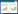文章信息
- 贺晓文, 边竞
- HE Xiaowen, BIAN Jing
- 鼠尾草酸通过抑制阿尔茨海默病小鼠脑内炎症反应减少β淀粉样蛋白沉积的机制
- Mechanism behind carnosic acid-induced reduction of amyloid-β protein deposition in brains of Alzheimer's disease
- 中国医科大学学报, 2023, 52(7): 590-594, 600
- Journal of China Medical University, 2023, 52(7): 590-594, 600
-
文章历史
- 收稿日期:2022-12-02
- 网络出版时间:2023-07-06 15:30:16
阿尔茨海默病(Alzheimer’s disease,AD)又称为老人失智症、老年痴呆症、脑退化症,是最常见的神经退行性疾病。目前,AD已成为世界上最常见的痴呆类型之一。且随着世界老龄化人口增加,AD发病率逐年上升。由于AD暂无有效的防治手段,给患者家庭和社会均带来沉重的经济负担。因此,找到AD的发病原因并寻找有效的预防手段尤其重要[1]。
AD的病因不明,其主要病理特征为神经元内tau蛋白过度磷酸化形成的神经原纤维缠结和胞外剪切生成的以β淀粉样蛋白(amyloid β-protein,Aβ)沉积形成的老年斑[2-3]。大量研究结果显示,炎症反应是AD发病的主要原因之一,脑内沉积的Aβ可通过与神经胶质细胞上的受体结合,激活星形胶质细胞和小胶质细胞,释放大量炎性细胞因子和趋化因子,炎性细胞因子包括NOD样受体蛋白3(NOD-like receptor protein 3,NLRP3)、肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)、白细胞介素(interleukin,IL)-6和IL-1β等。这些炎性细胞因子可进一步促进淀粉样前体蛋白(amyloid precursor protein,APP)向淀粉样蛋白剪切途径进行,促使Aβ生成增多,最终形成恶性循环,加快AD的进程[4-5]。
近年来,针对AD的治疗药物越来越多,主要包括胆碱酯酶抑制剂(多奈哌齐等)、谷氨酸受体拮抗剂(美金刚)以及甘露特纳,但是这些药物都无法达到预期的治疗效果,且存在潜在的不良反应[6-7]。鼠尾草酸(carnosic acid,CA)主要存在于鼠尾草和迷迭香等植物中,具有抗炎、抗氧化和抗菌等作用[8-10]。研究[11]发现,CA能够抑制多种皮肤炎症,且对人体无毒。此外,CA能够通过降低TNF-α的表达,抑制细胞内炎症反应[12]。因此,CA可能具有广泛的抗炎作用。然而,CA能否通过降低神经炎症反应减少Aβ生成和沉积数量,进而延缓AD的病理进程,尚未见报道。因此,本研究通过给予AD小鼠CA,研究CA对AD的影响,并探索其是否可以通过核因子κB(nuclear factor-κB,NF-κB)抑制小鼠大脑皮层免疫炎症反应,从而改善AD小鼠的认知功能障碍,最终延缓AD的发病进程。
1 材料与方法 1.1 材料 1.1.1 药品和试剂盒CA,购自中国MedChemExpress公司;NLRP3、TNF-α、IL-6、IL-1β、NF-κB、p-NF-κB、Aβ抗体、β-actin、二抗、Alexa Fluor 555标记的荧光二抗,购自美国Cell Signaling Technology公司。IL-1β、IL-6和TNF-α的ELISA试剂盒,购自美国Abcam公司。
1.1.2 仪器Morris水迷宫,购自中国安徽正华生物仪器设备有限公司;激光共聚焦显微镜,购自日本尼康公司;酶标仪,购自美国伯腾仪器有限公司。
1.1.3 实验动物和分组5月龄APP/PS1转基因小鼠(AD模型)18只,购自北京华阜康生物科技有限公司,体质量25~28 g。将18只小鼠分为对照组和CA组,每组9只。CA组:腹腔注射CA(20 mg·kg-1·d-1),连续注射2个月;对照组:腹腔注射相同体积的生理盐水,连续注射2个月。
1.2 方法 1.2.1 Morris水迷宫实验实验分为3个时期。训练期为2 d,3次/d,每次间隔1 h;可视平台期为5 d,3次/d,每次间隔1 h;第8天为隐蔽平台期,移走平台,记录小鼠穿越原来平台位置的次数。每只小鼠进行3次实验。
1.2.2 组织取材10%水合氯醛(10 g/3 μL)麻醉小鼠,待小鼠进入深度麻醉状态(手术镊子夹小鼠四肢未见反应即可),立刻将小鼠固定于解剖台上。剖开胸腔并剪去横膈膜,暴露心脏,在左心室左下角开一个小口,将灌流针由左心室插入主动脉,并用止血钳固定住针头,剪开右心耳,立即用0.9% NaCl灌流,灌流4~6 min,至肝脏边缘无血丝为止。迅速断头,剖取小鼠大脑,于冰上一分为二。一部分放入4%多聚甲醛中固定保存,用于免疫组织化学中的形态学检测;另一部分放入-80 ℃冰箱保存,用于蛋白提取、Western blotting等分子生物学检测。
1.2.3 Western blotting前额皮层组织称重,加入RIPA裂解液(内含蛋白酶抑制和磷酸酶抑制剂)后进行超声破碎。破碎后,放置冰上裂解3~4 h,4 ℃、12 000 r/min离心15 min,吸取上清,进行蛋白质定量后深度低温保存,用于Western blotting检测。10%SDS聚丙烯酰胺凝胶电泳分离胶,转膜完成后,PVDF膜用2%脱脂牛奶封闭,抗体4 ℃孵育过夜。第2天二抗室温孵育45 min,洗膜,ECL发光并显影。
1.2.4 免疫荧光染色切片水化后,用羊血清封闭2 h,一抗4 ℃孵育过夜。荧光二抗室温孵育2 h,PBS漂洗后,防荧光淬灭剂封片,显微镜下采集图像。
1.2.5 ELISA检测按照试剂盒操作,测定小鼠皮层中炎性细胞因子NLRP3、TNF-α、IL-6、IL-1β的含量。
1.3 统计学分析采用Prism7.0软件进行统计学分析。数据用x±s表示,组间比较采用t检验。P < 0.05为差异有统计学意义。
2 结果 2.1 CA对AD小鼠学习记忆能力的调控水迷宫实验结果显示,对照组小鼠和CA组小鼠寻找可视平台的时间无统计学差异(P > 0.05),说明2组小鼠的运动能力和视力无显著差异。隐蔽平台实验结果显示,与对照组相比,CA组小鼠逃避潜伏期明显缩短(P < 0.05),说明CA可提高小鼠的记忆能力,且可明显缓解AD小鼠的空间记忆损伤(P < 0.05)。见图 1。
|
| A, the time taken by mice to find the visual platform in the first two training days; B, escape latency of mice in the hidden platform experiment; C, the number of times and swimming routes of mice passing through the central area in the space exploration experiment. *P < 0.05 vs control group. 图 1 CA对AD小鼠空间学习记忆能力的影响 Fig.1 Effects of carnosic acid on spatial learning and memory abilities of AD mice |
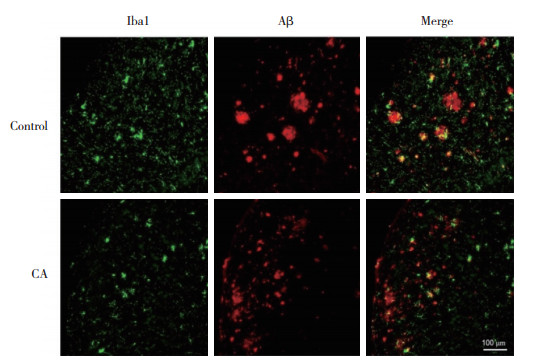
2.2 CA对AD小鼠大脑皮层中Aβ沉积数量和胶质细胞活化的影响
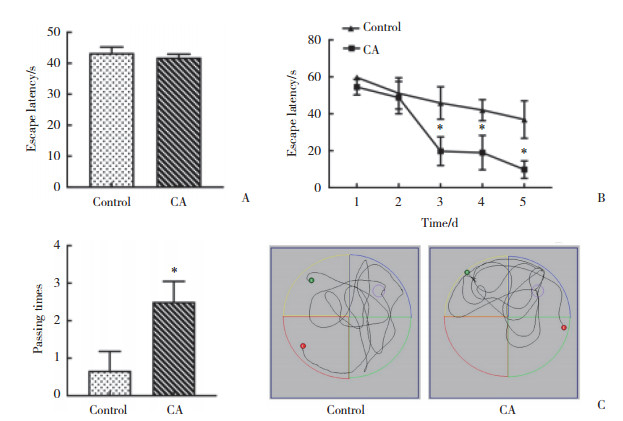
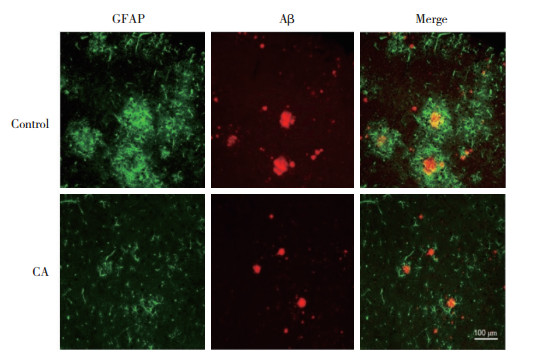
免疫荧光双标记染色结果显示,对照组小鼠的大脑皮层出现大量Aβ老年斑沉积,并且其周围的神经胶质细胞明显增多。与对照组相比,CA组小鼠大脑皮层中Aβ老年斑数量明显减少,星形胶质细胞和小胶质细胞明显减少。说明CA可能通过抑制星形胶质细胞和小胶质细胞的活化减少Aβ的生成,进而延缓AD的发病进程。见图 2、3。
|
| 图 2 皮层中Aβ老年斑沉积和星形胶质细胞的共定位情况 Fig.2 The co-localization of Aβ senile plaques and astrocytes in the cortical area |
|
| 图 3 皮层中Aβ老年斑沉积和小胶质细胞的共定位情况 Fig.3 The co-localization of Aβ senile plaques and microglia in the cortical area |
2.3 CA对AD小鼠大脑皮层中炎性细胞因子的影响
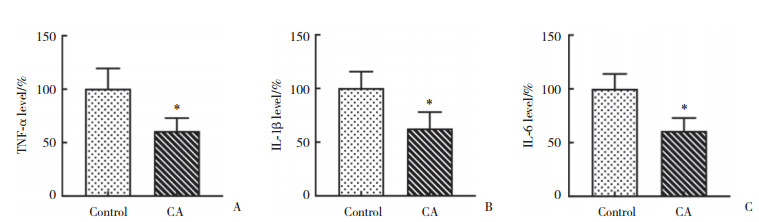
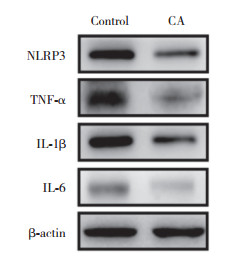
ELISA结果(图 4)显示,与对照组相比,CA组小鼠TNF-α、IL-6和IL-1β蛋白表达水平明显降低(P < 0.05)。Western blotting结果(图 5)进一步证实,与对照组相比,CA组小鼠NLRP3、TNF-α、IL-6和IL-1β的表达水平明显降低(P < 0.05),CA抑制NLRP3、TNF-α、IL-6和IL-1β的表达。说明CA可明显抑制AD小鼠脑内炎症反应。
|
| A, TNF-α; B, IL-1β; C, IL-6. *P < 0.05 vs control group. 图 4 ELISA检测CA对AD小鼠脑内炎性细胞因子的影响 Fig.4 Effects of carnosic acid on inflammatory factors in the brains of AD mice, as detected by ELISA |
|
| 图 5 Western blotting检测CA对AD小鼠脑内炎性细胞因子的影响 Fig.5 Effects of carnosic acid on inflammatory factors in the brains of AD mice, as detected by Western blotting |
2.4 CA通过NF-κB信号通路抑制AD小鼠脑内炎性细胞因子表达
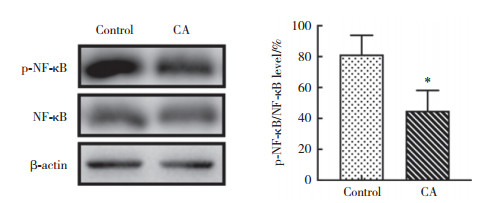
与对照组相比,CA组p-NF-κB的表达明显降低(P < 0.05)。说明CA可能通过NF-κB信号通路抑制AD小鼠免疫炎症反应,进而改善小鼠的学习记忆能力。见图 6。
|
| *P < 0.05 vs control group. 图 6 CA对AD小鼠脑内NF-κB信号通路的影响 Fig.6 Effects of carnosic acid on NF-κB signaling in the brains of AD mice |
3 讨论
Aβ形成过程中伴随着神经胶质细胞活化,产生大量的炎性细胞因子,包括TNF-α、IL-6和IL-1β等。而小胶质细胞持续激活削弱了其结合和吞噬Aβ的能力,并降低Aβ的降解,进一步增加Aβ的沉积数量,加快AD的发病进程。
NF-κB在神经系统中神经元损伤的细胞反应中发挥重要作用。NF-κB通过调节细胞因子和免疫应答基因的转录来调节免疫。在刺激作用下,NF-κB从细胞质转位到细胞核,诱导靶基因转录[13]。重要的是,AD患者死后脑退化细胞中可见明显的NF-κB活化。在胶质增生过程中,Aβ神经毒性依赖于NF-κB [14]。相反,阻断NF-κB可减轻IL-1β诱导的变性[15]。NF-κB抑制被认为是减少神经炎症损伤,从而延缓AD发病进程的潜在靶点。
CA是一种天然化合物,是从迷迭香中分离得到的一种酚类二萜,因其药理作用而受到越来越多的关注。研究[9]证实,CA可逆转Aβ引起的海马神经元损伤,进而起到保护神经元的作用。此外,CA可通过抑制神经胶质细胞产生的炎性细胞因子,改善AD小鼠的认知功能障碍。
本研究检测了炎性细胞因子NLRP3、TNF-α、IL-6和IL-1β的表达,结果发现,与对照组相比,CA组NLRP3、TNF-α、IL-6和IL-1β蛋白表达明显降低,p-NF-κB表达明显降低,提示CA可能通过NF-κB信号通路抑制炎性细胞因子的表达,进而抑制AD小鼠脑内的免疫炎症反应,最终延缓AD的病理进程。免疫荧光双标记染色结果进一步揭示,CA可抑制AD小鼠脑内Aβ的生成以及减少Aβ的沉积数量,抑制神经胶质细胞的活化。行为学实验证实,CA改善AD小鼠的认知功能障碍,为今后在临床上CA防治AD提供实验基础和理论依据。
综上所述,本研究结果表明,CA可通过抑制APP/PS1转基因小鼠脑内免疫炎症反应,进而减少AD小鼠脑内的Aβ沉积数量,最终改善AD的认知功能障碍。本研究为CA今后用于防治AD提供了有力的理论依据。
| [1] |
Alzheimer's ASSOCIATION. 2011 Alzheimer's disease facts and figures[J]. Alzheimers Dement, 2011, 7(2): 208-244. DOI:10.1016/j.jalz.2011.02.004 |
| [2] |
KAWAHARA M, KATO-NEGISHI M, TANAKA K. Cross talk between neurometals and amyloidogenic proteins at the synapse and the pathogenesis of neurodegenerative diseases[J]. Metallomics, 2017, 9(6): 619-633. DOI:10.1039/c7mt00046d |
| [3] |
HARDY J, SELKOE DJ. The amyloid hypothesis of Alzheimer's di-sease: progress and problems on the road to therapeutics[J]. Science, 2002, 297(5580): 353-356. DOI:10.1126/science.1072994 |
| [4] |
PILLAI JA, BENA J, BEBEK G, et al. Inflammatory pathway analytes predicting rapid cognitive decline in MCI stage of Alzheimer's disease[J]. Ann Clin Transl Neurol, 2020, 7(7): 1225-1239. DOI:10.1002/acn3.51109 |
| [5] |
YANG W, LIU Y, XU QQ, et al. Sulforaphene ameliorates neuroinflammation and hyperphosphorylated tau protein via regulating the PI3K/Akt/GSK-3β pathway in experimental models of Alzheimer's disease[J]. Oxid Med Cell Longev, 2020, 2020: 4754195. DOI:10.1155/2020/4754195 |
| [6] |
JIA J, WEI C, CHEN W, et al. Safety and efficacy of donepezil 10 mg/day in patients with mild to moderate Alzheimer's disease[J]. J Alzheimers Dis, 2020, 74(1): 199-211. DOI:10.3233/JAD-190940 |
| [7] |
WANG X, SUN G, FENG T, et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer's disease progression[J]. Cell Res, 2019, 29(10): 787-803. DOI:10.1038/s41422-019-0216-x |
| [8] |
KUO CF, SU JD, CHIU CH, et al. Anti-inflammatory effects of supercritical carbon dioxide extract and its isolated carnosic acid from Rosmarinus officinalis leaves[J]. J Agric Food Chem, 2011, 59(8): 3674-3685. DOI:10.1021/jf104837w |
| [9] |
GRACE MH, QIANG Y, SANG SM, et al. One-step isolation of carnosic acid and carnosol from rosemary by centrifugal partition chromatography[J]. J Sep Sci, 2017, 40(5): 1057-1062. DOI:10.1002/jssc.201601063 |
| [10] |
LIXANDRU BE, DRĂCEA NO, DRAGOMIRESCU CC, et al. Antimicrobial activity of plant essential oils against bacterial and fungal species involved in food poisoning and/or food decay[J]. Roum Arch Microbiol Immunol, 2010, 69(4): 224-230. |
| [11] |
CALNIQUER G, KHANIN M, OVADIA H, et al. Combined effects of carotenoids and polyphenols in balancing the response of skin cells to UV irradiation[J]. Molecules, 2021, 26(7): 1931. DOI:10.3390/molecules26071931 |
| [12] |
LIU M, ZHOU X, ZHOU L, et al. Carnosic acid inhibits inflammation response and joint destruction on osteoclasts, fibroblast-like synoviocytes, and collagen-induced arthritis rats[J]. J Cell Physiol, 2018, 233(8): 6291-6303. DOI:10.1002/jcp.26517 |
| [13] |
KABE Y, ANDO K, HIRAO S, et al. Redox regulation of NF-κB activation: distinct redox regulation between the cytoplasm and the nucleus[J]. Antioxid Redox Signal, 2005, 7(3/4): 395-403. DOI:10.1089/ars.2005.7.395 |
| [14] |
KIM YM, PARK S, CHOI SY, et al. Clusterin binding modulates the aggregation and neurotoxicity of amyloid-β (1-42)[J]. Mol Neurobiol, 2022, 59(10): 6228-6244. DOI:10.1007/s12035-022-02973-6 |
| [15] |
GUERRERO A, DE STROOPER B, ARANCIBIA-CÁRCAMO IL. Cellular senescence at the crossroads of inflammation and Alzhei-mer's disease[J]. Trends Neurosci, 2021, 44(9): 714-727. DOI:10.1016/j.tins.2021.06.007 |
 2023, Vol. 52
2023, Vol. 52