗等)。已有研究[10]显示约75%患者初步诊断时为非肌层浸润性膀胱癌。非肌层浸润性膀胱癌有复发或发展为肌层浸润性的趋势,因此定期膀胱镜下评估是必需的[11]。然而膀胱镜检查是侵入性检查,而且费用昂贵,因此寻找无创且经济的早期诊断方法尤为重要。
近年来,越来越多的血清肿瘤标志物(AFP、CEA、CA19-9等[12])被应用于临床前诊断及筛查。研究[13]表明TAP的产生与肿瘤发生、发展、转移及预后存在密切关系。TAP水平间接表明细胞癌变的数量和程度[5]。结直肠癌患者中TAP的检测具有较高的灵敏度和特异度,此外,TAP也被作为一种新的预后独立指标应用于结直肠癌患者化疗和临床监测中[14]。CK19作为上皮细胞分化的特异标志物,在肺癌、乳腺癌、结肠癌等恶性肿瘤中表达上调,与相关恶性肿瘤的发生、发展均有较明确的临床意义[7]。研究[15]表明CK19上调与肝癌患者的预后不良有关。
本研究结果显示,与膀胱良性疾病患者和健康人群相比,膀胱癌患者血清中TAP和CK19表达明显上调,与以往研究[16-17]结果一致。本研究结果显示良性组与对照组血清TAP和CK19表达水平无统计学差异(均P > 0.05),说明TAP和CK19的表达主要来源于肿瘤细胞,提示二者可作为膀胱癌标志物用于早期诊断。诊断价值分析结果显示,TAP与CK19联合检测的诊断价值高于单独检测,因此TAP与CK19联合检测有助于膀胱癌的早期诊断。
综上所述,膀胱癌患者血清中TAP及CK19高表达,二者表达水平与肿瘤大小相关,TAP及CK19有望成为膀胱癌诊断的肿瘤标志物;TAP和CK19联合检测有助于提高膀胱癌的诊断价值。本研究的不足之处:(1)样本量较小,尚需大样本、多中心研究完善检测体系;(2)入组膀胱癌患者术后未进行TAP和CK19表达水平的复检和系统的病程随访,无法通过TAP及CK19的检测水平评估患者预后情况,因此须进一步研究论证。
| [1] |
POWLES T, BELLMUNT J, COMPERAT E, et al. Bladder cancer: esmo Clinical Practice Guideline for diagnosis, treatment and follow-up[J]. Ann Oncol, 2022, 33(3): 244-258. DOI:10.1016/j.annonc.2021.11.012 |
| [2] |
SIEGEL RL, MILLER KD, FUCHS HE, et al. Cancer statistics, 2022[J]. CA A Cancer J Clin, 2022, 72(1): 7-33. DOI:10.3322/caac.21708 |
| [3] |
Bladder cancer: diagnosis and management of bladder cancer[J]. BJU Int, 2017, 120 (6): 755-765. DOI: 10.1111/bju.14045.
|
| [4] |
BABJUK M, BURGER M, COMPÉRAT EM, et al. European association of urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ)-2019 update[J]. Eur Urol, 2019, 76(5): 639-657. DOI:10.1016/j.eururo.2019.08.016 |
| [5] |
CHENG YJ, CHEN YB, ZANG GH, et al. Increased expression of TAP is predictive of poor prognosis in patients with non-small cell lung cancer[J]. Cancer Manag Res, 2020, 12: 1941-1946. DOI:10.2147/CMAR.S239593 |
| [6] |
LIU J, HUANG XN. Clinical application of serum tumor abnormal protein from patients with gastric cancer[J]. Asian Pac J Cancer Prev, 2015, 16(9): 4041-4044. DOI:10.7314/apjcp.2015.16.9.4041 |
| [7] |
VERMA V, RAO RN. Cytokeratin 19 expression in intrathoracic neo-plasms: first study utilizing cellblocks, evaluating the role of a rarely used cytokeratin for lung cancers[J]. Diagn Cytopathol, 2022, 50(3): 105-111. DOI:10.1002/dc.24927 |
| [8] |
YIN D, JIANG Y, WANG N, et al. The diagnostic value of serum hybrid capture 2 (CH2) HPV DNA in cervical cancer: a systematic review and meta-analysis[J]. Tumor Biol, 2014, 35(9): 9247-9253. DOI:10.1007/s13277-014-2214-4 |
| [9] |
DEGEORGE KC, HOLT HR, HODGES SC. Bladder cancer: diagnosis and treatment[J]. Am Fam Physician, 2017, 96(8): 507-514. |
| [10] |
HENTSCHEL AE, BEIJERT IJ, BOSSCHIETER J, et al. Bladder cancer detection in urine using DNA methylation markers: a technical and prospective preclinical validation[J]. Clin Epigenetics, 2022, 14(1): 19. DOI:10.1186/s13148-022-01240-8 |
| [11] |
JORDAN B, MEEKS JJ. T1 bladder cancer: current considerations for diagnosis and management[J]. Nat Rev Urol, 2019, 16(1): 23-34. DOI:10.1038/s41585-018-0105-y |
| [12] |
FENG F, TIAN YZ, XU GH, et al. Diagnostic and prognostic value of CEA, CA19-9, AFP and CA125 for early gastric cancer[J]. BMC Cancer, 2017, 17(1): 737. DOI:10.1186/s12885-017-3738-y |
| [13] |
CHENG YJ, FANG QR, CHEN YB, et al. High expression of tumor abnormal protein preoperatively predicts poor prognosis of patients with esophageal squamous cell carcinoma[J]. Front Surg, 2021, 8: 609719. DOI:10.3389/fsurg.2021.609719 |
| [14] |
WU XY, HUANG XN. Clinical application of serum tumor abnormal protein (TAP) in colorectal cancer patients[J]. Asian Pac J Cancer Prev, 2015, 16(8): 3425-3428. DOI:10.7314/apjcp.2015.16.8.3425 |
| [15] |
ZHUO JY, LU D, TAN WY, et al. CK19-positive hepatocellular carcinoma is a characteristic subtype[J]. J Cancer, 2020, 11(17): 5069-5077. DOI:10.7150/jca.44697 |
| [16] |
ZHANG LQ, GUO XX, MIN YZ, et al. Tumor abnormal protein (TAP) examination contributes to primary diagnosis of bladder cancer[J]. Int J Clin Exp Med, 2015, 8(10): 18528-18532. |
| [17] |
APOLLO A, ORTENZI V, SCATENA C, et al. Molecular characterization of low grade and high grade bladder cancer[J]. PLoS One, 2019, 14(1): e0210635. DOI:10.1371/journal.pone.0210635 |
 2023, Vol. 52
2023, Vol. 52